Abstract
Boesenbergia rotunda has been used as an antiobesity agent by suppressing adipogenesis. This study aimed to investigate the biological activity of B. rotunda on preadipocyte cells and to evaluate the effectiveness and safety of using B. rotunda extract in a capsaicin-loaded body-firming formulation. The antiadipogenesis of B. rotunda ethanolic extract was evaluated in 3T3-L1 preadipocyte cells. After the application of the B. rotunda extract-loaded body-firming formulation on the skin of volunteers for 28 d, thigh circumference, melanin index, and skin erythema were investigated. The results showed that the ethanolic extract of B. rotunda was not toxic toward 3T3-L1 cells at concentrations lower than 20 µg/mL, with antiadipogenesis of the B. rotunda extract occurring at a concentration of 1 µg/mL. The B. rotunda extract containing panduratin A was mixed with capsaicin body-firming products and successfully permeated into and through the skin. Applying this formulation to the thighs of the volunteers two times a day for 21 days led to a significant reduction in thigh circumference and melanin index. A slight elevation in skin erythema was observed, but there was no significant increase in redness or pain. In conclusion, the B. rotunda extract contained bioactive compounds that inhibited antiadipogenesis. The formulations containing B. rotunda extract and capsaicin showed potential as effective body-firming products.
1. Introduction
Topical body-firming products are formulated to target thigh circumference, skinfold thickness, and fat mass [1]. Subcutaneous adipose tissue primarily consists of adipocytes, a type of loose connective tissue beneath the skin. Various approaches have been employed to address subcutaneous fat, with liposuction as one option for removing excess adipose tissue [2]. However, the inherent limitations and safety concerns associated with liposuction must be understood to mitigate the risk of serious complications with unfavorable outcomes [3].
Capsaicin, an active compound in chili pepper (Capsicum frutescens Linn. [Solonaceae]), has been found to increase metabolic rate. The ingestion of chili pepper or its derivatives reduced abdominal fat [4] and increased energy expenditure via the activation of brown adipose tissue, which enhanced thermogenesis (the generation of heat from fat), ultimately aiding in fat reduction [5]. For topical application, capsaicin is usually mixed with cream or gel or used as patches in pain relief treatments. Capsaicin enhances the sensation of warmth and increases blood flow in the applied area, and this promotes the breakdown of fat cells through a process called lipolysis [6]. Combinations of other medical herbs are also used as alternative formulations for safe and effective topical application.
Boesenbergia rotunda (L.) Mansf. [Zingiberaceae] is recognized as a medicinal plant and contains various bioactive compounds, including pinostrobin, cardamon, boesenbergin, 5,7-dihydroxy-flavone, 1,8-cineole, and pandurate A [7]. Panduratin A is known for its active biological properties and also exhibits anti-inflammatory, antioxidative, antibacterial, and antiobesity activities [8]. Topical body-firming products containing panduratin A have also been reported to reduce thigh circumference and increase skin hydration and firmness [9].
The synergistic impacts of antiobesity agents from B. rotunda extract and capsaicin in body-firming products were examined for their effectiveness and safety for topical application. The biological activity of B. rotunda on 3T3-L1 preadipocytes was investigated to formulate an effective body-firming product. The bioactivity of 3T3-L1 preadipocyte cells was also assessed, and B. rotunda extract in a capsaicin-loaded body-firming formulation was prepared and evaluated for safety and efficacy in an in vivo human study.
2. Materials and Methods
2.1. Materials
Dried B. rotunda rhizome powder (Lot No. 6503120) was purchased from Vejpong Osot Co., Ltd., Bangkok, Thailand, while the ethanolic extract of B. rotunda (5.90% yield) was sourced from the Faculty of Pharmaceutical Sciences, Ubon Ratchathani University, Thailand [9]. Capsaicin resin was purchased from Specialty Natural Products Co., Ltd., Bangkok, Thailand, while cold-pressed coconut oil was obtained from Krungthep Chemi Co., Ltd., Bangkok, Thailand. Mouse 3T3-L1 preadipocytes, Dulbecco’s Modified Eagle’s Medium (DMEM), and bovine calf serum (BCS) were purchased from the American-Type Culture Collection (ATCC), Rockville, MD, USA. Fetal bovine serum (FBS), L-glutamine (GlutamaxTM), non-essential amino acids, penicillin, and streptomycin were purchased from Gibco BRL, MD, USA. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), 3-isobutyl-1-methylxanthine (IBMX), insulin, and Oil Red O solution were bought from Sigma-Aldrich, St. Louis, MO, USA, while dexamethasone was purchased from G-Biosciences, St. Louis, MO, USA. (±)Panduratin A (purity > 98%) was isolated and analyzed at Mahidol University, Thailand. All chemical agents were analytical-reagent-grade.
