Microencapsulation as a Route for Obtaining Encapsulated Flavors and Fragrances
Abstract
1. Introduction
- Extended shelf life;
- Improved stability during processing and in the final product, with a change in the structure from liquid to solid; liquidity, dispersibility, and dosage accuracy in the final product are improved;
- Gradual, controlled release of aroma compounds, prolonging exposure to odor or taste;
- Masking of taste and odor;
- Protection from external factors, separation of chemically unstable and highly volatile substances from environmental factors, protection from UV radiation, degradation reactions, from heat, oxidation, and dehydration;
- Improved safety by reducing the flammability of volatile substances.
2. Morphological Characterization of Capsules
2.1. Effect of the Encapsulation Process on Capsule Size
- Nanocapsules: <1 µm;
- Microcapsules: 1 µm–1000 µm;
- Millicapsules: >1 mm.
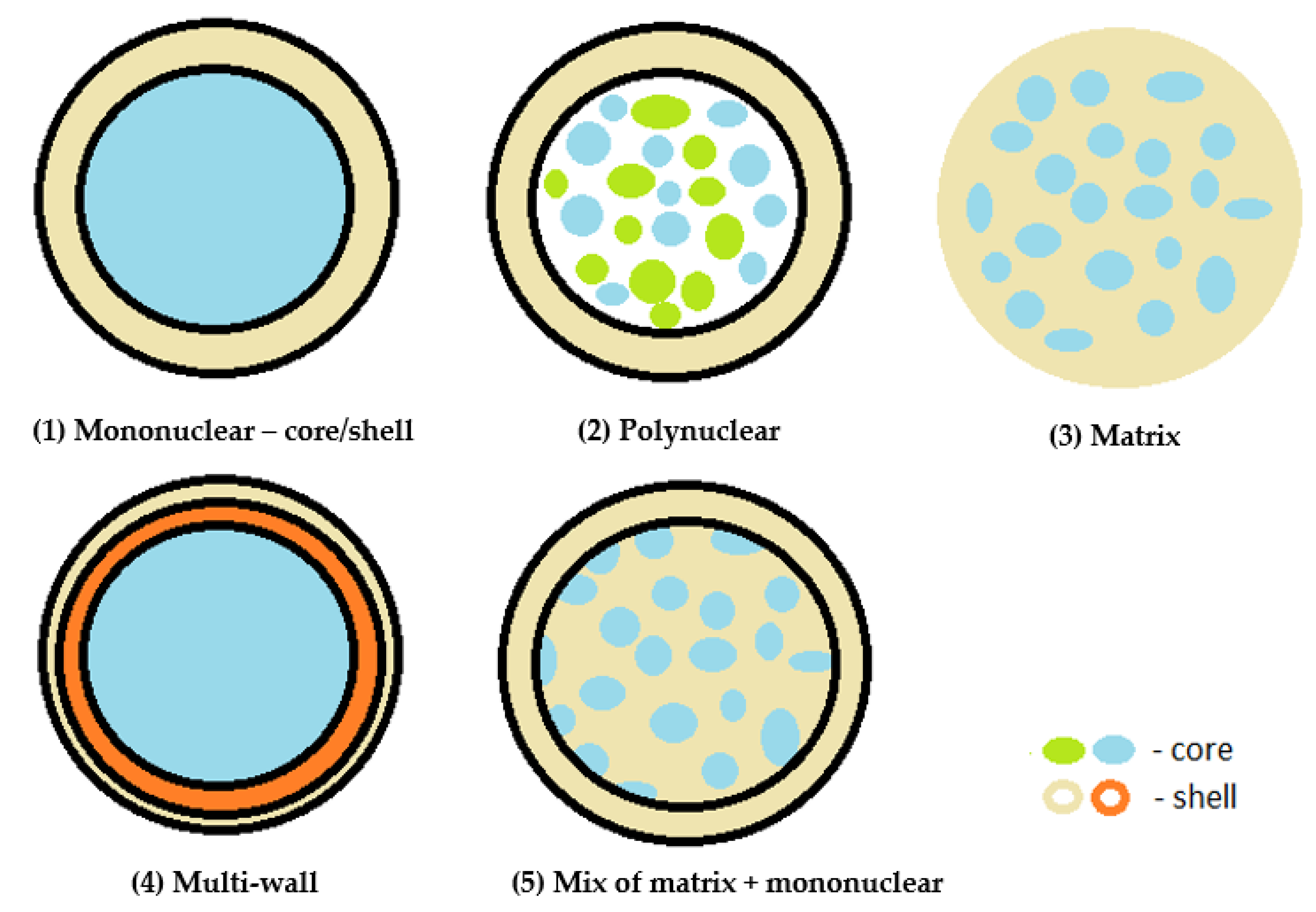
2.2. Effect of the Encapsulation Process on Capsule Structure
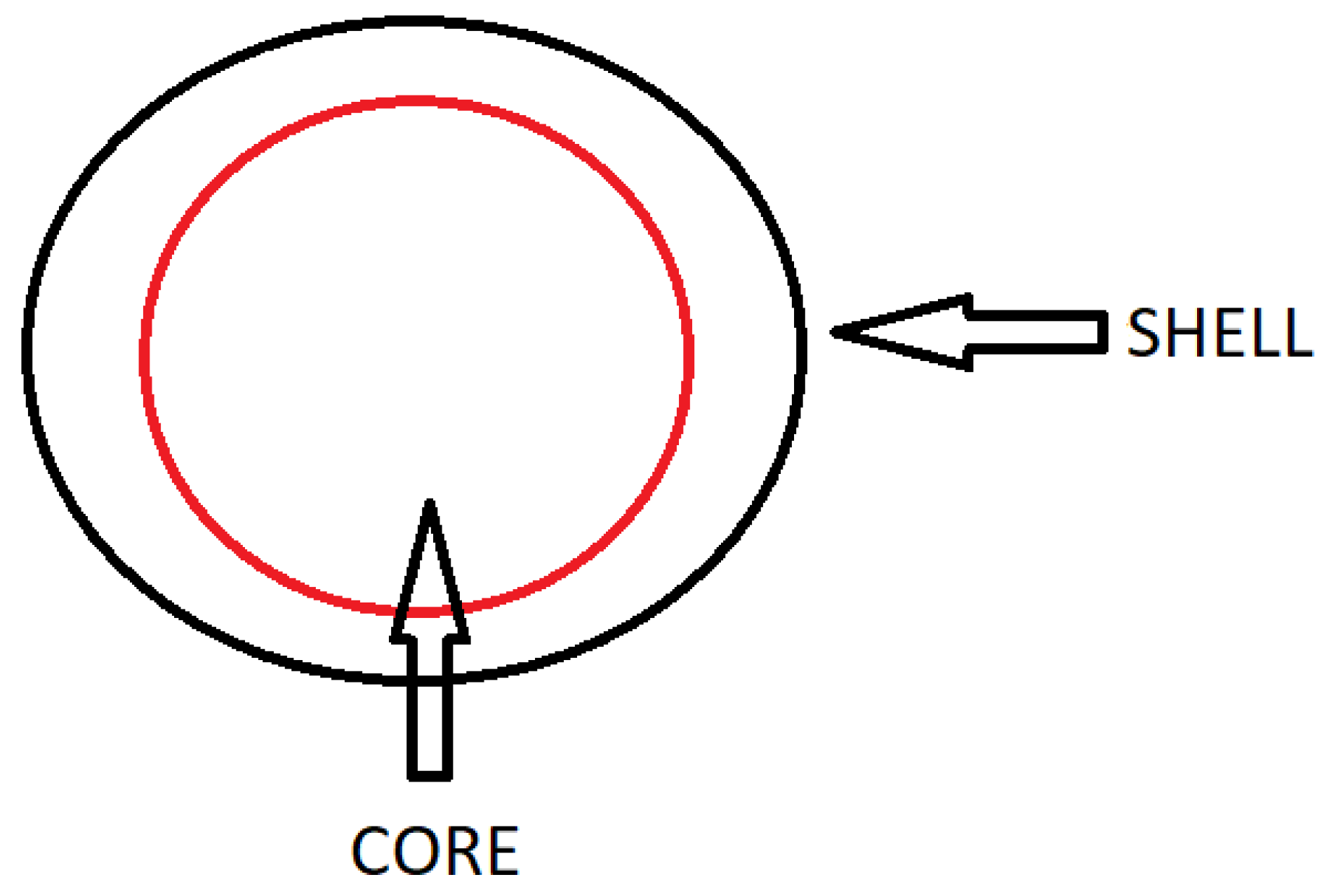
- Mononuclear, reservoir type—core/shell capsules and mononuclear encapsulates, in which a single shell is arranged around the core;
- Polynuclear capsules—contain multiple cores surrounded by the shell;
- Matrix encapsulation—the core is homogeneously distributed within the shell material. This is currently the most common type of encapsulation used in the pharmaceutical and food industries;
- Multi-wall—a microcapsule made up of several coatings;
- Coated matrix type—a combination of the matrix and mononuclear type.
2.3. Selection of the Coating Substance
- Polymeric, natural encapsulants, such as gum arabic, alginate, β-glucan, starch, plant protein and gelatin and synthetic encapsulants, e.g., polyesters (poly(lactide-co-glycolide) (PLGA);
