The Biological Role of Dead Sea Water in Skin Health: A Review
Abstract
1. Introduction
2. Mechanism of Action of DSW
2.1. Direct Action
2.2. Indirect Action
3. Biological Function of DSW and Related Complexes (Figure 1)
3.1. Dermatological Treatment
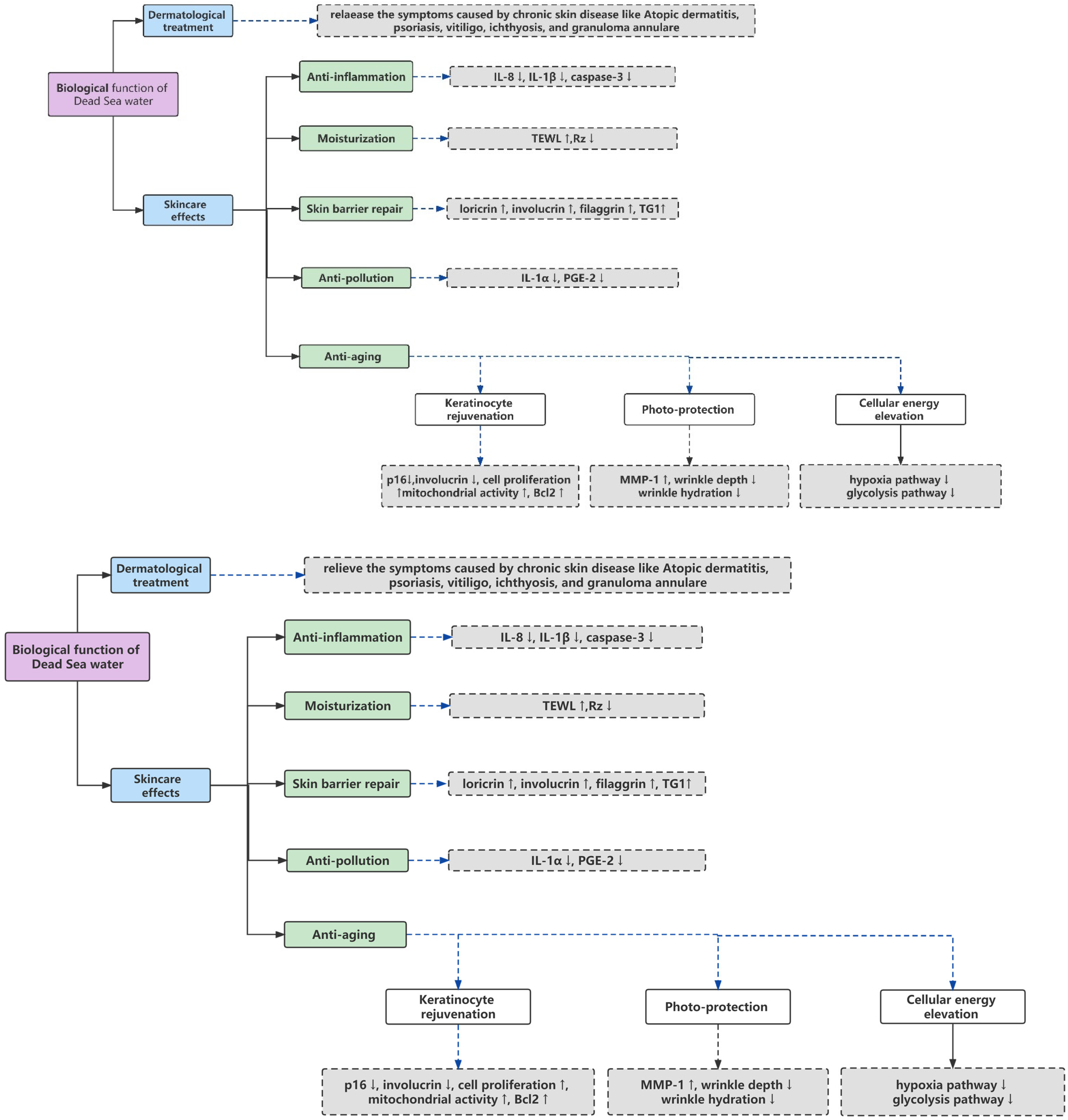
3.2. Skincare Effects
3.2.1. Moisturization
3.2.2. Anti-Inflammation
3.2.3. Skin Barrier Repair
3.2.4. Anti-Pollution
3.2.5. Anti-Aging
Keratinocyte Rejuvenation
Photo-Protection
Cellular Energy Elevation
4. Current Challenges for the Use of DSW
5. Future Direction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Even-Paz, Z.; Shani, J. The Dead Sea and psoriasis: Historical and geographic background. Int. J. Dermatol. 1989, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Buskila, D.; Abu-Shakra, M.; Neumann, L.; Odes, L.; Shneider, E.; Flusser, D.; Sukenik, S. Balneotherapy for fibromyalgia at the Dead Sea. Rheumatol. Int. 2001, 20, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ravn Jørgensen, A.-H.; Thomsen, S.F. Biologics for chronic inflammatory skin diseases: An update for the clinician. J. Dermatol. Treat. 2020, 31, 108–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Seité, S.; Adar, T. The use of balneotherapy in dermatology. Clin. Dermatol. 2018, 36, 363–368. [Google Scholar] [CrossRef]
- Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P. The role of thermal water in chronic skin diseases management: A review of the literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef]
- Maarouf, M.; Hendricks, A.J.; Shi, V.Y. Bathing additives for atopic dermatitis—A systematic review. Dermatitis 2019, 30, 191–197. [Google Scholar] [CrossRef]
- Denda, M.; Fuziwara, S.; Inoue, K. Influx of calcium and chloride ions into epidermal keratinocytes regulates exocytosis of epidermal lamellar bodies and skin permeability barrier homeostasis. J. Investig. Dermatol. 2003, 121, 362–367. [Google Scholar] [CrossRef]
- Bäsler, K.; Brandner, J.M. Tight junctions in skin inflammation. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 3–14. [Google Scholar] [CrossRef]
- Hudson, L.E.M. Integration of Wound-Induced Calcium Signals to Transcriptional Activation and Regulation of Cutaneous Wound Healing Responses; Newcastle University: Tyne, UK, 2015. [Google Scholar]
- Lee, S.E.; Lee, S.H. Skin barrier and calcium. Ann. Dermatol. 2018, 30, 265–275. [Google Scholar] [CrossRef]
- Schempp, C.M.; Dittmar, H.C.; Hummler, D.; Simon-Haarhaus, B.; Schöpf, E.; Simon, J.C.; Schulte-Mönting, J. Magnesium ions inhibit the antigen-presenting function of human epidermal Langerhans cells in vivo and in vitro. Involvement of ATPase, HLA-DR, B7 molecules, and cytokines. J. Investig. Dermatol. 2000, 115, 680–686. [Google Scholar] [CrossRef]