2.2. Cytotoxicity Study of B. rotunda on 3T3-L1 Cells
Mouse 3T3-L1 preadipocyte cells (2 × 103 cells/well) were cultured in a 96-well plate with a preadipocyte medium (DMEM with 10% BCS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin) under a humidified atmosphere (5% CO2, 95% RH, 37 °C) for 48 h until 70–80% cell confluency. The cells were then washed with phosphate-buffered saline (PBS) at pH 7.4 and treated with a differentiation medium (DMEM with 10% FBS, 0.5 mM of IBMX, 1.0 μM of dexamethasone, and 1.0 μg/mL of insulin) at 37 °C, 5% CO2, and 95% RH for 48 h of incubation. After the removal of the differentiation medium, an adipocyte maintenance medium (DMEM with 10% FBS and 1.0 μg/mL of insulin) was added to the cells every 48–72 h. Lipid droplet formation was completed over 8 d.
The cytotoxicity of the pre-treated B. rotunda extract was assessed at various concentrations (0–50 µg/mL) by dilution. After removing the preadipocyte medium from the cell plates, each concentration of B. rotunda extract was added, and the procedure was performed as described above. For post-treated cells with B. rotunda extract, lipid droplets of 3T3-L1 adipocyte cells were treated with different concentrations of the diluted extract for 48 h. Both preadipocyte and mature adipocyte 3T3-L1 cells were assessed for cell viability using the MTT assay. The treated cells were washed with PBS at pH 7.4 before mixing with the MTT solution (0.5 mg/mL) and left for 3 h. The medium was then replaced with 100 µL of DMSO to dissolve the formazan crystals that formed in the living cells. The absorbance was measured using a microplate reader (VICTOR NivoTM Multimode Plate Reader, PerkinElmer, Rodgau, Germany) at 550 nm, and the percentage of cell viability was calculated following Equation (1).
2.3. Antiadipogenesis Evaluation with Oil Red O Staining Assay
After treating the cells with the B. rotunda extract for 8 d, the lipid droplets were visualized by staining with an Oil Red O solution. In brief, the differentiated adipocytes were washed with PBS at pH 7.4 before fixing the cells with 10% formalin for 30 min and then washing again. The treated cells were then incubated with 60% isopropanol for 5 min. After removing the isopropanol, the cells were incubated with 0.5% (w/v) Oil Red O solution at 37 °C, 5% CO2, and 95% RH for 1 h. The stained cells were visualized using a microscope (Nikon ECLIPSE TE2000-S, Tokyo, Japan) [9].
2.4. Preparation of B. rotunda in Body-Firming Formulation
The dried B. rotunda rhizome powder was extracted with cold-pressed coconut oil in a ratio of 1:5 in an ultrasonic bath for 30 min. Maceration for 24 h was then performed with continuous stirring by a magnetic stirrer, followed by centrifugation at 4000 rpm for 15 min to collect the supernatant. After that, 25.0% (w/w) B. rotunda extract was mixed with 10.0% (w/w) nanovesicle-loading capsaicin in a gel base containing water, carbopol, glycerin, menthol, Viscolam AT 100P (sodium polyacryloyldimethyl taurate, hydrogenated polydecene, and trideceth-10), butylene glycol, germabenTM II, ethanol, and triethanolamine. The final capsaicin concentration in the body-firming formulation was 0.008% (w/w). The physicochemical properties of the formulation, including appearance, viscosity, pH, and panduratin A content, were then determined. The viscosity and pH of the body-firming formulations were determined with a rheometer (Thermo ScientificTM HAAKETM rheometer, Thermo Fisher Scientific, Bangkok, Thailand) and a pH meter (pH meter SevenDirect SD50, Mettler Toledo, Bangkok, Thailand). Panduratin A content was analyzed by HPLC.
2.5. HPLC Analysis
Both the ethanolic extract and the oily extract of B. rotunda contained panduratin A, a major bioactive compound. Briefly, each sample was diluted with 50 µL of ethanol and injected into an HPLC (Thermo Scientific™ Dionex™ UltiMate 3000 System, Germering, Germany), equipped with a C18 HPLC column (VertiSepTM GES, 4.6 × 250 mm, 5 µm pore size, Vertical Chromatography Co., Ltd., Bangkok, Thailand) and a 285 nm UV detector. The mobile phase consisted of a gradient system of acetonitrile and phosphoric acid (0.1% v/v), starting with 20% acetonitrile and reaching 80% in the final phase. The flow rate was set at 1.5 mL/min. Each run lasted for 60 min, and the retention time of panduratin A was around 54 min [9].
2.6. In Vitro Skin Permeation and Deposition Study
Abdominal porcine skin was obtained from an intrapartum stillborn animal at a local farm in Nakhon Pathom Province, Thailand. Study approval was obtained from the Investigational Review Board (07/2567/IACUC, Animal Experimentation Ethics Committee, Ubon Ratchathani University). The subcutaneous layers were removed using medical scissors, and skins measuring 600–700 µm in thickness were stored in a refrigerator at −20 °C until utilized.