- Inorganic encapsulants, e.g., SiO2, silica, which is a non-toxic, highly biocompatible, and mechanically stable substance that meets the requirements in pharmacy and biochemistry;
- Polymers (inorganic).
2.4. Effect of Encapsulation on Prolonging the Aroma Experience
- Impermeable sealed encapsulations;
- Semi-permeable encapsulates;
- Permeable open encapsulates. The coating on which the material is deposited can be salt or sugar and this process is cheap and sufficient in some cases, but unfavorable when considering the mixture of volatile compounds, as there is no barrier to oxidizing compounds.
3. Physicochemical Characterization of Microcapsules Obtained by Encapsulation Process
3.1. Size of the Capsules Obtained in the Encapsulation Process
3.2. Density
- True particle density
- Apparent particle density
- Effective particle density
- Porosity, €
3.3. Abrasion Resistance
3.4. Effectiveness and Efficiency of Encapsulation
4. Encapsulation Technologies
- I.
- Introduction of a core material, which will then be surrounded by a coating [21]. The active material can be either of the following:
- Liquid core:
- -
- Solution or melt;
- -
- Emulsion;
- -
- Suspension.
- Solid core (powder).
- II.
- Dispersion stage, where many different technologies are used to produce microcapsules, including the following [1,4,8,11,21]:
- Spraying;
- Dripping;
- Emulsification;
- Spray coating;
- Formation of suspension coating;
- Extrusion.
- III.
- The proper process of encapsulation, which can be divided into the following three groups, in terms of the transformations that take place [13]:
- Physical methods:
- -
- Solidification;
- -
- Evaporation.
- Physical and chemical methods:
- -
- Gelation;
- -
- Coacervation.
