Abstract
Probiotics developed for topical applications in humans have the potential to beneficially modulate microbial imbalances on the skin surface and thereby improve skin health. This study was conducted to determine whether topical formulations containing the human skin commensal Micrococcus luteus strain Q24 (BLIS Q24) are safe, tolerable and efficacious when used by healthy human subjects. M. luteus Q24 was assessed in vitro for haemolytic activity and its antibiotic susceptibility profile. Formulations of strain Q24 were evaluated for the preliminary safety and tolerability in healthy human participants. Forty-seven adults were randomly assigned to four single-site, single-blind randomised placebo or baseline controlled or active-controlled trials. Skin swab samples were collected for differential viable counts to monitor levels of probiotic colonisation. M. luteus Q24 was found to be non-haemolytic and susceptible to commonly used antibiotics. The M. luteus Q24 formulations were safe and tolerable and >90% of the participants reported improvements from baseline in the appearance (e.g., radiance and hydration) of their treated skin. Additionally, participants observed a reduction in pore size, skin clarity and enhanced skin softness. No adverse effects were reported. A dose-related significant increase was observed in the levels of M. luteus Q24 isolated from skin swabs of the probiotic-treated subjects. Placebo-controlled trials in human subjects involving the topical application of different doses of M. luteus Q24 formulations were supportive of the safety, tolerability and efficacy of probiotic M. luteus Q24. Self-reported skin health assessments by the subjects indicated that M. luteus Q24 has good potential as a probiotic for improving skin health quality.
Keywords:
topical probiotic; skin; Micrococcus luteus Q24; BLIS Q24; serum; safety; tolerability; efficacy; commensal probiotic 1. Introduction
The human skin interfaces with a wide variety of harmful agents ranging from sunlight, temperature shifts and noxious chemicals to a vast array of potentially malevolent microbes, some of which play key etiological roles in the development of common skin conditions such as acne, facial redness, eczema, psoriasis, impetigo, and atopic dermatitis [1,2,3]. Most contemporary skin products used for treatment of these conditions contain drugs (antibiotics, steroids) and various chemical agents, some of which contribute to further deterioration of the skin’s quality and vitality [4,5]. Skin blemishes and minor infections are typically delt with by applying makeup or other cosmetic products to obtain temporary relief, rather than treating the underlying problem [6]. The skin blemishes and pathologies typically re-emerge, resulting in further damage to the skin and associated adverse socioeconomic implications for the consumer.
One reason for poor skin quality is an imbalance or dysbiosis within the skin microbiome—the community of skin-dwelling microorganisms comprising bacteria, fungi and viruses [1]. Actinobacteria, Firmicutes, Proteobacteria and Bacteroidetes have been identified as the four main bacterial phyla and Corynebacterium, Propionibacterium and Staphylococcus are predominant genera [7,8,9]. Pioneering colonization at birth establishes the foundations for an individual’s skin microbiome [1,10]. Many microbial species accounting for inter and intra-personal variations are more commonly present on the face, forearms and palms of the hand, probably due to the specific niche, lifestyle factors, hygiene rituals and cosmetic products which are predominantly used in these areas [11,12]. Human skin disorders and diseases including acne, atopic dermatitis and psoriasis [13,14,15,16] have been linked to dysbiotic changes within the skin microbiome. Recent evidence suggests that the development of a dysbiosis within the usual synergistic relationships that normally exist between the microbes inhabiting the skin can influence the pathological progression of many skin diseases [9]. These observations have provided a scientific basis for research into the use of probiotic skin bacteria to beneficially modulate the composition of the skin microbiome in both the dermatological and cosmetic fields [3,17].
Some probiotic bacteria interfere with the proliferation and/or metabolic activity of other bacteria (including some potential pathogens) via their production of antibacterial molecules (metabolites and bacteriocins), some creating an unfavourable environment (e.g., by altering the pH or reducing key nutrient availability) and others selectively killing or interfering with the adhesion of competitors to epithelial cells [18]. The World Health Organization (WHO—2000) has defined probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”. Although this definition provides no specification regarding the route of delivery to the host, it has traditionally applied to the delivery of probiotics (especially Bifidobacterium or Lactobacillus) to the human gastrointestinal tract. More recently there is a fast-growing evidence base for the efficacy of probiotic strains sourced from and then primarily delivered to the oral cavity [19,20,21,22], vaginal tract [23,24], and skin epidermis [25,26]. The skin is the largest organ in the human body and the skin microbiome comprises a multitude of commensal and also many opportunistic pathogenic microbes.
The human skin commensal Micrococcus luteus (formerly Micrococcus lysodeikticus) is of historical interest in microbiology, since it played a prominent part in Fleming’s discovery of lysozyme, to which it shows exquisite sensitivity [27,28]. The species M. luteus is commonly found in fermented foods [29,30], in the environment and both internally and within the epidermal microbiotas of humans and other animals, including sheep [31], dogs and cattle [32]. M. luteus has Biosafety Level-1 classification by the American Type Culture Collection (ATCC).