In vitro skin permeation was studied using vertical Franz-type diffusion cells. The receptor medium, 12 mL of PBS at pH 7.4 with polysorbate 20 (5 g/L) and ethanol in a 1:1 ratio, was continuously stirred using a magnetic stirrer and maintained at 32 ± 0.5 °C. Each piece of skin was inserted between the donor and receptor compartments. One gram of the formulation was applied to the skin surface for 8 h, and the receptor medium was collected for the skin permeation study. Each treated piece of skin was then washed, cut into small pieces, and subjected to extraction with ethanol using an ultrasonic bath for 30 min. The quantity of panduratin A that permeated and was deposited into the skin was analyzed in triplicate by HPLC [9].
2.7. In Vivo Human Skin Study
Fifteen healthy volunteers (aged from 17 to 50 years old) agreed to participate in this clinical trial and signed the informed consent approved by the Investigational Review Board (UBU-REC-75/2566, Human Studies Ethics Committee, Ubon Ratchathani University). The inclusion criteria were a body mass index (BMI) higher than 24.00 and a fat composition in the thigh higher than 0. The exclusion criteria were skin disorders, taking antifungal medicines or antihistamine drugs, and patients with congenital disease. In this study, the volunteers had a BMI in the range of 24.80 to 36.10 and a distribution in the thigh of fat in the range of 0 to +4 (medium to high), determined with a segmental body composition analyzer (Tanita corporation® MC-980, Tokyo, Japan). The participants were not allergic to B. rotunda, capsaicin, or any other components in the firming cream formulations. During the study, the skin was not treated with other moisturizing products. Each volunteer was asked to apply 0.5 mL of the firming product on the thigh twice a day after taking a bath in the morning and evening for four consecutive weeks, with skin examinations performed every week.
Erythema and melanin contents were evaluated with the Mexameter® MX 18 probe of a multiprobe adapter 9 device (Courage-Khazaka Electronic GmbH, Cologne, Germany) after applying the formulation for 7, 14, 21, and 28 d. Skin appearance was observed using a Dino-Lite Edge digital microscope (AM7915 Series, Hsinchu City, Taiwan). Thigh circumference was also determined, with the percentage change calculated (%CT) using Equation (2).
The impact of the body-firming formulations on erythema (vascularity) and melanin content was assessed by calculating the erythema index percentage (% EI) and melanin index (% MI). Vascularity and melanin were considered to be 100%, as depicted in Equations (3) and (4), respectively.
2.8. Data Analysis
All data were expressed as mean ± standard deviation (S.D.). Statistical significance was assessed using a one-way ANOVA, followed by Tukey’s post hoc test for in vitro studies. For the in vivo human study, the Wilcoxon signed-rank test was employed, with a significance level set at p < 0.05.
3. Results
3.1. Bioactivity of B. rotunda Extracts on 3T3-L1 Cells
As depicted in Figure 1, the extract exhibited no toxicity on the 3T3-L1 cells in either the preadipocyte or mature adipocyte stages within a concentration range of 1–20 µg/mL. The cell viability percentage ranged from 95.31 to 101.14% of the control between these tested concentrations, indicating the safety of this range for further experiments.
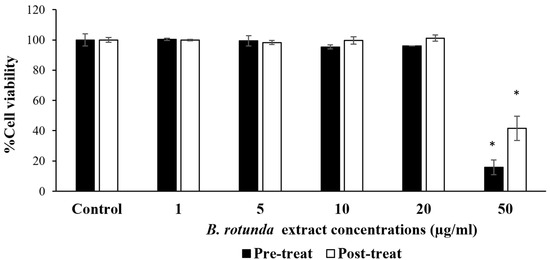
Figure 1.
Cell viability percentage of 3T3-L1 cells pre- and post-treatment with different concentrations of B. rotunda extract for 48 h. Data are presented as mean ± S.D. (n = 3). * denotes a significant difference from the control group (untreated cells) (p < 0.05).
As illustrated in Figure 2, 3T3-L1 preadipocytes served as the (+) control, while the differentiated 3T3-L1 cells stained with Oil Red O served as the (−) control. The lipid accumulation in adipocytes was visualized by staining the cells with Oil Red O, highlighting triglyceride accumulation in the adipocyte cells. Before adipogenic stimulation, the 3T3-L1 preadipocytes exhibited a fibroblast-like appearance. Following adipocyte differentiation, the number of Oil Red O-positive cells increased, and the lipid droplets showed size enlargement with a transition to round-shaped cells [10]. When assessing the antiadipogenic effect of the B. rotunda extract, it is important to note that the treated cells exhibited a lower number of lipid droplets compared to the differentiated cells ((−) control). Lipid accumulation diminished at B. rotunda extract concentrations of 1 and 20 µg/mL.