- Eliasse, Y.; Redoules, D.; Espinosa, E. Impact of Avène Thermal Spring Water on immune cells. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Nocera, T.; Jean-Decoster, C.; Georgescu, V.; Guerrero, D. Benefits of Avène thermal hydrotherapy in chronic skin diseases and dermatological conditions: An overview. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Tacheau, C.; Weisgerber, F.; Fagot, D.; Bastien, P.; Verdier, M.; Liboutet, M.; Sore, G.; Bernard, B. Vichy Thermal Spring Water (VTSW), a cosmetic ingredient of potential interest in the frame of skin ageing exposome: An in vitro study. Int. J. Cosmet. Sci. 2018, 40, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Rasmont, V.; Valois, A.; Gueniche, A.; Sore, G.; Kerob, D.; Nielsen, M.; Berardesca, E. Vichy volcanic mineralizing water has unique properties to strengthen the skin barrier and skin defenses against exposome aggressions. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 5–15. [Google Scholar] [CrossRef]
- Portugal-Cohen, M.; Cohen, D.; Ish-Shalom, E.; Laor-Costa, Y.; Ma’or, Z.e. Dead Sea minerals: New findings on skin and the biology beyond. Exp. Dermatol. 2019, 28, 585–592. [Google Scholar] [CrossRef]
- Levi-Schaffer, F.; Shani, J.; Politi, Y.; Rubinchik, E.; Brenner, S. Inhibition of proliferation of psoriatic and healthy fibroblasts in cell culture by selected Dead-sea salts. Pharmacology 1996, 52, 321–328. [Google Scholar] [CrossRef]
- Carbajo, J.M.; Maraver, F. Salt water and skin interactions: New lines of evidence. Int. J. Biometeorol. 2018, 62, 1345–1360. [Google Scholar] [CrossRef]
- Cohen, D.; Ma’or, Z.e.; Cohen, M.P.; Oron, M.; Kohen, R. Nrf2 Pathway Involvement in the Beneficial Skin Effects of Moderate Ionic Osmotic Stress–the Case of the Dead Sea Water. J. Cosmet. Dermatol. Sci. Appl. 2022, 12, 109–130. [Google Scholar]
- Sawada, Y.; Saito-Sasaki, N.; Mashima, E.; Nakamura, M. Daily Lifestyle and Inflammatory Skin Diseases. Int. J. Mol. Sci. 2021, 22, 5204. [Google Scholar] [CrossRef]
- Duong, T.A.; Valeyrie-Allanore, L.; Wolkenstein, P.; Chosidow, O. Severe cutaneous adverse reactions to drugs. Lancet 2017, 390, 1996–2011. [Google Scholar] [CrossRef]
- Marsakova, A.; Kudish, A.; Gkalpakiotis, S.; Jahn, I.; Arenberger, P.; Harari, M. Dead Sea climatotherapy versus topical steroid treatment for atopic dermatitis children: Long-term follow-up study. J. Dermatol. Treat. 2019, 31, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, T.; Petersen, A.; Houborg, H.I.; Rønsholdt, A.B.; Lybæk, D.; Steiniche, T.; Bregnhøj, A.; Iversen, L.; Johansen, C. Climatotherapy at the Dead Sea for psoriasis is a highly effective anti-inflammatory treatment in the short term: An immunohistochemical study. Exp. Dermatol. 2022, 31, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, O.; Ophir, J.; Brener, S.; Paran, D.; Wigler, I.; Efron, D.; Even-Paz, Z.; Politi, Y.; Yaron, M. Immediate and delayed effects of treatment at the Dead Sea in patients with psoriatic arthritis. Rheumatol. Int. 2000, 19, 77–82. [Google Scholar] [CrossRef]
- Bożek, A.; Reich, A. The reliability of three psoriasis assessment tools: Psoriasis area and severity index, body surface area and physician global assessment. Adv. Clin. Exp. Med. 2017, 26, 851–856. [Google Scholar] [CrossRef]
- Elewski, B.E.; Puig, L.; Mordin, M.; Gilloteau, I.; Sherif, B.; Fox, T.; Gnanasakthy, A.; Papavassilis, C.; Strober, B.E. Psoriasis patients with psoriasis Area and Severity Index (PASI) 90 response achieve greater health-related quality-of-life improvements than those with PASI 75–89 response: Results from two phase 3 studies of secukinumab. J. Dermatol. Treat. 2017, 28, 492–499. [Google Scholar] [CrossRef]