Micrococcus luteus strain Q24 (BLIS Q24) is a prototype skin probiotic strain developed for topical epidermal applications [27,28]. Originally isolated from the skin of a healthy human adult, M. luteus Q24 is a Gram-positive, spherical, saprotrophic, coagulase-negative, bacitracin susceptible bacterium that forms bright yellow colonies when grown on nutrient blood agar. Its survival on the skin is attributed to its ability to tolerate drying and high salt concentrations and its carotenoid pigmentation provides protection against UV -irradiation, all of which are common stressors operating on the surface of mammalian skin. M. luteus Q24 exhibits antimicrobial activity against a wide variety of microbes known to elicit pathological changes within human skin [29].
In view of its distinctive in vitro antimicrobial activity M. luteus Q24 has been given consideration for a variety of probiotic applications including (a) reducing the skin carriage of potentially pathogenic microbes, (b) reduction of body odour by limiting the growth of odiferous organisms, (c) provision of a useful adjunct for acne treatment and (d) treatment of atopic dermatitis, impetigo and tinea pedis (athlete’s foot) [33]. Currently, M. luteus Q24 is being developed for applications in topically administrable formats including serums and cream. In the cosmetic and pharmaceutical fields, the delivery of a living bacterium for topical applications is still a relatively novel concept [29]. It is of paramount importance that these novel probiotics be evaluated by robust testing via a topical route in human volunteers in order to assess their safety and tolerability.
The primary aim of the present studies was to evaluate the preliminary tolerability, safety and potential efficacy of M. luteus Q24 in healthy adult subjects following the topical application of cream and serum formulations containing M. luteus Q24 in different doses, durations and body sites (forearm and face). Safety considerations also included the in vitro testing of M. luteus Q24 for haemolytic activity and for its susceptibility to a relevant series of therapeutic antibiotics.
2. Materials and Methods
M. luteus Q24 has been deposited in the internationally recognized culture collections Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH—German Collection of Microorganisms and Cell Cultures DSMZ 17,172 (submission 10 March 2005).
M. luteus Q24 also has an approved cosmetic ingredient listing (INCI—International Nomenclature of Cosmetic Ingredients) with the Cosmetics, Toiletries and Fragrance Association (INCI name: Micrococcus/hydrolysed non-fat milk ferment CTFA File no. 10419). The biochemical profiling of M. luteus Q24 using ID 32 and API 50CH kits (Biomerieux, Marcy l’Etoile, France) has been reported earlier and this has indicated the absence of metabolic activities potentially adversely affecting the host [33].
M. luteus Q24 freeze dried raw ingredient powder was supplied by Blis Technologies Ltd., Dunedin, New Zealand. The (USP grade) base Medium Chain Triglyceride (MCT) oil for incorporation into the serum was obtained as a gift from Cremer Oleo, Hamburg, Germany. A colloidal hydrophobic silica oleogelator (>99.8%, USP grade) was a gift from Chemiplas, Auckland, New Zealand Limited. The dispersing and wetting agent polysorbate 80 (USP grade) was purchased from Lab Supply, Dunedin, New Zealand. Aqueous cream BP-SLS free (AFT Pharma, Auckland, New Zealand) was obtained from a local pharmacy. Dilutions for cell counts were prepared using analytical grade Phosphate Buffered Saline (PBS) (Oxoid, Dulbeco A) purchased from Thermofisher Scientific, Auckland, New Zealand. The sample dispersions were prepared using non-filter stomacher bags (BagLight Polysilk Transparent, 400 mL, Interscience, Auckland, New Zealand). Bacterial cultures were grown on human blood agar (hBa), sometimes also supplemented with 0.1% (w/v) calcium carbonate (hBaCa), sheep blood agar or mannitol salt agar medium, all purchased from Fort Richard Labs, Auckland, New Zealand. Distilled water was used for preparing buffers and agar medium. The sterile single wrapped COPAN FLOQ swabs used for sample collection were purchased from Fort Richards Labs, Auckland, New Zealand.
2.1. Assessment of Haemolytic Activity
The ability of M. luteus Q24 to lyse red blood cells was tested on two media: (i) human blood agar, consisting of Columbia agar base with either 5% v/v human blood (hBa) and (ii) 5% v/v sheep blood agar, according to the methodology described by Burton et al. [20].
2.2. Antibiotic Susceptibility Testing
M. luteus Q24 was assessed for susceptibility to the clinically important antibiotics Ampicillin, Clindamycin, Erythromycin, Gentamicin, Tetracycline and Vancomycin. The BD Phoenix 50 Automated system was used by an independent accredited laboratory (Southern Community Laboratories, Dunedin, New Zealand) following the recommendations established by the European Committee for Antimicrobial Susceptibility Testing (EUCAST) (2015) [34].
2.3. Preparation of Topical Formulations of M. luteus Q24
The required quantities of M. luteus Q24 freeze dried powder and aqueous cream BP-Sodium Lauryl Sulphate (SLS) free (composition: water, white soft paraffin, cetostearyl alcohol, light liquid paraffin, ceteareth-20, phenoxyethanol) were added to 400 mL stomacher bags (BagLight Polysilk, non-filter transparent bags, Interscience) and homogenised using a stomacher (Masticator Basic, IUL Instruments, Barcelona, Spain) to produce smooth homogenous suspensions of M. luteus Q24 (0.5%, 1%, 2.5% or 10% w/w of the final cream weight). The serum formulations were prepared by combining specified amounts of silica, polysorbate 80 (Tween 80), M. luteus Q24 and MCT oil in a beaker and mix with a high shear homogeniser (DS-160 DLAB, Dunedin, NZ) for 2–3 min. The resultant serum was a smooth homogeneous suspension containing 1% w/w M. luteus Q24.