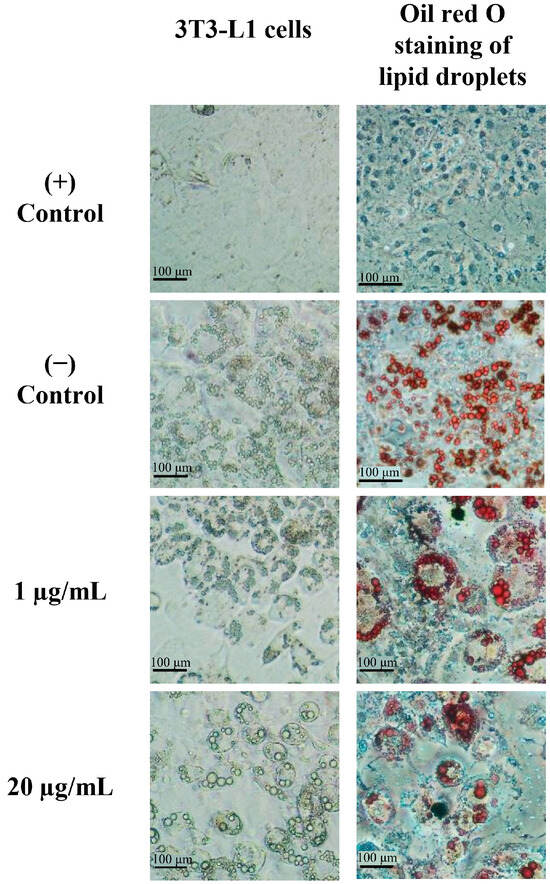
Figure 2.
Antiadipogenesis effect of B. rotunda on 3T3-L1 cells. The 3T3-L1 cells were cultured in normal conditions ((+) control), differentiated conditions ((−) control), and differentiated conditions with 1 and 20 µg/mL of B. rotunda, and oil droplets were stained with Oil Red O after treatment for 8 d.
3.2. Physicochemical Properties of B. rotunda in Capsaicin Body-Firming Formulations
The oily B. rotunda extract was a yellow-orange color with the odor of B. rotunda and coconut oil, with a panduratin A content of 1.18 ± 0.11 mg/mL. The B. rotunda extract mixed with the capsaicin body-firming formulation had a good appearance with suitable viscosity (77.61 ± 1.25 Pa), a neutral pH (7.13 ± 0.02), and a smooth texture when applied to the skin. The final concentration of panduratin A in the body-firming formulation was 0.03% (w/w). For the skin permeation and deposition study, the B. rotunda extract-loaded capsaicin body-firming product exhibited marked panduratin A permeation through the skin and was deposited into the skin after 8 h at concentrations of 5.08 ± 4.68 µg/cm2 and 0.83 ± 0.75 µg/cm2, respectively, suggesting the possibility of delivering active compounds through the skin into subcutaneous fat.
3.3. In Vivo Human Skin Study
After applying the B. rotunda extract-loaded capsaicin body-firming product for 28 d, the volunteers were monitored for changes in thigh circumference, melanin content, and skin erythema. No significant differences were recorded in the average body weight of the volunteers from the initial treatment day to Day 28 (data not shown). As shown in Figure 3, the thigh circumferences of all the volunteers significantly changed when measured on Days 21 and 28 compared with Day 1, ranging from −3.17 to +0.37 cm on Day 21 and −4.87 to +1.10 cm on Day 28. The mean values of the change in thigh circumference were −1.11 ± 1.19 cm on Day 21 and −0.91 ± 1.68 cm on Day 28. Therefore, this formulation provided a local effect on subcutaneous fat reduction in the tested area after application for more than 21 d.
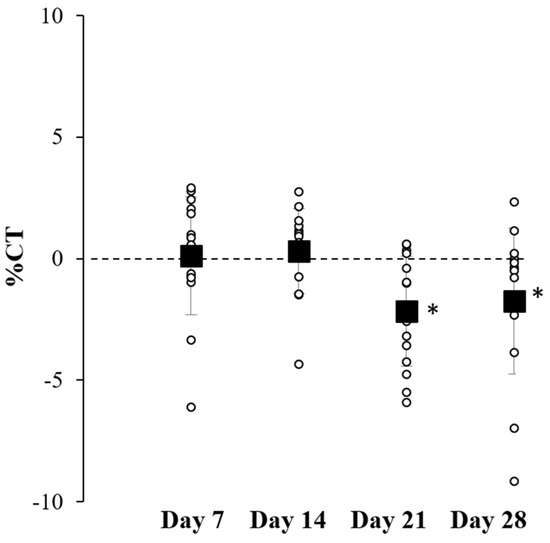
Figure 3.
Percentage change in thigh circumference (%CT) after applying B. rotunda extract in a body-firming product to the skin of healthy volunteers for 28 d. Symbols: ○ presents the value of each volunteer and ■ presents as mean ± S.D. (n = 15). * denotes a significant difference from Day 7 (p < 0.05).
Alterations in skin chromophore concentrations (melanin and hemoglobin) induced changes in both the melanin index (MI) and the erythema index (EI), as illustrated in Figure 4. The melanin index values showed a slight decrease after 7 days of application for all the formulations, while after 28 days, the percentage change in the melanin index (%MI) in the treated skin significantly differed from that in the untreated skin, with values ranging from 68.34% to 98.20%, suggesting a reduction in pigmentation. The erythema value represents irritant and allergic reactions. After formulation application, no significant change in the EI was observed in the treated skin, with values ranging from 77.11 to 117.99%. After using the formulation for 7, 14, 21, and 28 days, the average EI value for the skin treated with the B. rotunda extract in the capsaicin body-firming product slightly decreased, but with no significant difference from Day 1. A warm sensation was experienced after applying the formulation for 1–2 h with no pain or severe redness, indicating that the product did not induce skin irritation.