- Harari, M.; Shani, J.; Seidl, V.; Hristakieva, E. Climatotherapy of atopic dermatitis at the Dead Sea: Demographic evaluation and cost-effectiveness. Int. J. Dermatol. 2000, 39, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Portugal-Cohen, M.; Oron, M.; Merrik, E.; Ben-Amitai, D.; Yogev, H.; Zvulunov, A. A dead sea water-enriched body cream improves skin severity scores in children with atopic dermatitis. J. Cosmet. Dermatol. Sci. Appl. 2011, 1, 71. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research techniques made simple: Transepidermal water loss measurement as a research tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin barrier function in psoriasis and atopic dermatitis: Transepidermal water loss and temperature as useful tools to assess disease severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef]
- Harari, M.; Czarnowicki, T.; Fluss, R.; Ruzicka, T.; Ingber, A. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 554–559. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Bigliardi-Qi, M.; Buechner, S.; Rufli, T. Expression of μ-opiate receptor in human epidermis and keratinocytes. J. Investig. Dermatol. 1998, 111, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Nissen, J.; Avrach, W.; Hansen, E.; Stengaard-Pedersen, K.; Kragballe, K. Increased levels of enkephalin following natural sunlight (combined with salt water bathing at the Dead Sea) and ultraviolet A irradiation. Br. J. Dermatol. 1998, 139, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Harari, M.; Dreiher, J.; Czarnowicki, T.; Ruzicka, T.; Ingber, A. SCORAD 75: A new metric for assessing treatment outcomes in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; Harari, M.; Ruzicka, T.; Ingber, A. Dead Sea climatotherapy for vitiligo: A retrospective study of 436 patients. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, T.; Lybæk, D.; Johansen, C.; Iversen, L. Effect of Dead Sea climatotherapy on psoriasis; a prospective cohort study. Front. Med. 2020, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Carlin, C.S.; Feldman, S.R.; Krueger, J.G.; Menter, A.; Krueger, G.G. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J. Am. Acad. Dermatol. 2004, 50, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Harari, M.; Novack, L.; Barth, J.; David, M.; Friger, M.; Moses, S.W. The percentage of patients achieving PASI 75 after 1 month and remission time after climatotherapy at the Dead Sea. Int. J. Dermatol. 2007, 46, 1087–1091. [Google Scholar] [CrossRef]
- Proksch, E.; Nissen, H.P.; Bremgartner, M.; Urquhart, C. Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. Int. J. Dermatol. 2005, 44, 151–157. [Google Scholar] [CrossRef]
- Ma’Or, Z.; Yehuda, S.; Voss, W. Skin smoothing effects of Dead Sea minerals: Comparative profilometric evaluation of skin surface. Int. J. Cosmet. Sci. 1997, 19, 105–110. [Google Scholar] [CrossRef]
- Havas, F.; Krispin, S.; Cohen, M.; Loing, E.; Farge, M.; Suere, T.; Attia-Vigneau, J. A Dunaliella salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties. Mar. Drugs 2022, 20, 104. [Google Scholar] [CrossRef]