2.4. Enumeration of M. luteus Q24 in Formulations
In a microcentrifuge tube (Eppendorf, USA), 0.1 g of M. luteus Q24 cream or serum formulation was weighed and diluted with 0.9 g of pre-warmed (37 °C) sterile PBS supplemented with 0.1% v/v polysorbate 80. The mixture was homogenised by shaking using a vortex mixer (auto vortex) at 2800 rpm for 5 min at room temperature (20 °C ± 2 °C) to obtain a homogeneous dispersion. Samples (100 µL) were ten-fold serially diluted in sterile PBS, spread plated onto hBaCa agar and incubated at 37 °C in an atmosphere of 5% CO2 in air for 48 h. The M. luteus Q24 colonies were counted and recorded as colony-forming units (CFU)/g, using a Q-Count Automatic Colony Counter (Spiral Biotech, Auckland, New Zealand).
2.5. Stability of M. luteus Q24 in Formulations
The stability of M. luteus Q24 (1% w/w) in the aqueous cream and serum preparations was evaluated in samples stored as recommended by the International Conference of Harmonization (ICH) guidelines. The specified storage conditions were room temperature, 5 °C and 25 °C (60% relative humidity). Samples were obtained for analysis at specified time intervals to assess the viability of the M. luteus Q24 cells not only during the trial period but also longer term to help assess the shelf life of cells in the formulations.
2.6. Preliminary Cosmetic Safety, Tolerability and Colonisation Efficacy Trials
Four in-house trials were conducted in healthy human adult participants to assess colonisation efficiency, tissue responses, adverse events and participant reactions to the use of the M. luteus Q24 formulations. The formulations were first tested for the absence of microbial contaminants by an independent laboratory (Cawthron Institute, New Zealand). All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was reviewed by the Health and Disability Ethics Committee (HDEC) (New Zealand). This committee advised that since the scope of these currently proposed pilot trials were cosmetic-based rather than directed at health-related outcomes they were outside of the scope of HDEC review. Therefore, due to cosmetic product testing, ethics committee approval was not required. Further, since M. luteus is classified as a Risk Group 1 organism [35,36], and is a human commensal isolated from human skin, it was considered highly unlikely to pose any risk of infection in healthy human subjects. All of the other ingredients in the formulations are commonly used in cosmetic products and were of suitable cosmetic or pharmaceutical grade with the lowest safety risk. Furthermore, a product containing M. luteus Q24 (Live Probiotic Hydration Serum, Unconditionalskin.com accessed on 15 November 2022) is already marketed in New Zealand, with no reported adverse events to date.
2.7. Trial Design
Forty-seven subjects participated in four single-site, single-blind, randomised placebo or baseline controlled or active controlled trials. The study design and dosing regimens are shown in Figure 1 and details of the design are also in Table 1. Potential participants were enrolled if they met the following inclusion criteria: healthy adults 18−75 y of age having generally healthy skin and with mild to moderate fine lines and wrinkles. Participants were excluded if they had cut or wounded skin, had an active skin infection, had a history of autoimmune disease or were currently being treated with either antibiotics or anti-inflammatories (e.g., steroids, non-steroidal anti-inflammatory drugs).
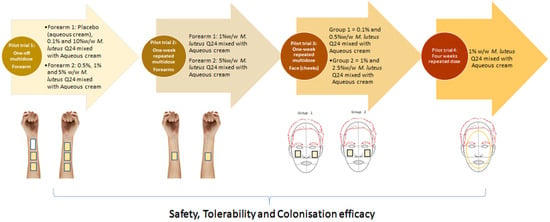
Figure 1.
Schematic diagram of M. luteus Q24 pilot trials on skin. Images created using MS PowerPoint.

Table 1.
Summary of M. luteus Q24 clinical trials.
The primary goal of these pilot trials was to evaluate the safety and tolerability of M. luteus Q24. The secondary outcome was the assessment of the probiotic colonisation efficacy by detection of M. luteus Q24 levels in samples of the skin microbiota. Trials conducted comprised one-off multi-dose on the forearm, one week repeated dose on the forearm, one week multidose repeated on the face (cheeks) and four-week single dose on the entire face of healthy adult participants (Table 1). In pilot trial 3, samples were also analysed for presumptive levels of staphylococci, potentially pathogenic bacteria sometimes found on human skin and often implicated in skin diseases.
Additionally, in the pilot trial, 4 participants were asked to respond to a set of questions related to their perception of their skin health after M. luteus Q24 application and their responses recorded for improvement in facial skin features (radiance, skin health, hydration, tightness/firmness (reduction in pore size), texture (smoothness/softness), pigmentation (clear skin) using a 5 point scale: score −2 (strongly disagree), −1 (disagree), 0 (no change), 1 (agree), 2 (strongly agree).
One participant applied the M. luteus Q24 serum to a dry skin patch on the forearm. Digital photography was taken with consent using the phone’s rear camera (12-megapixel) (Samsung Galaxy S7 edge) to document the condition of the participant’s skin patch pre and post application. Photographs were taken under standard settings such as distance, angle position, background, and lighting.