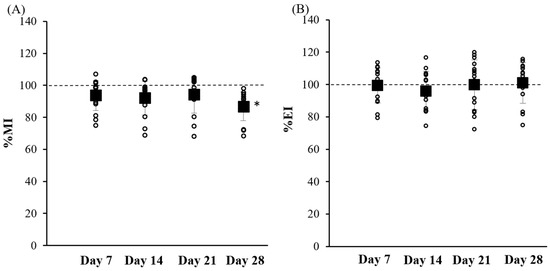
Figure 4.
(A) Melanin index (%MI) and (B) erythema index (%EI) values after applying the B. rotunda extract body-firming product to the skin of healthy volunteers for 28 d. Symbols: ○ presents the value of each volunteer and ■ presents as mean ± S.D. (n = 15). * denotes a significant difference from Day 7 (p < 0.05).
The changes in skin appearance after applying the formulation for 28 days are shown in Figure 5. No undesirable symptoms were found based on visual inspection. On Days 21 and 28, the skin images seem to be brighter and smoother than on the initial day. In this study, B. rotunda extract in capsaicin body-firming products was shown to be a safe and effective formulation.

Figure 5.
Images of changes in the skin after application of B. rotunda extract capsaicin-loaded body-firming product on Day 0, 7, 14, 21, and 28 (~133.6–151.2× magnification).
4. Discussion
The B. rotunda extract exhibited no cytotoxicity on preadipocytes or mature adipocytes, indicating the safety of this extract. A previous study also revealed that isolated panduratin A from B. rotunda did not exhibit cytotoxicity on skin cells (fibroblasts and keratinocytes), establishing its safety for use as a cosmeceutical ingredient [11]. For the antiadipogenesis effect, staining the treated cells with Oil Red O highlighted triglyceride accumulation in the adipocyte cells, and the antiadipogenic effect of the B. rotunda extract provided a low number of lipid droplets in the treated cells. This suggested that treating the 3T3-L1 adipocytes with the B. rotunda extract during adipogenesis markedly reduced triglyceride accumulation, thereby highlighting its antiobesity potential [8].
Five major compounds, namely alpinetin, pinocembrin, pinostrobin, 4-hydroxypanduratin A, and panduratin A, were identified in the B. rotunda extract via the HPLC chromatogram reported [12]. Among these, pinostrobin, 4-hydroxypanduratin A, and isopanduratin A have demonstrated anti-adipogenic effects [13,14]. In our study, panduratin A was used as a marker of the B. rotunda extract and strongly supported antiadipogenic properties in obese mice. Panduratin A was also used as the bioactive component of the B. rotunda ethanolic extract in 3T3 cells, HepG2 cells, animal models [8,15] and human studies [9]. Previous studies demonstrated that panduratin A, isolated from B. rotunda, possessed antiadipogenesis and lipolysis effects on 3T3-L1 cells, manifested by inhibiting the transformation process from preadipocytes to mature adipocytes through intercellular adipogenesis, ultimately resulting in decreased lipid content in the adipocytes [9,16]. However, pinostrobin is also a major constituent of B. rotunda (Figure S1) and provides antiobesity activity by suppressing adipogenesis and reducing cellular lipid droplets. Pinostrobin-treated 3T3-L1 cells showed lower levels of lipid metabolism-mediating proteins (C/EBPα, PPARγ, and SREBP-1c) and cellular triglyceride content [14]. Therefore, the bioactive components of B. rotunda could be a phytopharmaceutical source for obesity control and subcutaneous fat reduction.
To enhance their effectiveness, we incorporated the synergistic effects of antiobesity agents derived from B. rotunda extract and capsaicin in the formulation of body-firming products. An oily B. rotunda extract containing active panduratin A was successfully mixed with the capsaicin body-firming formulation, exhibiting a good appearance, suitable physical properties, and the possibility of delivering active compounds through the skin into subcutaneous fat. B. rotunda is used as a natural ingredient in food and as an ingredient in traditional massage oil [17]. Its extraction via edible coconut oil confirms its safety for skin application. At a concentration of 25% w/w, the B. rotunda extract produced a gel with a satisfying physical appearance and panduratin A content of 0.034% w/w. A previous study showed that 0.029% w/w of isolated panduratin A-loaded microspicule serum showed a potential lipolytic effect for reducing human subcutaneous fat mass [9]. For capsaicin, the lowest concentration of capsaicin cream available on the market is 0.0125%, which is indicated for pain relief [18]. However, since firming lotion is used over a wide body area and on healthy customers, we have designed it with a reduced concentration to prevent side effects, but the formulation still provides a warm sensation. Therefore, applying this formulation to the thighs of the volunteers for more than 21 days provided a local effect on subcutaneous fat reduction in the tested area.