- Xu, Y.; Harvey, P.J. Carotenoid production by Dunaliella salina under red light. Antioxidants 2019, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Ma’Or, Z.; Meshulam-Simon, G.; Yehuda, S.; Gavrieli, J.; Sea, D. Antiwrinkle and skin-moisturizing effects of a mineral-algal-botanical complex. J. Cosmet. Sci. 2000, 51, 27–36. [Google Scholar]
- Wang, B.; Amerio, P.; Sauder, D.N. Role of cytokines in epidermal Langerhans cell migration. J. Leukoc. Biol. 1999, 66, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tarnowska, M.; Briançon, S.; Resende de Azevedo, J.; Chevalier, Y.; Bolzinger, M.A. Inorganic ions in the skin: Allies or enemies? Int. J. Pharm. 2020, 591, 119991. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Lee, H.B.; Shin, J.H.; Kim, E.K. Water-retentive and anti-inflammatory properties of organic and inorganic substances from Korean sea mud. Nat. Prod. Commun. 2010, 5, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, L.M.; Nachat, R.; Groot, K.R.; Klement, J.F.; Uitto, J.; Djian, P.; Määttä, A.; Watt, F.M. Mice deficient in involucrin, envoplakin, and periplakin have a defective epidermal barrier. J. Cell Biol. 2007, 179, 1599–1612. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Song, C.; Liu, L.; Chen, J.; Hu, Y.; Li, J.; Wang, B.; Bellusci, S.; Chen, C.; Dong, N. Evidence for the critical role of the PI3K signaling pathway in particulate matter-induced dysregulation of the inflammatory mediators COX-2/PGE2 and the associated epithelial barrier protein Filaggrin in the bronchial epithelium. Cell Biol. Toxicol. 2020, 36, 301–313. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Benítez-Angeles, M.; Sánchez-Hernández, R.; Morales-Lázaro, S.L.; Hiriart, M.; Morales-Buenrostro, L.E.; Torres-Quiroz, F. TRPV4: A physio and pathophysiologically significant ion channel. Int. J. Mol. Sci. 2020, 21, 3837. [Google Scholar] [CrossRef]
- Richard, F.; Creusot, T.; Catoire, S.; Egles, C.; Ficheux, H. Mechanisms of pollutant-induced toxicity in skin and detoxification: Anti-pollution strategies and perspectives for cosmetic products. In Annales Pharmaceutiques Françaises; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging—Contributors and inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef]
- Portugal-Cohen, M.; Oron, M.; Cohen, D.; Ma’or, Z. Antipollution skin protection–a new paradigm and its demonstration on two active compounds. Clin. Cosmet. Investig. Dermatol. 2017, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.K. Skin aging & modern age anti-aging strategies. PharmaTutor 2019, 7, 22–70. [Google Scholar]
- Soroka, Y.; Ma’or, Z.; Leshem, Y.; Verochovsky, L.; Neuman, R.; Brégégère, F.M.; Milner, Y. Aged keratinocyte phenotyping: Morphology, biochemical markers and effects of Dead Sea minerals. Exp. Gerontol. 2008, 43, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Chervonsky, A.V. Apoptotic and effector pathways in autoimmunity. Curr. Opin. Immunol. 1999, 11, 684–688. [Google Scholar] [CrossRef]
- Karaźniewicz-Łada, M.; Główka, A. A review of chromatographic methods for the determination of water-and fat-soluble vitamins in biological fluids. J. Sep. Sci. 2016, 39, 132–148. [Google Scholar] [CrossRef]
- Danis, J.; Mellett, M. Nod-like receptors in host defence and disease at the epidermal barrier. Int. J. Mol. Sci. 2021, 22, 4677. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef]