2.7.1. Sample Collection
A sterile swab (COPAN FLOQ flocked swabs) was lightly dampened with a solution of sterile PBS + polysorbate 80 (0.1% w/w) and preliminary microbiota samples were collected from the skin of each participant’s right and left forearm. Holding firmly 4 cm from the flocked tip, the swab was rubbed over a 2 cm × 2 cm area of the non-hairy underside of the forearm (or cheek and forehead in the follow-up trials 2, 3 and 4 outlined below), in a zig-zag motion for 4–8 s. The flocked end of the swab was then placed into a microcentrifuge tube containing 1ml of sterile PBS + 0.1% w/w polysorbate 80, the flock stick was cut off and the cap was sealed. These tubes were stored at −20 °C until analysed for M. luteus Q24 content. The skin surface was then allowed to dry and six different formulations (0.1 g each) were then applied once only using the tip of a finger to a specified area on the right and left forearm (3 formulations on each forearm) (Figure 1). Skin swab samples were collected 24 h post application following the same procedure as described for the pre-treatment swabs.
All participants were instructed to apply the treatment as the final step of their cosmetic routine at night and to avoid cleaning or washing the treated area for at least 8 h post application.
2.7.2. Microbial Analysis
The frozen swab samples were defrosted at room temperature and then vortex mixed for 1 min at 2600 rpm in a Class II biohazard hood prior to 10-fold serially diluting in PBS. Samples (50 µL) of the initial suspension and each serial dilution were spread plated on hBaCa and (specifically for trial 3) on mannitol salt agar (for detection of mannitol fermentaion positive staphylococci) and incubated at 37 °C in air supplemented with 5% CO2 for 48 h. A Q-Count Automatic Colony Counter (Spiral Biotech, New Zealand) was used to enumerate the bacterial concentrations in the samples in terms of colony forming units (CFU/mL).
2.7.3. Tolerability Assessment Questionnaire
All subjects were asked to document if they experienced irritation (stinging, tingling, redness), itching, burning or erythema associated with the use of the preparations. A four point scale of 1 = none, 2 = slight, 3 = moderate, 4 = severe was implemented.
2.7.4. Statistical Analysis
Statistical analysis and graphing were performed using Prism 9.4.0 (GraphPad Software). Data were analysed by one-way analysis of variance (ANOVA) with correction for multiple comparisons using Bartlett’s or Brown-Forsythe’s method, as appropriate.
3. Results
3.1. Haemolytic Activity
No haemolytic activity against either human or sheep erythrocytes was detected for M. luteus Q24.
3.2. Antimicrobial Susceptibility Testing
M. luteus Q24 was sensitive to a variety of commonly utilised antibiotics (Table 2) a finding consistent with previous reports that this species is highly susceptible to commonly prescribed therapeutic antibiotics and with only very limited risk of development of antibiotic resistance.

Table 2.
Antibiotic susceptibility of M. luteus Q24.
3.3. Preparation, Enumeration and Stability Assessment of M. luteus Q24 Formulations
Several topical formulations were prepared using different concentrations of M. luteus Q24 in either aqueous cream or oil serum. In both the aqueous cream and oil serum samples, one hundred percent of the M. luteus Q24 inoculum (as CFU/g) was detected, confirming that the analytical sampling method was suitable for M. luteus Q24 enumeration in both formulations. The count of M. luteus Q24 in the 1% w/w oil serum formulation was found to be stable at 25 °C/60% relative humidity with <0.25 log reduction in the cell count detected after 24 months (Figure 2). This established that the levels of M. luteus Q24 in this formulation were retained throughout the study period. On the other hand, the M. luteus Q24 in aqueous cream had a relatively poor shelf life at room temperature, with ≥3-log reduction occurring within 1 month and even under refrigerated storage with ≥1.4 log reduction within 3 months and ≥2.2 log reduction after 12 months, the formulation was therefore not considered commercially viable and no further data points were collected. The presence of preservative agents in the aqueous formulations was considered to have rendered these preparations unsuitable for retention of M. luteus Q24 viability.
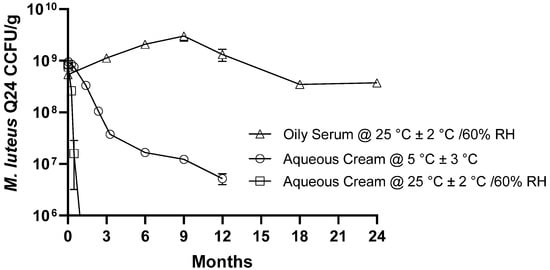
Figure 2.
Effect of storage conditions on the microbial stability (viable cell count) of M. luteus Q24 serum. Data points are means (n = 3 ± SD).
3.4. Cosmetic Safety and Tolerability Trials
3.4.1. Pilot Trial 1: One-Off Multidose—Forearm
The different doses of M. luteus Q24 were generally well tolerated with 90–100% of the participants reporting no adverse effects (Figure 3).
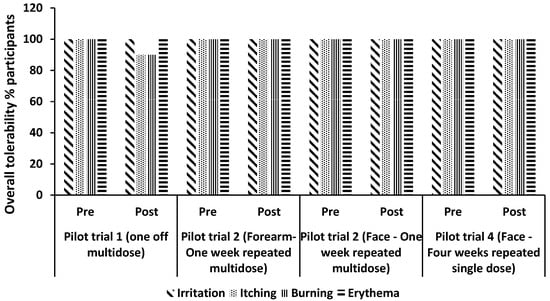
Figure 3.
