Collagenase and Tyrosinase Inhibitory Activities and Stability of Facial Cream Formulation Containing Cashew Leaf Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. Determination of Total Phenolic Content
2.3. Determination of Total Flavonoid Content
2.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical-Scavenging-Capacity Assay
2.5. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.6. Determination of Tyrosinase-Inhibition Activity
2.7. Determination of Collagenase Inhibition Activity
2.8. Formulation of Facial Cream
2.9. Stability Studies
2.10. Statistical Analysis
3. Results and Discussion
3.1. Percentage Yield of Cashew Leaves
3.2. Total Phenolic Content, Flavonoid Content and Antioxidant Activity
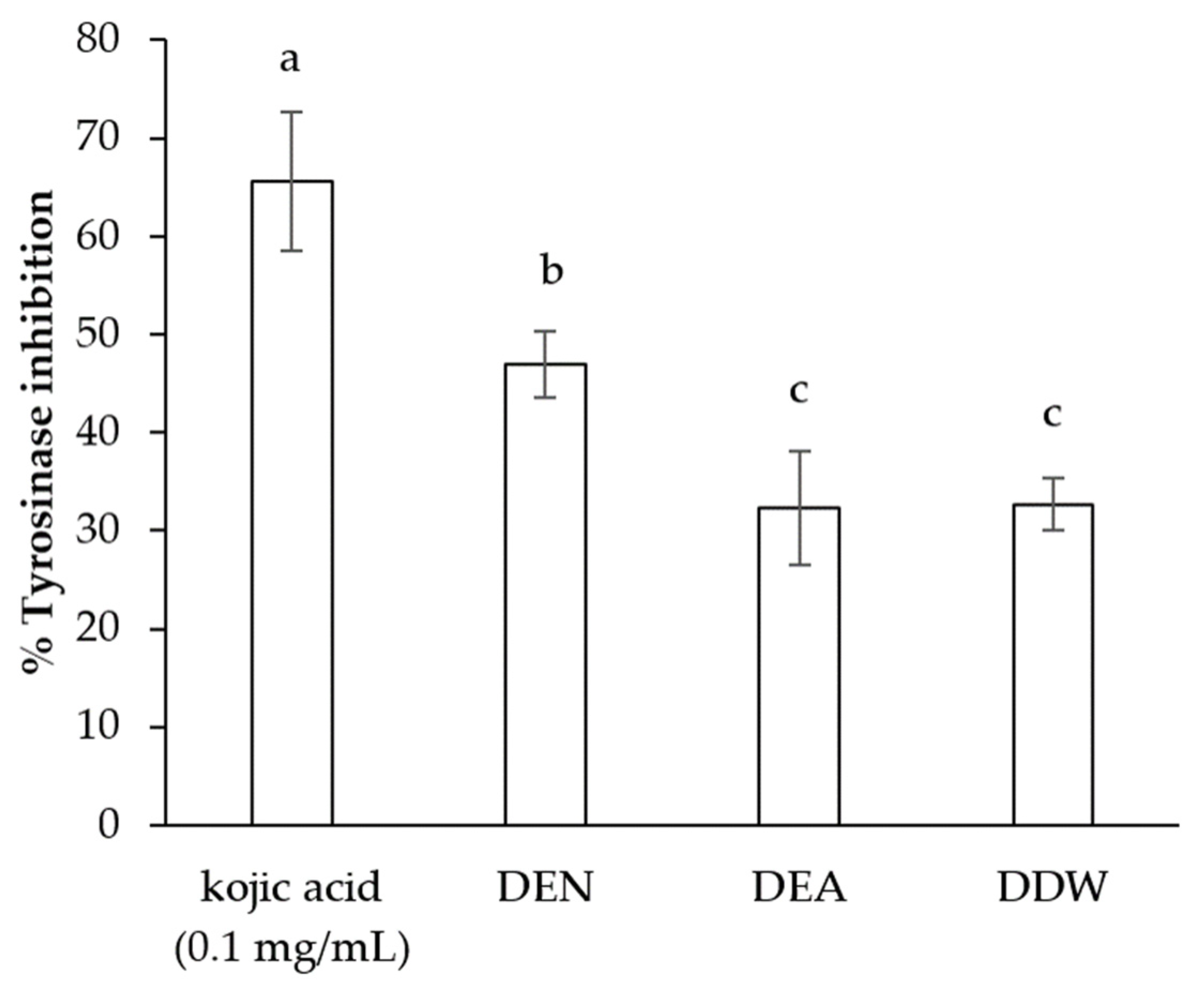
3.3. Tyrosinase Inhibition Activity
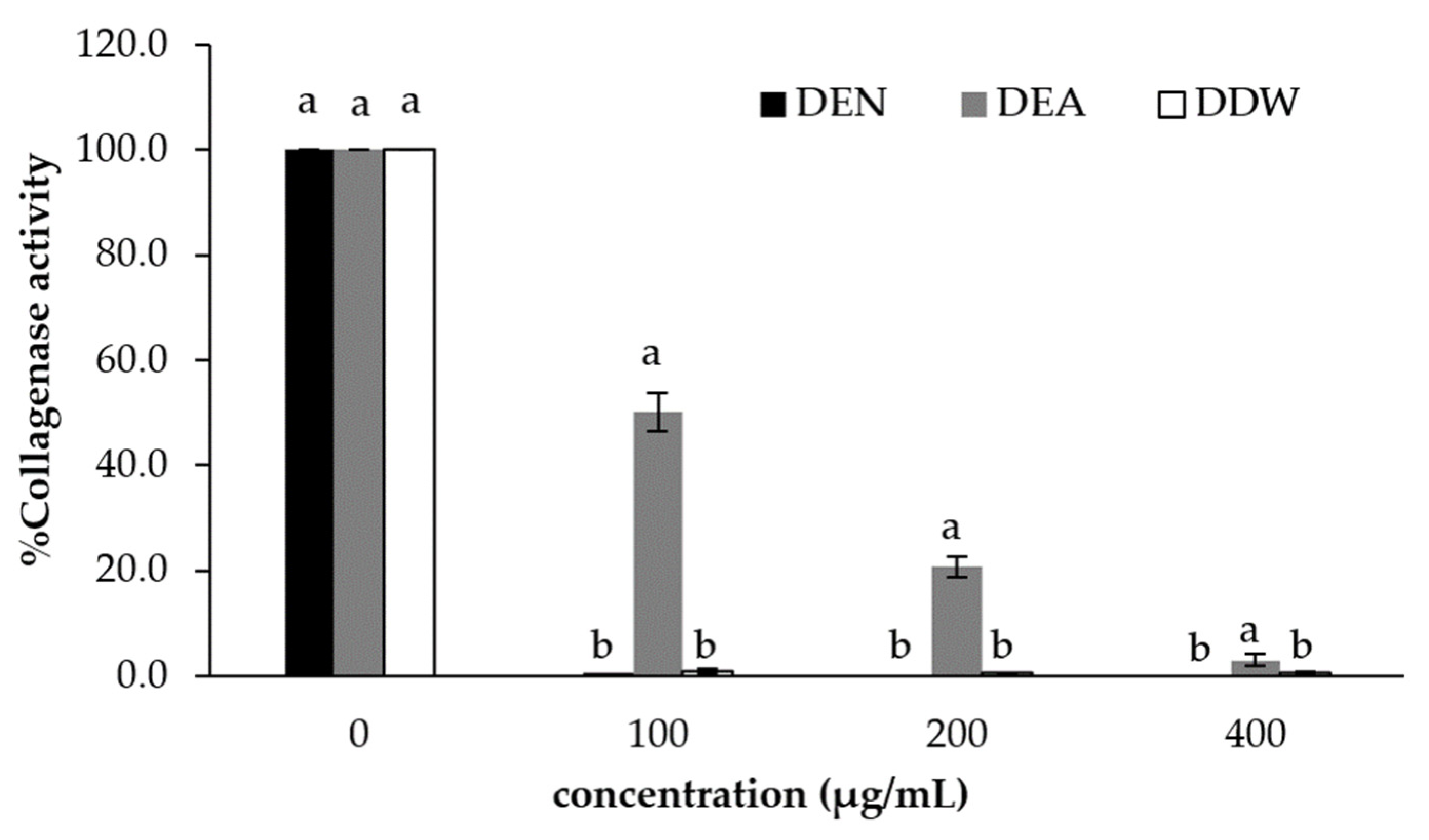
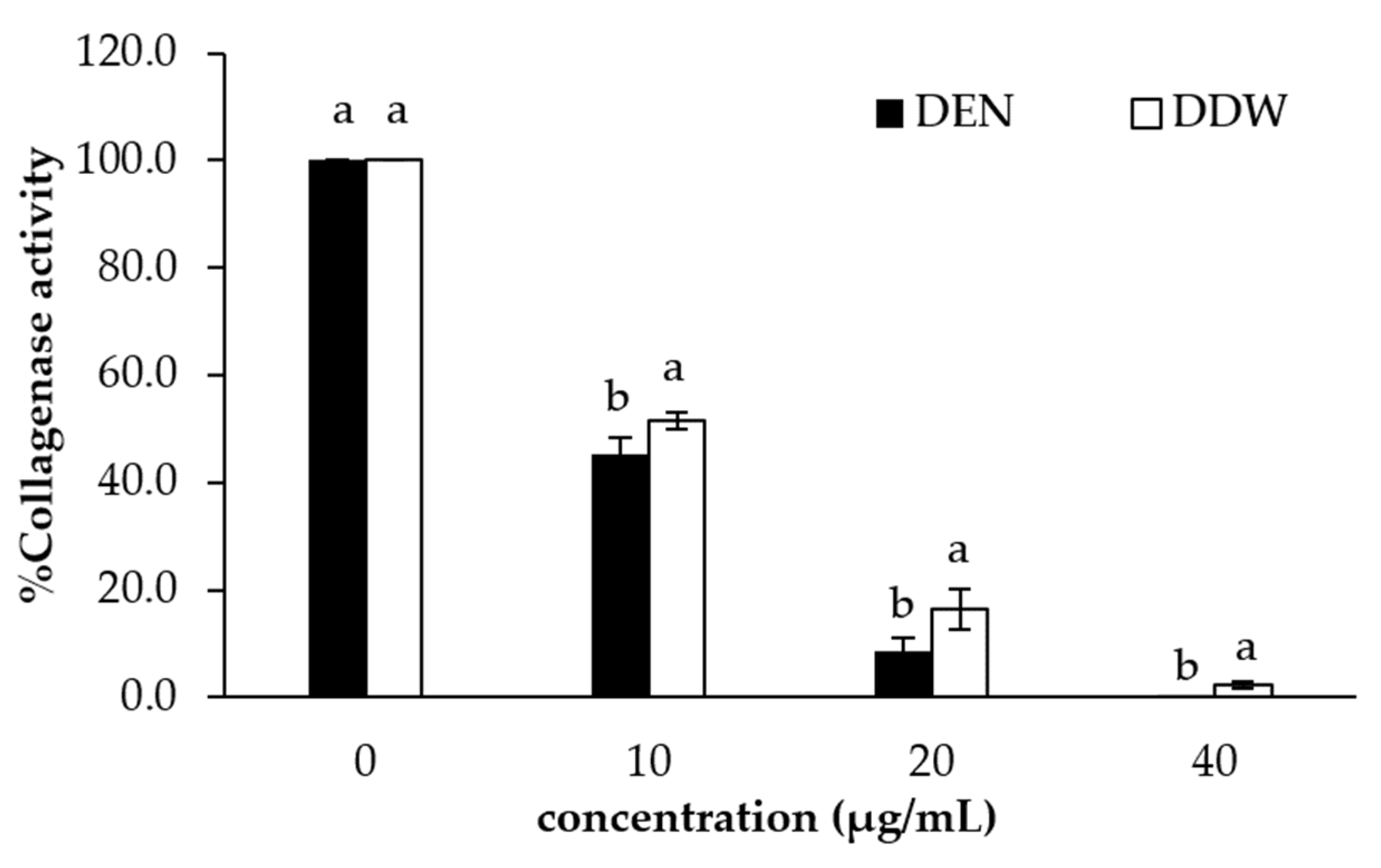
3.4. Collagenase Inhibition Activity
3.5. Stability Test of Facial Cream Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabelo, M.C.; Fontes, C.P.M.L.; Rodrigues, S. Enzyme synthesis of oligosaccharides using cashew apple juice as substrate. Bioresour. Technol. 2009, 100, 5574–5580. [Google Scholar] [CrossRef] [PubMed]
- Zepka, L.Q.; Borsarelli, C.D.; da Silva, M.A.A.P.; Mercadante, A.Z. Thermal degradation kinetics of carotenoids in a cashew apple juice model and its impact on the system color. J. Agric. Food Chem. 2009, 57, 7841–7845. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.B.; Sarandy, M.M.; Gonçalves, R.V.; Novaes, R.D.; da Costa, C.G.; Leite, J.P.V.; do Carmo Gouveia Peluzio, M. Antioxidant and anti-inflammatory effects of Anacardium occidentale L. and Anacardium microcarpum D. extracts on the liver of IL-10 knockout mice. Evid.-based Complement. Altern. Med. 2020, 2020, 3054521. [Google Scholar] [CrossRef]