- Lee, T.-H.; Kang, T.-H. DNA oxidation and excision repair pathways. Int. J. Mol. Sci. 2019, 20, 6092. [Google Scholar] [CrossRef]
- Cohen, D.; Portugal-Cohen, M. Safe Retinol-Like Skin Biological Effect by a New Complex, Enriched with Retinol Precursors. J. Cosmet. Dermatol. Sci. Appl. 2020, 10, 59. [Google Scholar]
- Wang, M.; Charareh, P.; Lei, X.; Zhong, J.L. Autophagy: Multiple Mechanisms to Protect Skin from Ultraviolet Radiation-Driven Photoaging. Oxidative Med. Cell. Longev. 2019, 2019, 8135985. [Google Scholar] [CrossRef]
- Wineman, E.; Portugal-Cohen, M.; Soroka, Y.; Cohen, D.; Schlippe, G.; Voss, W.; Brenner, S.; Milner, Y.; Hai, N.; Ma’or, Z. Photo-damage protective effect of two facial products, containing a unique complex of Dead Sea minerals and Himalayan actives. J. Cosmet. Dermatol. 2012, 11, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: The implication in UV-based phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gutop, E.; Diatlova, A.; Linkova, N.; Orlova, O.; Trofimova, S.; Khavinson, V. Aging of skin fibroblasts: Genetic and epigenetic factors. Adv. Gerontol. Uspekhi Gerontol. 2019, 32, 908–914. [Google Scholar]
- Cook, M.K.; Kaszycki, M.A.; Richardson, I.; Taylor, S.L.; Feldman, S.R. Comparison of two devices for facial skin analysis. J. Cosmet. Dermatol. 2022, 21, 7001–7006. [Google Scholar] [CrossRef]
- Overy, D.; Correa, H.; Roullier, C.; Chi, W.-C.; Pang, K.-L.; Rateb, M.; Ebel, R.; Shang, Z.; Capon, R.; Bills, G. Does osmotic stress affect natural product expression in fungi? Mar. Drugs 2017, 15, 254. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Heterologous protein expression in Pichia pastoris: Latest research progress and applications. ChemBioChem 2018, 19, 7–21. [Google Scholar] [CrossRef]
- Portugal-Cohen, M.; Dominguez, M.F.; Oron, M.; Holtz, R. Dead Sea minerals-induced positive stress as an innovative resource for skincare actives. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 22. [Google Scholar] [CrossRef]
- Eckersley, A.; Ozols, M.; O’Connor, C.; Bell, M.; Sherratt, M.J. Predicting and characterising protein damage in the extracellular matrix. J. Photochem. Photobiol. 2021, 7, 100055. [Google Scholar] [CrossRef]
- Heinemann, U.; Schuetz, A. Structural features of tight-junction proteins. Int. J. Mol. Sci. 2019, 20, 6020. [Google Scholar] [CrossRef]
- Seo, S.H.; Kim, S.-E.; Lee, S.E. ER stress induced by ER calcium depletion and UVB irradiation regulates tight junction barrier integrity in human keratinocytes. J. Dermatol. Sci. 2020, 98, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Portugal-Cohen, M.; Soroka, Y.; Ma’or, Z.; Oron, M.; Zioni, T.; Brégégère, F.M.; Neuman, R.; Kohen, R.; Milner, Y. Protective effects of a cream containing Dead Sea minerals against UVB-induced stress in human skin. Exp. Dermatol. 2009, 18, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.A.; Russell, E.V.; Latimer, J.A. Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure and oxidative stress. Br. J. Dermatol. 2013, 169, 9–14. [Google Scholar] [CrossRef]