Participant tolerability assessment for each attribute: Itching, Burning, Irritation, and Erythema. The distribution percent (%) of scores is plotted against each trial. Score 1 = none, 2 = slight, 3 = moderate, 4 = severe.
Analysis of the skin swab samples showed that an increase in the level of M. luteus Q24 in the formulation resulted in a corresponding increase in its colonisation within the skin microbiota (as represented by the relative numbers of M. luteus Q24 colonies) (Figure 4). In comparison to the placebo, a non-significant but 2 to 6-fold increase in the colony counts of M. luteus Q24 was observed. A clear dose–response effect was confirmed by a linear correlation (r2 = 0.90) between M. luteus Q24 dose and M. luteus Q24 colonies on the forearm.
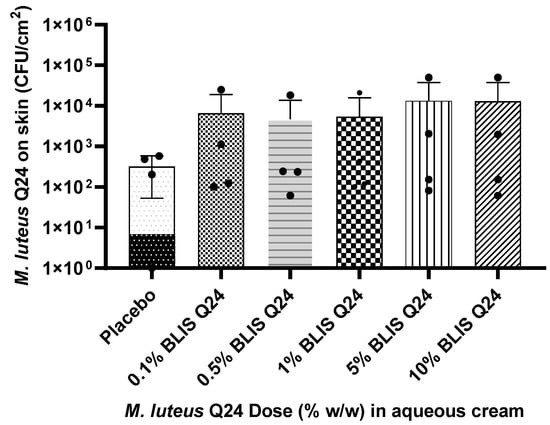
Figure 4.
Influence of M. luteus Q24 dosage level on the subsequent colonisation levels of the probiotic detected after 24 h following one-off application on the forearms of adult participants (n = 13) Data points are means ± SD. Dots on the graphs represents distribution of individual data points.
3.4.2. Pilot Trial 2: One-Week Repeated Multidose—Forearm
As a follow-up to the one-off multidose study, a further assessment of tolerability was carried out by repeated application of doses of serum formulations containing 1% and 5% w/w of M. luteus Q24 once a night at bedtime for 7 days on the forearm of adult participants. Once again it was found that with an increase in the dose of M. luteus Q24 in the formulation there was a corresponding increase in M. luteus colonisation levels (Figure 5). In comparison to the pre-application skin microbiota swab samples, a significant increase in M. luteus Q24-like colonies was observed in the swabs collected post application (Figure 5) indicative of increased colonisation levels and improved persistence of the probiotic colonization upon repeated application. A stronger linear correlation (r2 = 0.92) between dose and viable Q24-like micrococcus cells on the skin was also observed. Compared to the single dose pilot trial 1, a 2–4 fold increase in colonisation levels was observed for different serum doses indicating that enhanced colonisation occurred upon repeated dosing with M. luteus Q24.
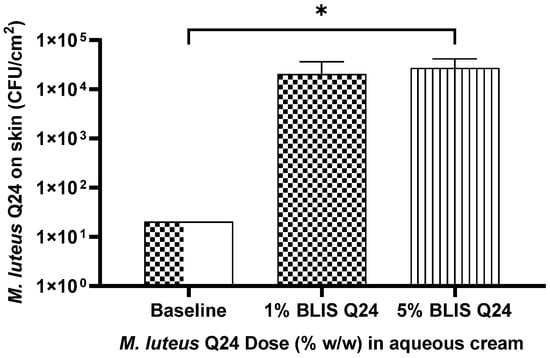
Figure 5.
Influence of M. luteus Q24 dosage level on the subsequent colonisation levels of the probiotic detected 24 h post last dose following one week pre sleep application on the forearms of adult participants (n = 11). Data points are means ± SD. Only groups showing a significant difference (p ≤ 0.05) are indicated with * on the graph.
3.4.3. Pilot Trial 3: One-Week Repeated Multidose—Face (Cheeks Only)
Once the different doses and repeated dosing (one-off versus 7 days) of M. luteus Q24 application on the less sensitive body area(i.e., forearm) had been found to be safe and tolerable, the participants were then asked to apply low dose (0.1% and 0.5% w/w) and high dose (1% and 2.5%w/w) of M. luteus Q24 in serum on the right and left side of face/cheek (split face model) every night for one week. No adverse tolerability reactions were reported by the participants. This outcome was particularly important since the proposed application of the probiotic product is as a facial cosmetic (Figure 6).
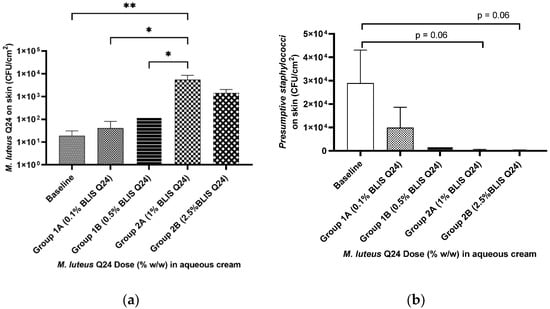
Figure 6.