- Olajide, O.A.; Aderogba, M.A.; Fiebich, B.L. Mechanisms of anti-inflammatory property of Anacardium occidentale stem bark: Inhibition of NF-jB and MAPK signaling in the microglia. J. Ethnopharmacol. 2013, 145, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Andarwulan, N.; Kurniasih, D.; Apriady, R.A.; Rahmat, H.; Roto, A.V.; Bolling, B.W. Polyphenols, carotenoids, and ascorbic acid in underutilized medicinal vegetables. J. Funct. Foods. 2012, 4, 339–347. [Google Scholar] [CrossRef]
- Kubo, I.; Nitoda, T.; Tocoli, F.E.; Green, I.R. Multifunctional cytotoxic agents from Anacardium occidentale. Phytother. Res. 2011, 25, 38–45. [Google Scholar] [CrossRef]
- Nugroho, A.E.; Malik, A.; Pramono, S. Total phenolic and flavonoid contents, and in vitro anti-hypertension activity of purified extract of Indonesian cashew leaves (Anacardium occidentale L.). Int. Food Res. J. 2013, 20, 299–305. [Google Scholar]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medical plants. J. Pharmacogn. Phytochem. 2013, 1, 169–182. [Google Scholar]
- Agidew, M.G. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar] [CrossRef]
- Shobana, G.; Keerthana, K.; John, N.A.A.; Felicita, E.S. In vitro antioxidant potentials of aqueous extract of Anacardium occidentale L. Int. J. Pharm. Pharm. Sci. 2016, 5, 1458–1467. [Google Scholar]
- Ojezele, M.O.; Agunbiade, S. Phytochemical constituents and medicinal properties of different extracts of Anacardium occidentale and Psidium guajava. Asian J. Biomed. Pharm. Sci. 2013, 3, 20–23. [Google Scholar]
- Desai, D.; Raorane, C.; Patil, S.; Gadgil, R.; Patkar, D. Anacardium occidentale: Fountain of phytochemicals; the qualitative profiling. World J. Pharm. Res. 2017, 6, 585–592. [Google Scholar] [CrossRef]
- Shukri, M.A.M.; Alan, C. Analysis of phenolics in Anacardium occidentale shoot extracts using a reversed-phase high performance liquid chromatography tandem mass spectrometry (RP-HPLC-MS). J. Trop. Agric. Food Sci. 2010, 38, 221–230. [Google Scholar]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef]