- Chemical methods:
- -
- Polymerization;
- -
- Cross-linking.
- IV.
- Scale-up and down processing [21].
4.1. The Most Common Methods Used to Encapsulate Flavors and Fragrances
4.1.1. Spray Drying
- Good solubility in water;
- Good emulsifying properties;
- Low viscosity at high concentrations (<500 cps at >45% concentration);
- Low hygroscopicity;
- Taste and/or odor release under the right conditions;
- Low-cost and accessible material;
- Neutrality in taste;
- Stability.
- Preparation of an emulsion or slurry from the main material and carrier. The emulsion is usually formed at high mixing speeds or using colloid mills, under high pressure. The product is then processed further by various mechanical means, such as high-pressure homogenization, microfluidization, and ultrasonic emulsification. These methods are used to stabilize the emulsion at least for a certain period of time. The viscosity of the emulsion affects the subsequent drying step and moderate values give the best encapsulation results. Emulsions with too high viscosity can clog the feeder nozzle, or settle on the walls of the chamber [27].
- Atomization and dispersion of the emulsion. The emulsion is pumped into the drying chamber through an atomizer. Various techniques are used to atomize the emulsion and among the most common are high-pressure nozzles and centrifugal wheels. The atomizer separates the emulsions into small droplets (the size of the droplet