A previous study found that the topical application of isolated panduratin A from B. rotunda loaded into a microsphere serum on the skin for 14 days resulted in a denser network of collagen and elastic fibers in the dermis and subcutis, suggesting a measurable increase in firmness due to the stimulation of lipolysis and the inhibition of lipid synthesis by the extract [9]. Panduratin A, previously identified as a novel natural adenosine monophosphate-activated protein kinase (AMPK) activator, promotes increased fatty acid oxidation by activating genes associated with fatty acid oxidation and also hinders lipid synthesis by reducing the phosphorylation of the sterol regulatory element-binding protein-1c (SREBP-1c) and peroxisome proliferator-activated receptor gamma (PPARγ). These effects culminate in weight loss and a reduction in fat pad mass [19]. Therefore, topical products containing active compounds from B. rotunda play important roles in reducing subcutaneous fat in the application area.
Several studies have examined the effectiveness of capsaicin-based body-firming products. A randomized controlled trial found that taking capsinoid significantly reduced abdominal fat in overweight individuals compared to a placebo group [4]. Capsaicin enhanced lipid oxidation and promoted weight loss when used in a mouse model [20]. Topical capsaicin formulations, including creams, lotions, and patches, contain capsaicin within a range of 0.025–0.1% (w/w). These formulations are commonly used without a prescription for the management of neuropathic and musculoskeletal pain. However, high concentrations of capsaicin are often linked with a burning sensation, potentially resulting in poor patient compliance [21,22]. The incorporation of capsaicin with other active ingredients, including black pepper seed extract, sweet orange peel, ginger root extract, green tea extract, cinnamon bark extract, and caffeine, resulted in an observed improvement in cellulite and a reduction in thigh circumference of 1.2 cm after 4 weeks [23]. Therefore, incorporating active compounds from B. rotunda in low concentrations of capsaicin-loaded body-firming products could provide an effective skin-tightening treatment without causing severe skin burns.
Additionally, applying B. rotunda extract in capsaicin-loaded body-firming products to the skin resulted in a slight decrease in melanin index, suggesting a reduction in pigmentation. Bioactive components in B. rotunda, such as (+)-panduratin A, (−)-isopanduratin A2, 4-hydroxypanduratin A, and capsaicin, were reported to have inhibitory effects on melanogenesis biosynthesis and tyrosinase activity. These compounds function as depigmenting agents, inhibiting melanin synthesis and reducing melanin accumulation in the skin. Furthermore, their antityrosinase activity hinders the catalyzed reaction of tyrosine hydroxylation to dihydroxyphenylalanine (DOPA), thereby inhibiting the accumulation of melanin as the end product [24,25,26].
B. rotunda is recognized as a food and medicinal plant which is developed into health products with various health claims. B. rotunda is edible, has low toxicity, and is safe to use in cosmetic products. However, its use in a capsaicin-loaded body-firming formulation might pose a great risk of side effects. Studies showed burning sensations and redness at capsaicin concentrations of 0.05–8.00% [27,28]. Even a low concentration of 0.0125% caused burning in 67% of knee osteoarthritis patients [18]. Discomfort from capsaicin may lead to discontinuing the use of the body-firming lotion before it has exerted its full therapeutic benefit. Therefore, studies on the safety of this formulation should be evaluated.
In terms of the safety of this formulation, no undesirable symptoms were found based on visual inspection. The erythema values of the skin after applying the formulation were not significantly different from those of untreated skin. For skin surface visualization, a dermatoscope was used as a readily available diagnostic method for visualizing the fine details of the outer layer of the skin that are not normally seen with the naked eye. A normal skin surface texture is composed of a series of transverse and diagonal primary lines, which intersect to form quadrilaterals and triangles [29]. After applying the formulation to the skin for 28 days, normal skin appearances were found, presenting a uniform texture, luminosity, no excessive shine, smoothness, and no apparent pores [30]. However, the images showed a little bit of a difference between magnifications, which should be evaluated with advanced visualization techniques in the future.
Although a warm sensation was experienced after applying the formulation for 1–2 h, no pain or severe redness was found. This indicated that the product did not induce skin irritation. The topical application of capsaicin compounds caused an intense burning sensation at both low and high doses, with a delay in the onset of mechanical hypersensitivity also observed. However, this compound is generally used for analgesia treatment in patients with neuropathic pain [31].
An improvement in skin properties was also observed after applying this product for 21 and 28 days. B. rotunda extract and its active compound, panduratin A, as potent nutraceuticals for enhancing skin hydration and barrier function, attributed to their roles in cornified envelope formation and filaggrin processing [32]. Many active ingredients in this formulation, especially coconut oil, are commonly used as components in anti-aging cosmetic products due to their moisturizing and antioxidant properties and ability to strengthen the skin barrier to support collagen regeneration [33]. Therefore, B. rotunda extract in capsaicin body-firming products is a safe and effective formulation.