- Costa, A.R.; AlmeiBezerra, J.W.; da Silva, T.G.; Pereira, P.S.; de Oliveira Borba, E.F.; Braga, A.L.; Fonseca, V.J.A.; de Menezes, S.A.; da Silva, F.S.H.; de Sousa Fernandes, P.A.; et al. Phytochemical profile and anti-Candida and cytotoxic potential of Anacardium occidentale L. (cashew tree). Biocatal. Agric. Biotechnol. 2021, 37, 102192. [Google Scholar] [CrossRef]
- Jennifer, C.; Stephie, C.M.; Abhishri, S.B.; Shalini, B.U. A review on skin whitening property of plant extracts. Int. J. Pharma Bio Sci. 2012, 3, 332–347. [Google Scholar]
- Oresajo, C.; Pillai, S.; Yatskayer, M.; Puccetti, G.; McDaniel, D.H. Antioxidants and skin aging: A review. Cosmet. Dermatol. 2009, 20, 563–570. [Google Scholar]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: An update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Ciganović, P.; Jakimiuk, K.; Tomczyk, M.; Končić, M.Z. Glycerolic licorice extracts as active cosmeceutical ingredients: Extraction optimization, chemical characterization, and biological activity. Antioxidants 2019, 8, 445. [Google Scholar] [CrossRef]
- Ola, A.R.B.; Metboki, G.; Lay, C.S.; Sugi, Y.; Rozari, P.D.; Darmakusuma, D.; Hakim, E.H. Single production of kojic acid by Aspergillus flavus and the revision of flufuran. Molecules 2019, 24, 4200. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, productions, biological activities, pharmacokinetics, and delivery systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef] [PubMed]
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the use of kojic acid—A skin-lightening ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 1–11. [Google Scholar]
- Hussin, M.; Abdul Hamid, A.; Abas, F.; Ramli, N.S.; Jaafar, A.H.; Roowi, S.; Majid, N.A.; Pak Dek, M.S. NMR-based metabolomics profiling for radical scavenging and anti-aging properties of selected herbs. Molecules 2019, 24, 3208. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Mungmai, L.; Preedalikit, W.; Aunsri, N.; Peerakam, N. Bioactivity test and GC–MS analysis of different solvent extracts from Perilla frutescens (Linn.) Britton and cosmetic product application for sensitive skin. Sci. Tech. Rmutt J. 2019, 9, 78–93. [Google Scholar]