depends on the pore size of the atomizer) and sprays into the hot air in the chamber. The following three methods of atomizer air atomization are possible: co-current, counter-current and mixed. For fragrance compounds, co-current air is commonly used. Then, the moisture evaporates from the emulsion droplets, leading to the entrapment of the main material in the coating [38,40,41].
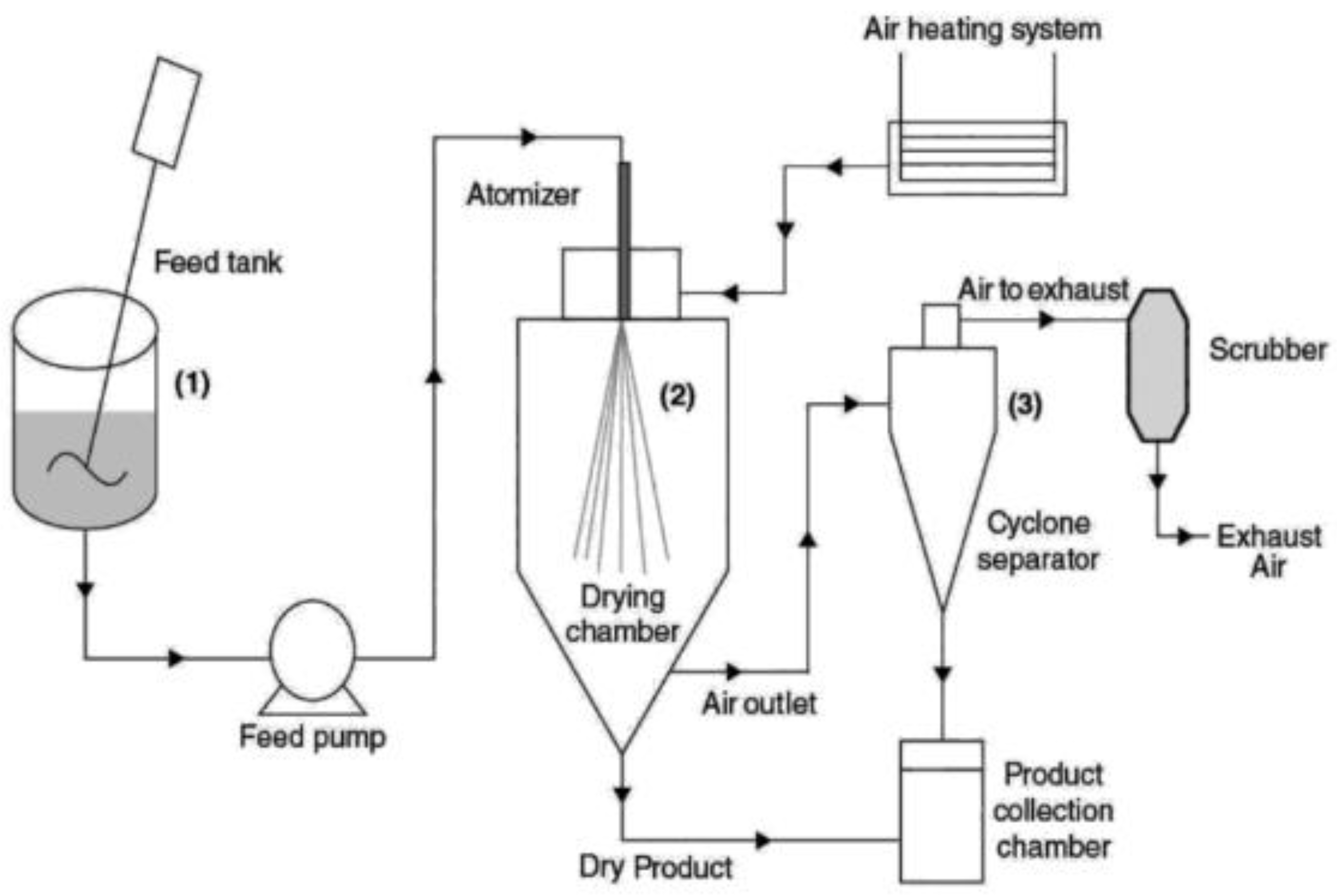
4.1.2. Extrusion
4.1.3. Microfluidization Method
4.1.4. Pan Coating
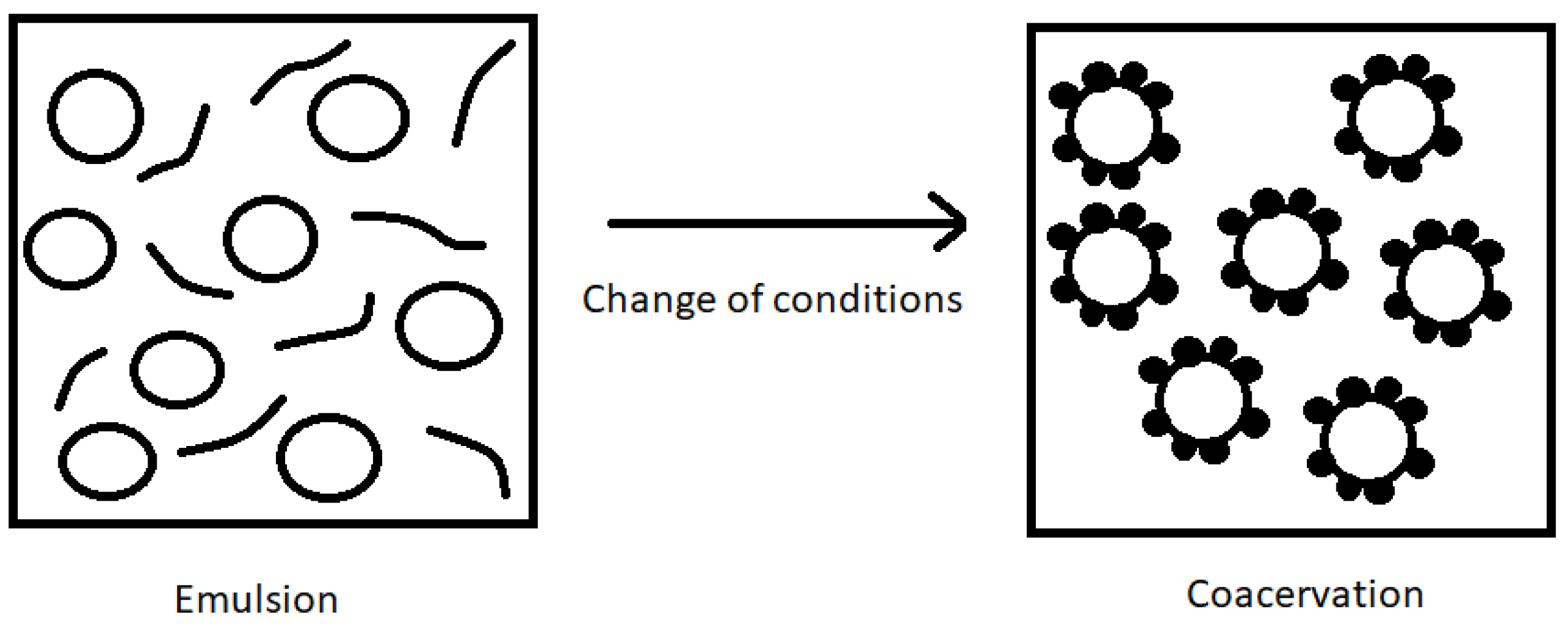
4.1.5. Coacervation Method
- Preparation of an aqueous solution of two or more polymers;
- Emulsification of the aqueous phase of the polymers with the hydrophobic phase of the core. The active ingredient should not dissolve in water, as this may lead to losses, reducing the efficiency of the coacervation method (the maximum solubility of the core material in water is 20 mg/mL);
- Change in the environmental conditions of the solution in order to proceed with coacervation and phase separation. A shell is formed around the core, and the phases are separated into a coacervate phase, with the core material surrounded by a carrier material, and an equilibrium phase (water);
- Cooling of the system and addition of a cross-linking substance to harden the shell.
- Animal: gelatin, whey, egg albumin and silk fibroin;
- Plant-based: soy protein, pea protein, wheat protein, lentil protein and chia protein.