5. Conclusions
The results demonstrated that the B. rotunda extract was not cytotoxic at a concentration of 20 µg/mL and showed antiadipogenesis against 3T3-L1 preadipocyte cells. The B. rotunda extract containing panduratin A was successfully mixed with a capsaicin gel base as a topical body-firming product and could permeate into and through the skin. In the in vivo human study, the application of this product to the thighs of the volunteers for 21 days showed significant reductions in thigh circumference and melanin index, with no pain or severe redness observed, indicating that the product was safe to use. The B. rotunda extract exhibited a potent bioactive inhibition of adipogenesis. Combining B. rotunda extract and capsaicin showed promise as an effective body-firming product for possible commercial applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/2079-9284/11/1/24/s1, Figure S1: HPLC chromatogram of (A) Panduratin A standard, (B) Pinostrobin standard, (C) B. rotunda ethanolic extract and (D) B. rotunda oily extract.
Author Contributions
Conceptualization, P.S., S.D. and W.R.; methodology, P.S., K.J., S.D. and W.R.; investigation, P.S., T.S., K.J., S.D. and W.R.; writing—original draft preparation, W.R.; writing—review and editing, P.S., S.D. and W.R.; visualization, P.S. and W.R.; supervision, P.O.; funding acquisition, T.S., W.R. and P.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agricultural Research Development Agency (public organization) (ARDA), grant number CRP6505030260, and the Faculty of Pharmaceutical Sciences, Ubon Ratchathani University, grant number 0604.11-6/2566.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Human Studies Ethics Committee, Ubon Ratchathani University (protocol code UBU-REC-75/2566 and date of approval: 13 June 2023) for studies involving humans. The animal study protocol was approved by the Institutional Review Board of the Animal Experimentation Ethics Committee, Ubon Ratchathani University (protocol code 07/2567/IACUC and date of approval: 4 December 2023), for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
The authors would like to thank the Faculty of Pharmaceutical Sciences, Ubon Ratchathani University and the Faculty of Pharmacy, Silpakorn University for their financial support and facility support. The authors would like to thank Sunhapas Soodvilai for the (±)Panduratin A (purity > 98%). We also thank Kanyarat Nokngam and Chittrawan Inhom as the research assistants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Escalante, G.; Bryan, P.; Rodriguez, J. Effects of a topical lotion containing aminophylline, caffeine, yohimbe, l-carnitine, and gotu kola on thigh circumference, skinfold thickness, and fat mass in sedentary females. J. Cosmet. Dermatol. 2019, 18, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Mittal, B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J. Med. Res. 2019, 149, 571–573. [Google Scholar] [PubMed]
- Dixit, V.V.; Wagh, M.S. Unfavourable outcomes of liposuction and their management. Indian J. Plast. Surg. 2013, 46, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Snitker, S.; Fujishima, Y.; Shen, H.; Ott, S.; Pi-Sunyer, X.; Furuhata, Y.; Ohinata, K. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: Possible pharmacogenetic implications. Am. J. Clin. Nutr. 2009, 89, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Kawai, Y.; Iwanaga, T.; Saito, M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr. 2012, 95, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J.; Caterina, M.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef]
- Kim, M.S.; Pyun, H.B.; Hwang, J.K. Effects of orally administered fingerroot (Boesenbergia pandurata) extract on oxazolone-induced atopic dermatitis-like skin lesions in hairless mice. Food Sci. Biotechnol. 2013, 22, 257–264. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.S.; Sa, B.K.; Kim, M.B.; Hwang, J.K. Boesenbergia pandurata attenuates diet-induced obesity by activating AMP-activated protein kinase and regulating lipid metabolism. Int. J. Mol. Sci. 2012, 13, 994–1005. [Google Scholar] [CrossRef]
- Jitsaeng, K.; Duangjit, S.; Sritananuwat, P.; Tansathien, K.; Opanasopit, P.; Rangsimawong, W. Potential Lipolytic Effect of Panduratin A Loaded Microspicule Serum as a Transdermal Delivery Approach for Subcutaneous Fat Reduction. Biol. Pharm. Bull. 2023, 46, 1770–1777. [Google Scholar] [CrossRef]
- Nobusue, H.; Endo, T.; Kano, K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res. 2008, 332, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Rangsimawong, W.; Jitsaeng, K.; Duangjit, S.; Sritananuwat, P.; Opanasopit, P. Development of isolated panduratin A-loaded solid lipid nanoparticles as a transdermal delivery system for cosmeceutical products. Sci. Eng. Health Stud. 2023, 17, 23050003. [Google Scholar]