- Chandler, S.F.; Dodds, J.H. The effect of phosphate nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Rep. 1983, 2, 205–208. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ali, E. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissue. J. Pharm. Sci. 2009, 3, 277–281. [Google Scholar]
- Duan, X.; Jiang, Y.; Su, X.; Zhang, Z.; Shi, J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2007, 101, 1365–1371. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Zinger, A.; Adir, O.; Alper, M.; Simon, A.; Poley, M.; Tzror, C.; Yaari, Z.; Krayem, M.; Kasten, S.; Nawy, G.; et al. Proteolytic nanoparticles replace a surgical blade by controllably remodeling the oral connective tissue. ACS Nano. 2018, 12, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A novel moisturizer extracted from freshwater macroalga [Rhizoclonium hieroglyphicum (C.Agardh) Kützing] for skin care cosmetic. Chiang Mai J. Sci. 2014, 41, 1195–1207. [Google Scholar]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huong-Huynh, L.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatic. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Dharamveer; Mishra, B.; Siddiqui, H.H. Pharmacognostical and phytochemical studies on Anacardium occidentale Linn. Leaves. Res. J. Pharm. Technol. 2013, 6, 75–79. [Google Scholar]
- Pin, K.Y.; Luqman Chuah, A.; Abdull Rashih, A.; Mazura, M.P.; Fadzureena, J.; Vimala, S.; Rasadah, M.A. Antioxidant and anti-inflammatory activities of extracts of betel leaves (Piper betle) from solvents with different polarities. J. Trop. For. Sci. 2010, 22, 448–455. [Google Scholar]
- Markom, M.; Hassan, M.; Wan Daud, W.R.; Singh, H.; Jahim, J.M. Evaluation of Piper betle on platelet activating factor (PAF) receptor binding activities. Malays. J. Sci. 2007, 26, 79–83. [Google Scholar]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; García, O.P.; Rosado, J.L.; Goñi, I. The contribution of fruits and vegetables to dietary intake of poly-phenols and antioxidant capacity in a Mexican rural diet: Importance of fruit and vegetable variety. Int. Food Res. J. 2011, 44, 1182–1189. [Google Scholar] [CrossRef]