4.1.6. Emulsion Methods
4.1.7. Polymerization
5. Application of Encapsulates Containing Flavors and Fragrances
6. Conclusions
- Additional costs;
- Increased complexity of the production process and/or supply chain;
- Unwanted consumer information (visual or tactile) about encapsulated foods;
- Stability of capsules during the processing and storage of the food product [12].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saifullah, M.; Islam Shishir, M.R.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and armoma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Trojanowska, A.; Giamberini, M.; Tsibranska, I.; Nowak, M.; Marciniak, Ł.; Jastrzab, R.; Tylkowski, B. Microencapsulation in food chemistry. JMSR 2017, 3, 265–271. [Google Scholar]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Gulumser, T. The role of microcapsules in masking bad odors of cotton fabrics. Industria Textila. 2017, 68, 275–282. [Google Scholar] [CrossRef]
- Winkler, M.; Kopf, G.; Hauptvogel, C.; Neu, T. Fate of artificial musk fragrances associated with suspended particulate matter (SPM) from the River Elbe (Germany) in comparison to other organic contaminants. Chemosphere 1998, 37, 1139–1156. [Google Scholar] [CrossRef]
- Horst, S.; Johannes, P. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; Wiley-VCH: Weinhem, NY, USA, 2016. [Google Scholar]
- Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Encapsulation of flavours and fragrances into polymeric capsules and cyclodextrins inclusion complexes: An update. Molecules 2020, 25, 5878–5911. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.R.; Ambrosi, A.; Di Luccio, M. Encapsulated essential oils: A perspective in food preservation. Future Food 2022, 5, 100126. [Google Scholar] [CrossRef]
- Mishra, M.K. Handbook of Encapsulation and Controlled Release; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zhu, G.Y.; Xiao, Z.B.; Zhou, R.J.; Yi, F.P. Fragrance and flavor microencapsulation technology. Adv. Mater. Res. 2012, 535–537, 440–445. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Mamusa, M.; Resta, C.; Sofroniou, C.; Baglioni, P. Encapsulation of volatile compounds in liquid media: Fragrances, flavors, and essential oils in commercial formulations. Adv. Colloid Interface Sci. 2021, 298, 102544. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, F.; Kermasha, S.; Inteaz Al, B. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [PubMed]
- Wandrey, C.; Bartkowiak, A.; Harding, S.E. Materials for Encapsulation. Encapsulation Technologies for Active Food Ingredients and food Processing; Springer: New York, NY, USA, 2009; pp. 31–100. [Google Scholar]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. JFF 2015, 19, 868–881. [Google Scholar] [CrossRef]
- He, L.; Hu, J.; Deng, W. Preparation and application of flavor and fragrance capsules. Polym. Chem. 2018, 9, 4926–4946. [Google Scholar] [CrossRef]
- Daneshniya, M.; Nezhad, H.J.; Maleki, M.H.; Jalali, V.; Behrouzian, M. A Review of encapsulation of bioactive peptides with antimicrobial and antioxidant activity. Int. J. Acad. Eng. Res. 2020, 4, 7. [Google Scholar]
- Temiz, U.; Öztürk, E. Encapsulation methods and use in animal nutrition. Selcuk J. Agr. Food Sci. 2018, 32, 624–631. [Google Scholar] [CrossRef]
- Poncelet, D.; Oxley, J. Introduction on Microencapsulation. Available online: https://www.youtube.com/watch?v=MNMbki8W1W8 (accessed on 16 October 2020).
- Ghosh, S.K. Functional Coatings and Microencapsulation: A General Perspective; Ghosh, S.K., Ed.; Functional coatings by polymer microencapsulation; John Wiley & Sons: New York, NY, USA, 2006; pp. 12–25. [Google Scholar]
- Zuidam, N.J.; Shimoni, E. Overview of microencapsulates for use in food products or processes and methods to make them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, USA, 2010; pp. 3–29. [Google Scholar]
- Gökmen, S.; Palamutoğlu, R.; Sarıçoban, C. Encapsulation applications in food industry. J. Food Technol. 2012, 7, 36–50. [Google Scholar]
- Tarhan, Ö.; Gökmen, V.; Harsa, Ş. Applications of nanotechnology in the field of food science and technology. Nutr. J. 2010, 35, 219–225. [Google Scholar]
- Bruyninck, K.; Dusselier, M. Sustainable chemistry considerations for the encapsulation of volatile compounds in laundry-type applications. ACS Sustain. Chem. Eng. 2019, 7, 8041–8054. [Google Scholar] [CrossRef]
- Salvador Cesa, F.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Weissbordt, J. Microcapsule Practical Characterization. Available online: https://www.youtube.com/watch?v=oS2iXHGwMog. (accessed on 21 February 2022).
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Standard ISO 13320:2020; Technical Committee: ISO/TC 24/SC 4 Particle characterization. ICS: 19.120 Particle size analysis. Sieving. January 2020.
- Rosenberg, M.; Kopelman, I.J.; Talmon, Y. A Scanning electron microscopy study of microencapsulation. J. Food Sci. 2006, 50, 139–144. [Google Scholar] [CrossRef]
- Procedure DIN 66165-1.
- Getachew, A.T.; Chun, B.S. Optimization of coffee oil flavor encapsulation using response surface methodology. Food Sci. Technol. 2016, 70, 126–134. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; Alvim, I.D.; Vissotto, F.Z.; de Aguirre, J.M. Influence of carrier agents on the physicochemical properties of blackberry powder produced by spray drying. Int. J. Food Sci. 2012, 47, 1237–1245. [Google Scholar] [CrossRef]