Influence of M. luteus Q24 dosage level on the subsequent colonisation levels of (a) M. luteus Q24and (b) presumptive staphylococci, detected 24h post last dose following one week of pre-sleep application on the face of adult participants (n = 9). Low dose group (n = 5) High dose group (n = 4). Data points are means ± SD. Only groups showing a significant difference are indicated on the graph with one * indicates p ≤ 0.05 and ** indicates p ≤ 0.01.
Good colonisation efficacy was demonstrated in the face swab samples following the use of both the low and high doses of M. luteus Q24 (Figure 6a). Increased dosage levels of M. luteus Q24 resulted in increased colonisation levels (r2 = 0.78), however due to large variation in the data, a significant difference was not observed in group 2B. Three of the 12 participants did not complete the trial due to reasons not related to the study (e.g., non-compliance due to travel) and therefore not included in the analysis. A significant difference between the groups was observed with a prominent dose–response trend indicating that an increase in dose may lead to increased colonisation.
In addition to the enumeration of M. luteus Q24-like colonies, the skin swab samples were also tested for presumptive staphylococci by plating on Mannitol—salt agar (a selective medium for the isolation and identification of S. aureus). No specific follow up S. aureus identification steps were however carried out. The use of this medium also facilitated the differentiation of M. luteus Q24 colonies from those of Staphylococcus sp. Interestingly, a weakly significant difference was observed for the groups applying 1% and 2.5% M. luteus Q24 in that they exhibited a reduction in presumptive staphylococcal counts compared to baseline, with a strong correlation between dose and the decline in the presumptive pathogenic staphylococcal counts (r2 = 0.94) (Figure 6b).
3.4.4. Pilot Trial 4: Four Weeks Repeated Dose—Face (Full Face)
A consumer experience focused study was carried out involving once daily application of 1% M. luteus Q24 for 4 weeks. As was observed in the previous pilot studies, the participants reported no adverse tolerability responses (Figure 3). A significant increase in the M. luteus population levels was observed post 28 days of the trial compared to the baseline levels (Figure 7a). The proportion of participants having facial swabs yielding M. luteus Q24 increased from 25% (baseline)) to 75% (Day 28 post trial) (Figure 7b).
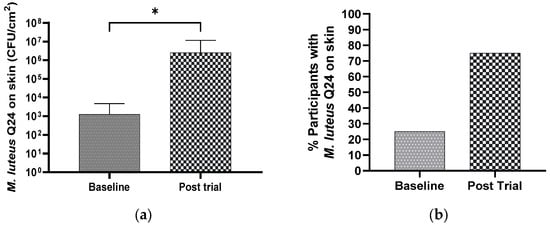
Figure 7.
Effect of M. luteus Q24 serum formulation (1% w/w) on the (a) detection levels of M. luteus Q24 like colonies and (b) percentage of participants colonized 24 h post the last dose of twice daily application for 4 weeks on the face of adult participants (n = 13). Only groups showing a significant difference (p ≤ 0.05) are indicated with * in the graph.
The participant survey (Figure 8) indicated that more than 67% considered there to be an improvement in their skin appearance, with comments including that their skin looks more radiant, healthy and hydrated. A majority (58%) of the participants observed a reduction in pore size, skin clarity and enhanced skin softness. Although no quantitative measurements were obtained, these responses were considered to be encouraging and were consistent with the parallel assessments made of the safety of strain Q24, its tolerability and the skin quality improvement rendered by its topical application.
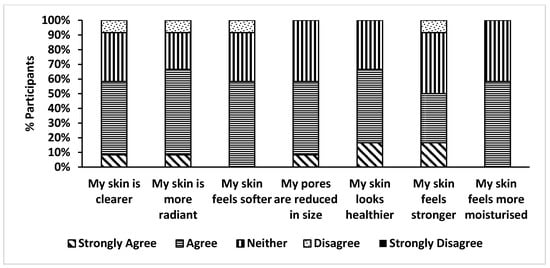
Figure 8.
Self-reported Skin health assessment.
As a footnote to this study, one participant who had a persisting dry patch on the skin on the forearm voluntarily applied the serum to this patch for 28 days (Figure 9). Colonization by M. luteus Q24 was demonstrated together with a 50% reduction in the relative count of potential pathogenic staphylococci. Photographs showed that marked change in the appearance of the skin appeared to have occurred from relatively dry and scaly tissue to considerably more hydrated tissue during the period of application of the M. luteus Q24 serum. Although these observations highlight the potential for M. luteus Q24 in promoting skin hydration, they were based on only one observation and were not placebo-controlled therefore they warrant a follow-up placebo controlled trial with a larger cohort to confirm these findings.
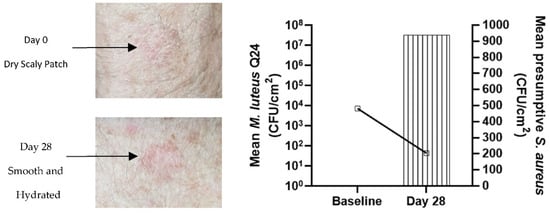
Figure 9.
Improvement in the appearance of dry scaling patch on the forearm (left) and increase in M. luteus Q24 count and decrease in presumptive pathogenic staphylococci count (right) after application of M. luteus Q24 oil serum for 28 days.