- Mungmai, L.; Preedalikit, W.; Pintha, K.; Tantipaiboonwong, P.; Aunsri, N. Collagenase and melanogenesis inhibitory effects of Perilla frutescens pomace extract and its efficacy in topical cosmetic formulations. Cosmetics 2020, 7, 69. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from me-dicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Jaiswal, Y.S.; Tatke, P.A.; Gabhe, S.Y.; Ashok, V. Antioxidant activity of various extracts of leaves of Anacardium occidentale (cashew). Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 112–119. [Google Scholar]
- Şöhretoğlu, D.; Sari, S.; Barut, B.; Özel, A. Tyrosinase inhibition by some flavonoids: Inhibitory activity, mechanism by in vitro and in silico studies. Bioorg. Chem. 2018, 81, 168–174. [Google Scholar] [CrossRef]
- Kishore, N.; Twilley, D.; van Staden, A.B.; Verma, P.; Singh, B.; Cardinali, G.; Kovacs, D.; Picardo, M.; Kumar, V.; Lall, N. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom ty-rosinase. J. Nat. Prod. 2018, 81, 49–56. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I.; Yokokawa, Y. Tyrosinase inhibitors from Anacardium occidentale fruits. J. Nat. Prod. 1994, 57, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.X.; Hu, Y.H.; Yang, M.H.; Lui, F.J.; Qiu, L.; Zhou, X.W.; Chen, Q.X. Irreversible competitive inhibitory kinetics of cardol triene on mushroom tyrosinase. J. Agric. Food Chem. 2010, 58, 12993–12998. [Google Scholar] [CrossRef]
- Yu, X.P.; Su, W.C.; Wang, Q.; Zhuang, J.X.; Tong, R.Q.; Chen, Q.X.; Chen, Q.H. Inhibitory mechanism of cardanols on tyrosinase. Process Biochem. 2016, 51, 2230–2237. [Google Scholar] [CrossRef]
- Chatatikun, M. Chiabchalard, A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement. Altern. Med. 2017, 17, 487–495. [Google Scholar]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharm. Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