- Comunian, T.A.; da Silva, A.G.; Bezerra, E.O.; Moraes, I.C.F.; Hubinger, M.D. Encapsulation of pomegranate seed oil by emulsification followed by spray drying: Evaluation of different biopolymers and their effect on particle proper. Food Bioproc. Tech. 2019, 13, 53–66. [Google Scholar] [CrossRef]
- Lee, Y.; Ra Ji, Y.; Lee, S.; Choi, M.J.; Cho, Y. Microencapsulation of probiotic Lactobacillus acidophilus KBl409 by extrusion technology to enhance survival under simulated intestinal and freeze-drying conditions. J. Microbiol. Biotechnol. 2019, 29, 721–730. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Fortea, M.I.; Trabal, J.; Rodríguez-López, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Stability of microencapsulated strawberry flavour by spray drying, freeze drying and fluid bed. Powder Technol. 2019, 347, 179–185. [Google Scholar] [CrossRef]
- Altay, Ö.; Köprüalan, Ö.; İlter, I.; Koç, M.; Ertekin, F.K.; Jafari, S.M. Spray drying encapsulation of essential oils; process efficiency, formulation strategies, and applications. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Costa, S.S.; Machado, B.A.S.; Martin, A.R.; Bagnara, F.; Ragadalli, S.A.; Costa Alves, A.R. Drying by spray drying in the food industry: Micro-encapsulation, process parameters and main carriers used. Afr. J. Food Sci. 2015, 9, 460–470. [Google Scholar]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray drying for the encapsulation of oils—A review. Molecules 2020, 25, 3873–3889. [Google Scholar] [CrossRef]
- Patel, R.P.; Patel, M.P.; Suthar, A.M. Spray drying technology: An overview. Afr. J. Food Sci. 2009, 2, 44–47. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Pignatelllo, R.; Musumeci, T. Biomaterials: Physics and Chemistry—New Edition Edited; IntechOpen: London, UK, 2018. [Google Scholar]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of bioactive compounds by “extrusion” technologies: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–19. [Google Scholar] [CrossRef]
- Tackenberg, M.W.; Krauss, R.; Schuchmann, H.P.; Kleinebudde, P. Encapsulation of orange terpenes investigating a plasticisation extrusion process. J. Microencapsul. 2015, 32, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Risch, S.J. Encapsulation: Overview of Uses and Techniques; ACS Symposium Series: Washington, DC, USA, 1995; pp. 2–7. [Google Scholar]
- Porzio, M. Advances in flavor encapsulation. IFT 2012, 6. [Google Scholar]
- Reineccius, G.A. Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 269–294. [Google Scholar]
- Rabanel, J.M.; Banquy, X.; Zouaoui, H.; Mokhtar, M. Technology in microencapsulation methods for cell therapy. Biotechnol. Prog. 2009, 25, 946–963. [Google Scholar] [CrossRef]
- Dendukuri, D.; Doyle, P.S. The synthesis and assembly of polymeric microparticles using Microfluidics. Adv. Mater. 2009, 21, 4071–4086. [Google Scholar] [CrossRef]
- Jyothi, N.V.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.H. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Przybysławska, M.; Winnicka, K. Technologies of microencapsulation. Farm Pol. 2012, 283–289. [Google Scholar]
- Sagiri, S.S.; Anis, A.; Pal, K. Review on encapsulation of vegetable oils: Strategies, preparation methods, and applications. Polym. Plast. Technol. Eng. 2016, 55, 291–311. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food system. LWT. 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Bansode, S.S.; Banarjee, S.K.; Gaikwad, D.D.; Jadhav, S.L.; Thorat, R.M. Microencapsulation: A review. Int. J. Pharm. Sci. Rev. Res. 2010, 1, 38–43. [Google Scholar]
- Sonawane, S.H.; Bhanvase, B.A.; Sivakumar, M.; Potdar, S.B. Current overview of encapsulation. In Encapsulation of Active Molecules and Their Delivery System; [s.l.]; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 1–8. [Google Scholar]
- Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Kassozi, V.; Nakisozi, H.; Van der Meeren, P. Recent advances in food colloidal delivery systems for essential oils and their main components. Trends Food Sci.Technol. 2020, 99, 474–486. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Stasse, M.; Laurichess, E.; Ribaut, T.; Anthony, O.; Héroguez, V.; Schmitt, V. Formulation of concentrated oil-in-water-in-oil double emulsions for fragrance encapsulation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124564. [Google Scholar] [CrossRef]
- Sachen, K.N.; Singh, B.; Rama, R.K. Controlled drug delivery through microencapsulation. Malays. J. Pathol. 2006, 4, 65–81. [Google Scholar]
- Park, Y.; Yeo, Y. Microencapsulation technology. Encycl. Pharm. Technol. 2007, 3, 2315–2325. [Google Scholar]
- Vishwakarma, G.S.; Gautam, N.; Babu, J.N.; Mittal, S.; Jaitak, V. Polymeric encapsulates of essential oils and their constituents: A review of preparation techniques, characterization, and sustainable release mechanisms. Poly. Rev. 2016, 56, 668–701. [Google Scholar] [CrossRef]
- European Patent Office, United States Patent and Trademark Office, Cooperative Patent Classification, Scheme and Definitions 2020, (n.d.).