- Singh, P.; Jain, K.; Desai, C.; Tiwari, O.; Madamwar, D. Microbial community dynamics of extremophiles/extreme environment. In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2019; pp. 323–332. [Google Scholar]
- Yaniv, Z.; Koltai, H. Calotropis procera, Apple of Sodom: Ethnobotanical review and medicinal activities. Isr. J. Plant Sci. 2018, 65, 55–61. [Google Scholar] [CrossRef]
- Imosemi, I.O. Evaluation of the toxicity, medicinal use and pharmacological actions of Calotropis procera. Ejpmr 2016, 3, 28–36. [Google Scholar]
- Portugal-Cohen, M.; Ish-Shalom, E.; Mallon, R.; Corral, P.; Michoux, F. Apple of Sodom (Calatropis procera) callus extract, a novel skincare active and its biological activity in skin models when combined with Dead Sea water. J. Cosmet. Dermatol. Sci. Appl. 2018, 8, 73–91. [Google Scholar] [CrossRef]
- Kottmeier, C.; Agnon, A.; Al-Halbouni, D.; Alpert, P.; Corsmeier, U.; Dahm, T.; Eshel, A.; Geyer, S.; Haas, M.; Holohan, E.; et al. New perspectives on interdisciplinary earth science at the Dead Sea: The DESERVE project. Sci. Total Environ. 2016, 544, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Anton, B.P.; DasSarma, P.; Martinez, F.L.; DasSarma, S.L.; Al Madadha, M.; Roberts, R.J.; DasSarma, S. Genome Sequence of Salarchaeum sp. strain JOR-1, an extremely halophilic archaeon from the Dead Sea. Microbiol. Resour. Announc. 2020, 9, e01505-19. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Lebre, P.H.; De Maayer, P.; Cowan, D.A. Xerotolerant bacteria: Surviving through a dry spell. Nat. Rev. Microbiol. 2017, 15, 285–296. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. A perspective on biotechnological applications of thermophilic microalgae and cyanobacteria. Bioresour. Technol. 2019, 278, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, M. Isolation and characterization of extremely halotolerant Bacillus species from Dead Sea black mud and determination of their antimicrobial and hydrolytic activities. Afr. J. Microbiol. Res. 2017, 11, 1303–1314. [Google Scholar]
- Al-Karablieh, N. Antimicrobial Activity of Bacillus Persicus 24-DSM Isolated from Dead Sea Mud. Open Microbiol. J. 2017, 11, 372–383. [Google Scholar] [CrossRef]
- Oren, A.; Ginzburg, M.; Ginzburg, B.; Hochstein, L.; Volcani, B. Haloarcula marismortui (Volcani) sp. nov., nom. rev., an extremely halophilic bacterium from the Dead Sea. Int. J. Syst. Evol. Microbiol. 1990, 40, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shin, J.Y.; Hwang, S.J.; Kim, Y.S.; Kim, Y.M.; Gil, S.Y.; Jin, M.H.; Lee, S.H. Effect of Halophilic bacterium, Haloarcula vallismortis, extract on UV-induced skin change. J. Soc. Cosmet. Sci. Korea 2015, 41, 341–350. [Google Scholar]
- Callewaert, C.; Ravard Helffer, K.; Lebaron, P. Skin Microbiome and its Interplay with the Environment. Am. J. Clin. Dermatol. 2020, 21, 4–11. [Google Scholar] [CrossRef]
- Dimitriu, P.A.; Iker, B.; Malik, K.; Leung, H.; Mohn, W.; Hillebrand, G.G. New insights into the intrinsic and extrinsic factors that shape the human skin microbiome. MBio 2019, 10, e00839-19. [Google Scholar] [CrossRef]
- Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Etten, E.V.; Horwitz, P.; Kozyrskyj, A. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef]