| Test Material | Total Phenolic Content (mg GAE/g Extract) | Total Flavonoid Content (mg QE/g Extract) | DPPH Assay (mg GAE/g Extract) | Reducing Power Assay (mg GAE/g Extract) |
|---|---|---|---|---|
| DEN | 41.39 ± 1.47 b | 7.63 ± 0.07 a | 152.04 ± 2.40 a | 37.90 ± 1.07 a |
| DEA | 0.91 ± 0.03 c | 0.32 ± 0.06 c | 31.43 ± 1.52 c | 0.53 ± 0.03 c |
| DDW | 111.00 ± 0.78 a | 3.88 ± 0.03 b | 144.21 ± 3.68 b | 35.59 ± 0.66 b |
| Parameter | Initiation | RT | 4 °C | 45 °C | H/C Cycle |
|---|---|---|---|---|---|
| Viscosity (pas) | 1025 ± 3.06 | 1030.33 ± 4.16 | 1020.33 ± 13.58 | 1036.00 ± 5.57 | 1044.67 ± 10.26 |
| Color | Pale green | Pale green | Pale green | Pale green | Pale green |
| Homogeneity | Good | Good | Good | Good | Good |
| Phase separation | No | No | No | No | No |
| Feel on skin | good | good | good | good | good |
| pH | 6.01 | 6.12 | 6.22 | 6.35 | 6.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisuksomwong, P.; Kaenhin, L.; Mungmai, L. Collagenase and Tyrosinase Inhibitory Activities and Stability of Facial Cream Formulation Containing Cashew Leaf Extract. Cosmetics 2023, 10, 17. https://doi.org/10.3390/cosmetics10010017
Srisuksomwong P, Kaenhin L, Mungmai L. Collagenase and Tyrosinase Inhibitory Activities and Stability of Facial Cream Formulation Containing Cashew Leaf Extract. Cosmetics. 2023; 10(1):17. https://doi.org/10.3390/cosmetics10010017
Chicago/Turabian StyleSrisuksomwong, Pawalee, Lalita Kaenhin, and Lapatrada Mungmai. 2023. "Collagenase and Tyrosinase Inhibitory Activities and Stability of Facial Cream Formulation Containing Cashew Leaf Extract" Cosmetics 10, no. 1: 17. https://doi.org/10.3390/cosmetics10010017
APA StyleSrisuksomwong, P., Kaenhin, L., & Mungmai, L. (2023). Collagenase and Tyrosinase Inhibitory Activities and Stability of Facial Cream Formulation Containing Cashew Leaf Extract. Cosmetics, 10(1), 17. https://doi.org/10.3390/cosmetics10010017