- Geffroy, C.; Schreiber, S.S.; Goodall, M.J.; Fadel, A.; Harrison, I.M. Encapsulation of Perfumes. US2015/0044262 A1, 12 February 2015. [Google Scholar]
- Brahms, J.; Lei, Y.; Wieland, J.; Xu, L.; Popplewell, L.M. Reloadable Microcapsules. WO2017192648A1, 9 November 2017. [Google Scholar]
- Morrison, E. Ibuprofen Nanoparticle Carriers En-capsulated with Hermatic Surfactant Films. US20170296496A1, 23 August 2007. [Google Scholar]
- Singh, N.; Sheikh, J. Microencapsulation and its application in production of functional textiles. IJFTR 2020, 45, 495–509. [Google Scholar]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Pithanthanakul, U.; Vatanyoopaisarn, S.; Thumthanaruk, B.; Puttanlek, C.; Uttapap, D.; Kietthanakorn, B.; Rungsardthong, V. Encapsulation of fragrances in zein nanoparticles and use as fabric softener for textile application. Flavour Fragr. J. 2021, 36, 365–373. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Gómez, M. Systematic review of encapsulation and shape-stabilization of phase change materials. J Energy Storage. 2020, 30, 101495–101516. [Google Scholar] [CrossRef]
- Azevedo, S.G.; de Andrade, C.P.; D’Ambros, N.C.d.S.; Pérez, M.T.M.; Manzato, L. Biotechnological Applications of Nanoencapsulated Essential Oils: A Review. Polymers 2022, 14, 5495. [Google Scholar]
- Yang, Z.; Peng, Z.; Li, J.; Li, S.; Kong, L.; Li, P.; Wang, Q. Development and evaluation of novel flavour microcapsules containing vanilla oil using complex coacervation approach. Food Chem. 2014, 145, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Afonso, C.; Fernandes, I.; Barreiro, M.F.; Costa, P.; Rodrigues, A.E. Chitosan-cellulose particles as delivery vehicles for limonene fragrance. Ind. Crop. Prod. 2019, 139, 111407. [Google Scholar] [CrossRef]
- Sadovoy, A.V.; Lomova, M.V.; Antipina, M.N.; Braun, N.A.; Sukhorukov, G.B.; Kiryukhin, M.V. Layer-by-layer assembled multilayer shells for encapsulation and release of fragrance. ACS Appl. Mater. Interfaces 2013, 5, 8948–8954. [Google Scholar] [CrossRef]
- Kwamman, Y.; Klinkesorn, U. Influence of oil load and maltodextrin concentration on properties of tuna oil microcapsules encapsulated in two-layer membrane. Dry. Technol. 2014, 33, 854–864. [Google Scholar] [CrossRef]
- Minemoto, Y.; Hakamata, K.; Adachi, S.; Matsuno, R. Oxidation of linoleic acid encapsulated with gum arabic or maltodextrin by spray-drying. J. Microencapsul. 2002, 19, 181–189. [Google Scholar] [CrossRef]
- Chang, D.; Abbas, S.; Hayat, K.; Xia, S.; Zhang, X.; Xie, M.; Kim, J.M. Original article: Encapsulation of ascorbic acid in amorphous maltodextrin employing extrusion as affected by matrix/core ratio and water content. Int. J. Food Sci. 2010, 45, 1895–1901. [Google Scholar] [CrossRef]
- Varavinit, S.; Chaokasem, N.; Shobsngob, S. Studies of flavor encapsulation by agents produced from modified Sago and Tapioca starches. Starch—Stärke 2001, 53, 281–287. [Google Scholar] [CrossRef]
- Edris, A.; Bergnståhl, B. Encapsulation of orange oil in a spray dried double emulsion. Nahrung. Food. 2001, 45, 133–137. [Google Scholar] [CrossRef] [PubMed]
- de Melo Ramos, F.; Silveira Júnior, V.; Prata, A.S. Assessing the vacuum spray drying effects on the properties of orange essential oil microparticles. Food Bioproc Technol. 2019, 12, 1917–1927. [Google Scholar] [CrossRef]
- Karrar, E.; Ali Mahdi, A.; Sheth, S.; Mohamed Ahmed, I.A.; Faisal Manzoor, M.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef]
- Nguyen, P.T.N.; Nguyen, H.T.A.; Hoang, Q.B.; Nguyen, T.D.P.; Nguyen, T.V.; Cang Mai, H. Influence of spray drying parameters on the physicochemical characteristics of microencapsulated orange (Citrus sinensis L.) essential oil. Mater. Today: Proc. 2022, 60, 2026–2033. [Google Scholar] [CrossRef]
- Bajac, J.; Nikolovski, B.; Lončarević, I.; Petrović, J.; Bajac, B.; Đurović, S.; Petrović, L. Microencapsulation of juniper berry essential oil (Juniperus Communis L.) by spray drying: Microcapsule characterization and release kinetics of the oil. Food Hydrocoll. 2022, 125, 107430. [Google Scholar] [CrossRef]
- Bušić, A.; Komes, D.; Belščak-Cvitanović, A.; Vojvodić Cebin, A.; Špoljarić, I.; Mršić, G.; Miao, S. The potential of combined emulsification and spray drying techniques for encapsulation of polyphenols from rosemary (Rosmarinus officinalis L.) Leaves. FTB 2018, 56, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Felix, P.H.C.; Birchal, V.S.; Botrel, D.A.; Marques, G.R.; Borges, S.V. Physicochemical and thermal stability of microcapsules of cinnamon essential oil by spray drying. J. Food Process. Preserv. 2017, 41, 12919–12928. [Google Scholar] [CrossRef]
- Abdin, M.; El-Beltagy, A.E.; El-sayed, M.E.; Naeem, M.A. Production and characterization of sodium alginate/gum arabic based films enriched with Syzygium cumini seeds extracts for food application. J. Food Process. Preserv. 2022, 30, 1615–1626. [Google Scholar] [CrossRef]


| SHELL MATERIAL | CORE MATERIAL | ||
|---|---|---|---|
| • Gums: gum arabic, sodium alginate and carrageenan | Gas | Liquid | Solid |
| • Carbohydrates: starch, dextran and sucrose | Forms: solution, dispersion and emulsion | ||
| • Celluloses: carboxymethylcellulose and methylcellulose | |||
| • Lipids: beeswax, stearic acid and phospholipids | |||
| • Proteins: gelatin and albumin | Composition of core material | ||
| • Flavor and fragrance | |||
| • Drug or active constituent | |||
| Composition of coating material | • Additives such as diluents | ||
| • Inert polymer | • Stabilizers | ||