- Tan, B.C.; Tan, S.K.; Wong, S.M.; Ata, N.; Rahman, N.A.; Khalid, N. Distribution of Flavonoids and Cyclohexenyl Chalcone Derivatives in Conventional Propagated and In Vitro-Derived Field-Grown Boesenbergia rotunda (L.) Mansf. Evid. Based Complement. Altern. Med. 2015, 2015, 451870. [Google Scholar] [CrossRef]
- Elrayess, M.A.; Almuraikhy, S.; Kafienah, W.; Al-Menhali, A.; Al-Khelaifi, F.; Bashah, M.; Jaganjac, M. 4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic. Biol. Med. 2017, 104, 129–137. [Google Scholar] [CrossRef] [PubMed]
- San, H.T.; Khine, H.E.E.; Sritularak, B.; Prompetchara, E.; Chaotham, C.; Che, C.T.; Likhitwitayawuid, K. Pinostrobin: An Adipogenic Suppressor from Fingerroot (Boesenbergia rotunda) and Its Possible Mechanisms. Foods 2022, 11, 3024. [Google Scholar] [CrossRef]
- Kim, D.; Lee, M.S.; Jo, K.; Lee, K.E.; Hwang, J.K. Therapeutic potential of panduratin A, LKB1-dependent AMP-activated protein kinase stimulator, with activation of PPARalpha/delta for the treatment of obesity. Diabetes Obes. Metab. 2011, 13, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Chumchoochart, W.; Sutthanut, K. Anti-obesity potential of glutinous black rice bran extract: Anti-adipogenesis and lipolysis induction in 3T3-L1 adipocyte model. Songklanakarin J. Sci. Technol. 2020, 42, 284–291. [Google Scholar]
- Thummajitsakul, S.; Silprasit, K. Classification of some Boesenbergia and Alpinia extracts and their medicinal products based on chemical composition, antioxidant activity, and concentration of some heavy metals. Songklanakarin J. Sci. Technol. 2021, 43, 160–168. [Google Scholar]
- Kosuwon, W.; Sirichatiwapee, W.; Wisanuyotin, T.; Jeeravipoolvarn, P.; Laupattarakasem, W. Efficacy of symptomatic control of knee osteoarthritis with 0.0125% of capsaicin versus placebo. J. Med. Assoc. Thai 2010, 93, 1188–1195. [Google Scholar] [PubMed]
- Tan, E.-C.; Lee, Y.-K.; Chee, C.-F.; Heh, C.-H.; Wong, S.-M.; Christina Thio, L.P.; Foo, G.-T.; Khalid, N.; Abd Rahman, N.; Karsani, S.A.; et al. Boesenbergia rotunda: From Ethnomedicine to Drug Discovery. Evid. Based Complement. Altern. Med. 2012, 2012, 473637. [Google Scholar]
- Zhang, L.L.; Yan Liu, D.; Ma, L.Q.; Luo, Z.D.; Cao, T.B.; Zhong, J.; Zhu, M.X. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 2007, 100, 1063–1070. [Google Scholar] [CrossRef]
- Altman, R.; Barkin, R.L. Topical therapy for osteoarthritis: Clinical and pharmacologic perspectives. Postgrad. Med. 2009, 121, 139–147. [Google Scholar] [CrossRef]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef]
- Rao, J.; Paabo, K.E.; Goldman, M.P. A double-blinded randomized trial testing the tolerability and efficacy of a novel topical agent with and without occlusion for the treatment of cellulite: A study and review of the literature. J. Drugs Dermatol. 2004, 3, 417–425. [Google Scholar]
- Nanok, K.; Sansenya, S. α-Glucosidase, α-amylase, and tyrosinase inhibitory potential of capsaicin and dihydrocapsaicin. J. Food Biochem. 2020, 44, e13099. [Google Scholar] [CrossRef]
- Yoon, J.H.; Shim, J.S.; Cho, Y.; Baek, N.I.; Lee, C.W.; Kim, H.S.; Hwang, J.K. Depigmentation of melanocytes by isopanduratin A and 4-hydroxypanduratin A isolated from Kaempferia pandurata ROXB. Biol. Pharm. Bull. 2007, 30, 2141–2145. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Kim, H.S.; Kim, H.K.; Kim, J.W.; Yoon, J.H.; Cho, Y.; Hwang, J.K. Inhibitory effect of panduratin A isolated from Kaempferia panduarata Roxb. on melanin biosynthesis. Phytother. Res. 2010, 24, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Rice, A.S.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 1, CD007393. [Google Scholar] [PubMed]
- Chrubasik, S.; Weiser, T.; Beime, B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother. Res. 2010, 24, 1877–1885. [Google Scholar] [CrossRef]
- Serup, J.; Jemec, G.B.E.; Grove, G. Handbook of Non-Invasive Methods and the Skin, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Oliveira, R.; Ferreira, J.; Azevedo, L.F.; Almeida, I.F. An Overview of Methods to Characterize Skin Type: Focus on Visual Rating Scales and Self-Report Instruments. Cosmetics 2023, 10, 14. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.W.; Rhim, D.B.; Kim, C.; Hwang, J.K. Effect of Standardized Boesenbergia pandurata Extract and Its Active Compound Panduratin A on Skin Hydration and Barrier Function in Human Epidermal Keratinocytes. Prev. Nutr. Food Sci. 2015, 20, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; Thi, T.T.; Nguyen, H.T.; Lao, T.D.; Binh, N.T.; Nguyen, Q.D. Anti-Aging Effects of a Serum Based on Coconut Oil Combined with Deer Antler Stem Cell Extract on a Mouse Model of Skin Aging. Cells 2022, 11, 597. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).