- Dawson, T.L., Jr. Malassezia: The forbidden kingdom opens. Cell Host Microbe 2019, 25, 345–347. [Google Scholar] [CrossRef]
- Brandwein, M.; Fuks, G.; Israel, A.; Sabbah, F.; Hodak, E.; Szitenberg, A.; Harari, M.; Steinberg, D.; Bentwich, Z.; Shental, N. Skin microbiome compositional changes in atopic dermatitis accompany Dead Sea climatotherapy. Photochem. Photobiol. 2019, 95, 1446–1453. [Google Scholar] [CrossRef]
- Chng, K.R.; Tay, A.S.L.; Li, C.; Ng, A.H.Q.; Wang, J.; Suri, B.K.; Matta, S.A.; McGovern, N.; Janela, B.; Wong, X.F.C.C. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat. Microbiol. 2016, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, S.; Giryes, H.; Halevy, S.; Neumann, L.; Flusser, D.; Buskila, D. Treatment of psoriatic arthritis at the Dead Sea. J. Rheumatol. 1994, 21, 1305–1309. [Google Scholar] [PubMed]
- Chadzopulu, A.; Adraniotis, J.; Theodosopoulou, E. The therapeutic effects of mud. Prog. Health Sci. 2011, 1, 132–136. [Google Scholar]
- Sukenik, S.; Buskila, D.; Neumann, L.; Kleiner-Baumgarten, A. Mud pack therapy in rheumatoid arthritis. Clin. Rheumatol. 1992, 11, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Codish, S.; Abu-Shakra, M.; Flusser, D.; Friger, M.; Sukenik, S. Mud compress therapy for the hands of patients with rheumatoid arthritis. Rheumatol. Int. 2005, 25, 49–54. [Google Scholar] [CrossRef]
- Hamed, S.; Almalty, A.-M. Skin Tolerance of Three Types of Dead Sea Mud on Healthy Skin: A Short-Term Study. J. Cosmet. Sci. 2018, 69, 269–278. [Google Scholar]
- Hamed, S.; Almalty, A.M.; Alkhatib, H.S. The cutaneous effects of long-term use of Dead Sea mud on healthy skin: A 4-week study. Int. J. Dermatol. 2021, 60, 332–339. [Google Scholar] [CrossRef]
- Abu-Al-Basal, M.A. Histological evaluation of the healing properties of Dead Sea black mud on full-thickness excision cutaneous wounds in BALB/c mice. Pak. J. Biol. Sci. PJBS 2012, 15, 306–315. [Google Scholar] [CrossRef]
- Ma’or, Z.; Henis, Y.; Alon, Y.; Orlov, E.; Sørensen, K.B.; Oren, A. Antimicrobial properties of Dead Sea black mineral mud. Int. J. Dermatol. 2006, 45, 504–511. [Google Scholar] [CrossRef]
| Main Elements | Name | Dead Sea Water (mg/L) |
|---|---|---|
| Na+ | Sodium | 2295 |
| K+ | Potassium | 1440 |
| Ca2+ | Calcium | 27,620 |
| Mg2+ | Magnesium | 67,120 |
| Sr2+ | Strontium | 516 |
| Cl− | Chlorine | 2300 |
| Br− | Bromine | 38,000 |
| SiO2 | Silicate | <20 |
| Li+ | Lithium | 30 |
| Mn2+ | Manganese | 6 |
| Zn2+ | Zinc | ≤2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, D.; Ma, X.; Yan, X.; Bao, X. The Biological Role of Dead Sea Water in Skin Health: A Review. Cosmetics 2023, 10, 21. https://doi.org/10.3390/cosmetics10010021
Dai D, Ma X, Yan X, Bao X. The Biological Role of Dead Sea Water in Skin Health: A Review. Cosmetics. 2023; 10(1):21. https://doi.org/10.3390/cosmetics10010021
Chicago/Turabian StyleDai, Daoxin, Xiaoyu Ma, Xiaojuan Yan, and Xijun Bao. 2023. "The Biological Role of Dead Sea Water in Skin Health: A Review" Cosmetics 10, no. 1: 21. https://doi.org/10.3390/cosmetics10010021
APA StyleDai, D., Ma, X., Yan, X., & Bao, X. (2023). The Biological Role of Dead Sea Water in Skin Health: A Review. Cosmetics, 10(1), 21. https://doi.org/10.3390/cosmetics10010021