| • Plasticizer | • Release rate enhancers | ||
| • Coloring agent | |||
| Method | Advantages | Disadvantages |
|---|---|---|
| Spray drying |
|
|
| Extrusion |
|
|
| Microfluidization method |
|
|
| Pan coating |
|
|
| Coacervation |
|
|
| Emulsion methods |
|
|
| Polymerization |
|
|
| Core Material | Shell Material | Methodology | Goal | Morphology | Ref. |
|---|---|---|---|---|---|
| Vanilla oil | Chitosan/arabic gum | Complex coacervation | Controlled release and thermostability product for spice application in food industries | Spherical shape and smooth surface, 94.2% efficiency (VO/CS 2:1) | [72] |
| Limonene fragrance | Chitosan/cellulose | Freezing/ thawing/stirring process | A fragrant component widely used in the flavor and fragrance industries; encapsulation prolonged the release of fragrances | Spherical shapes, with an average diameter of 2 mm, 51.3% efficiency | [73] |
| Fragrances: D-limonene, Claritone, Amarocit, Rose Oxide-High Cis, methyl salicylate, 1-octanal, 1-octanol, hydrocitronitrile, Majantol and ethyl 2-methylbutanoate | Bovine serum albumin and tetramethylrhodamine isothiocyanate-labeled BSA (TRITC-BSA) | Layer-by-layer (LbL) | Fragrance encapsulation. Controlling the release of fragrances; both TA and BSA are relatively cheap and available compounds | The encapsulation efficiency depends on the water solubility; the less water-soluble the ingredient, the smaller its losses upon LbL coating of emulsion in the filtration cell and the higher its relative content in released fragrance | [74] |
| Pink fruity fragrances and white floral fragrances | Protein, carbohydrate and lipid | Liquid–liquid dispersion | Fabric softener application and long-lasting property in textile applications | Spherical shape with an average size of 100–300 nm, efficiency of 69–75% | [69] |
| Tuna oil in water emulsion stabilized by lecithin-chitosan membrane, using an electrostatic layer-by-layer deposition process | Maltodextrin | Spray drying of two-layer emulsion | High oil-loaded microcapsules that may lead to a wide range of applications in food products | Spherical particles (except for oil:maltodextrin 1:1), efficiency of 89% for oil:maltodextrin 1:4 ratio and 56% for ratio of 1:1 | [75] |
| Linoleic acid | Gum arabic or maltodextrin | Spray drying | Evaluation of influence of the encapsulation process on the stability of linoleic acid towards oxidation | Particles with an average size of 0.68 µm (gum arabic) or 1.68 µm (maltodextrin), efficiency of 75–99% (gum arabic) and 35–50% (maltodextrin) | [76] |
| Ascorbic acid | Maltodextrin | Extrusion | Vitamin encapsulation | Crystals with sharp edges, efficiency of 96% and load 19% | [77] |
| Orange terpenes | Maltodextrin and sucrose | Extrusion | Flavor encapsulation | Partly crystalline samples, about 1 mm particle size, efficiency of 34.5–67.3%; load 4.1% | [44] |
| Lemon oil | Sago and tapioca starch, gum arabic and stearic acid | Spray drying | Encapsulated agent for food industry | Efficiency of 49–59% | [78] |
| Orange oil in water emulsion | Lactose and caseinate | Spray drying | Application in different types of food or pharmaceutical products, where maximum protection for flavors or slow release are required | Particle size of 30.9 µm. Efficiency of 44.5% | [79] |
| Orange essential oil | Octenyl succinic anhydride, modified starch and maltodextrin | Vacuum spray drying | Application of vacuum spray drying | Efficiency of 99.89% | [80] |
| Gurum seed oil | Gum arabic, maltodextrin, pullulan and whey protein isolate | Spray drying | Evaluating the potential of combining maltodextrin with gum arabic and whey protein isolate | Efficiency of 97.38% | [81] |
| Citrus sinensil L. (essential oil) | Maltodextrin | Spray drying | Evaluating the factors affecting microencapsulation | Efficiency of 89.94% | [82] |
| Juniperus communis L. (essential oil) | Gum arabic, maltodextrin, sodium alginate and whey protein concentrate | Spray drying | Food flavoring agent and preservative | Efficiency of 82.66% | [83] |
| Rosemary (essential oil) | Maltodextrin and whey protein concentrate | Spray drying | The potential of combined emulsification and spray drying procedures to encapsulate polyphenolic components from rosemary | Efficiency of 27.09–42.93% | [84] |
| Cinnamon (essential oil) | Gum arabic, maltodextrin and whey protein concentrate | Spray drying | Effect of shell materials used on encapsulation efficiency | Efficiency of 13.8–50.1% | [85] |
| Syzygium Cumin Seed (essential oil) | Gum arabic | Spray drying | Antioxidant | Improvement of water vapor permeability; prolongation of oil oxidation | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłosowska, A.; Wawrzyńczak, A.; Feliczak-Guzik, A. Microencapsulation as a Route for Obtaining Encapsulated Flavors and Fragrances. Cosmetics 2023, 10, 26. https://doi.org/10.3390/cosmetics10010026
Kłosowska A, Wawrzyńczak A, Feliczak-Guzik A. Microencapsulation as a Route for Obtaining Encapsulated Flavors and Fragrances. Cosmetics. 2023; 10(1):26. https://doi.org/10.3390/cosmetics10010026
Chicago/Turabian StyleKłosowska, Agnieszka, Agata Wawrzyńczak, and Agnieszka Feliczak-Guzik. 2023. "Microencapsulation as a Route for Obtaining Encapsulated Flavors and Fragrances" Cosmetics 10, no. 1: 26. https://doi.org/10.3390/cosmetics10010026
APA StyleKłosowska, A., Wawrzyńczak, A., & Feliczak-Guzik, A. (2023). Microencapsulation as a Route for Obtaining Encapsulated Flavors and Fragrances. Cosmetics, 10(1), 26. https://doi.org/10.3390/cosmetics10010026