4. Discussion
The skin is the largest organ of the body and it is home to billions of microbes, many of which preferentially colonise (and sometimes evoke infections) in different skin sites [18]. There are a growing number of consumers seeking healthy natural alternatives such as probiotics to help optimise their skin health. The health benefits associated with maintaining a balanced population of “good” (beneficial) microbes within the various microbiota associated with the human body has been highlighted over many years especially, with respect to the gastrointestinal tract and the role of probiotics in achieving this is now well documented. Recently, some studies have indicated that there is a link between adverse skin conditions and poor gut health and that the use of high quality probiotics and the eating of probiotic rich foods can enhance the levels of beneficial bacteria in the gut and thereby improve skin health [29]. Although the concept of improving skin health via modulation of the gut microbiota has considerable merit perhaps a more direct approach to the maintenance of healthy skin is the direct epidermal application of probiotics that are naturally adapted to colonise this site and that have been shown to be capable of contributing to the maintenance of microbial homeostasis within the epidermal ecosystem [36].
Probiotic products specifically designed for topical applications such as the Live Probiotic Hydration Serum (Unconditional Skincare co., Blis Technologies Limited, New Zealand) are now attracting increased interest as non- antibiotic alternatives having the potential to relieve and prevent common skin disorders [29]. It is now evident that the use of some of these products can result in the establishment of persisting populations of probiotic microbes within the skin microbiota. Some probiotics may also serve to boost local skin immunity, thereby contributing to enhanced defenses against future exposures to harmful microbes [37]. Currently, there are several so-called topical “probiotic” products that do not actually contain live bacteria and thus should not be referred to as probiotics, but as postbiotics [37]. Some other probiotic products proposed for use on the skin are comprised of bacteria sourced from the gastrointestinal tract or environmental sources. In some cases, Lactobacillus and Bifidobacterium yoghurt culture strains have been utilized. The probiotic functionality of these bacteria that predominantly belong to the gut microbiome is presently rather equivocal within the epidermal ecosystem. These live probiotic products also suffer the disadvantage of undesirable short shelf life (≤6 months) and require refrigerated storage and are thus of less commercial significance. Furthermore, the safety assessment of many of these products has been based upon their GRAS status as intestinal probiotics rather than on clinical assessments following their epidermal application.
The probiotic development of the skin commensal M. luteus strain Q24 was based on the hypothesis similarly to that applied to probiotics for skin, vaginal or oral application that skin health benefits may potentially be achieved by the epidermal application of a harmless bacterium originally found to be persistently present on healthy human skin. The strain has been shown in vitro in agar-based competition assays to be capable of strongly inhibiting a wide range of potential skin pathogens, including methicillin-resistant S. aureus, as well as bacteria implicated in the aetiology of acne, body odour and impetigo and the fungal agents of Tinea pedis (Athlete’s foot) [33]. The oil based M. luteus Q24 formulations evaluated in this study have shown to have excellent shelf life stability (2 y) under the room temperature storage condition (Figure 2). The M. luteus Q24 formulations did not induce any adverse events in any of the trial participants and the various doses, durations and frequencies of applications were very well tolerated. A clear dose–response effect on the recovery of viable M. luteus was observed in samples collected from one-off forearm, one-week repeated multidose—forearm and one-week repeated multidose on cheeks depicted by a linear correlation between M. luteus Q24 dose and M. luteus Q24 colonies on the different body sites and different dosing regimen. The data from trial 3 (Figure 6) also indicated that the repeated use of a cream containing a relatively smaller (i.e., 1% w/w) dose had a similar colonisation efficacy to that obtained from the one-off administration of the higher (i.e., 2.5% w/w) dose formulation.
The recovery of M. luteus Q24 post 7 seven days in subjects of trial 3 One-week repeated multidose—Face (cheeks only (Figure 6a) is very encouraging since the facial skin can be relatively more sensitive than that present elsewhere on the body and also it tends to have more exposure to environmental vagaries and also to the impacts of applications of cosmetic products and cleansing routines. It has been previously documented that M. luteus Q24 inhibits the in vitro growth of various epidermal pathogens including S. aureus [33]. In the present study, it has been shown that M. luteus Q24 may also contribute to the reduction in staphylococci populations in situ following its probiotic application to the skin (Figure 6b).
The full face 4 week trial showed significant increase in M. luteus Q24 with participants reported improvement in a number of cosmetic parameters. The trial however suffers the limitation of absence of a placebo arm that would help to highlight that the beneficial effects are indeed related to M. luteus Q24. Nevertheless, these observations now encourage the further evaluation of the potential role of M. luteus Q24 as a probiotic. It would in particular be useful for the management of a wide variety of microbial dysbiosis of the human skin ranging from impetigo and atopic dermatitis, both known to be caused at least in part by pathogenic staphylococci. It is to be noted that not all staphylococci are pathogenic and some species such as Staphylococcus epidermidis are known to be skin commensals with both benign and pathogenic traits [38]. Therefore, it would be interesting to look at the change in skin microbiome in presence of M. luteus Q24 using advanced analytical tools such as whole genome sequencing to show that it has is no detrimental effect on other skin commensals.
In addition to reporting safety and tolerability, participants were also surveyed about their experience using the product and any benefits that they observed during the trial. The set of questions was related to general health and well-being of the skin and participants did not change their routine cosmetic applications. Participants reported an improvement from the baseline and felt that their skin looks more radiant, healthy and hydrated with a reduction in pore size, skin clarity and enhanced skin softness. Overall, we have demonstrated that M. luteus Q24 formulations were well tolerated on the forearm, forehead and face by healthy adult volunteers of different ages, sex, and skin types.
5. Conclusions
Human participants have been used to assist with the evaluation of the application format and cosmetic outcomes associated with the epidermal application of different doses of probiotic M. luteus Q24. Either cream or serum formulations were utilized and in some cases, placebo preparations were utilized. Preliminary data has been obtained relating to the influence of repeat dosing and its application to different body sites. The findings support the safety of epidermal application of M. luteus Q24, with associated very high tolerability and good colonisation efficacy. Self-reported skin health assessments are supportive of its potential beneficial role in improving skin health quality.
6. Patents
Some of the work included in this paper has been part of the provisional patent “Topical composition and use thereof”—WO 2022/185238 A1.
Author Contributions
Conceptualization, R.J., J.R.T., A.L.V. and J.D.F.H.; methodology, R.J. and A.L.V.; formal analysis, A.L.V.; investigation, A.L.V. and R.J.; writing—original draft preparation, R.J.; writing—review and editing, R.J., A.L.V., J.D.F.H. and J.R.T.; project administration, R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement:
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was reviewed by the Health and Disability Ethics Committee (New Zealand). “Ethical review and approval were waived for this study as the review board advised that since the scope of pilot trials were cosmetic-based rather than directed at health-related outcomes they were outside of the scope of HDEC review.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study”, “Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
All authors are employees of Blis Technologies limited, the manufacturer of Micrococcus luteus Q24 probiotics. The authors declare no conflict of interest.
References
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Aly, R. Microbial Infections of Skin and Nails. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Nakatsuji, T.; Gallo, R.L. The Role of the Skin Microbiome in Atopic Dermatitis. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2019, 122, 263–269. [Google Scholar] [CrossRef]
- Bandyopadhyay, D. Topical Antibacterials in Dermatology. Indian J. Dermatol. 2021, 66, 117. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Roga, G. Topical Steroid-Damaged Skin. Indian J. Dermatol. 2014, 59, 456. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Woolery-Lloyd, H.; Friedman, A. “Natural” Ingredients in Cosmetic Dermatology. J. Drugs Dermatol. 2009, 8, s5–s9. [Google Scholar]
- Niemeyer-van der Kolk, T.; van der Wall, H.E.C.; Balmforth, C.; Van Doorn, M.B.A.; Rissmann, R. A Systematic Literature Review of the Human Skin Microbiome as Biomarker for Dermatological Drug Development. Br. J. Clin. Pharmacol. 2018, 84, 2178–2193. [Google Scholar] [CrossRef]
- Sfriso, R.; Gempeler, M.; Voegeli, R.; Campiche, R. Revealing the secret life of skin—With the microbiome you never walk alone. Int. J. Cosmet. Sci. 2020, 42, 116–126. [Google Scholar] [CrossRef]
- Tavaria, F.K. Topical Use of Probiotics: The Natural Balance. Porto Biomed. J. 2017, 2, 69–70. [Google Scholar] [CrossRef]
- Kong, H.H. Skin Microbiome: Genomics-Based Insights into the Diversity and Role of Skin Microbes. Trends Mol. Med. 2011, 17, 320–328. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Ursell, L.K.; Clemente, J.C.; Rideout, J.R.; Gevers, D.; Caporaso, J.G.; Knight, R. The Interpersonal and Intrapersonal Diversity of Human-Associated Microbiota in Key Body Sites. J. Allergy Clin. Immunol. 2012, 129, 1204–1208. [Google Scholar] [CrossRef]
- Schommer, N.N.; Gallo, R.L. Structure and Function of the Human Skin Microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef]
- Egert, M.; Simmering, R.; Riedel, C.U. The Association of the Skin Microbiota with Health, Immunity, and Disease. Clin. Pharmacol. Ther. 2017, 102, 62–69. [Google Scholar] [CrossRef]
- Clausen, M.L.; Edslev, S.M.; Andersen, P.S.; Clemmensen, K.; Krogfelt, K.A.; Agner, T. Staphylococcus Aureus Colonization in Atopic Eczema and Its Association with Filaggrin Gene Mutations. Br. J. Dermatol. 2017, 177, 1394–1400. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef]
- Rather, I.A.; Bajpai, V.K.; Kumar, S.; Lim, J.; Paek, W.K.; Park, Y.-H. Probiotics and Atopic Dermatitis: An Overview. Front. Microbiol. 2016, 7, 507. [Google Scholar] [CrossRef]
- Dréno, B.; Araviiskaia, E.; Bedesca, E.; Gontijo, G.; Sanchez, V.M.; Xiang, L.F.; Martin, R.; Bieber, T. Microbiome in Healthy Skin, Update for Dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Taverniti, V.; Minuzzo, M.; Arioli, S.; Stuknyte, M.; Karp, M.; Mora, D. Oral Bacteria as Potential Probiotics for the Pharyngeal Mucosa. Appl. Microbiol. 2010, 76, 3948. [Google Scholar] [CrossRef]
- Burton, J.P.; Wescombe, P.A.; Moore, C.J.; Chilcott, C.N.; Tagg, J.R. Safety Assessment of the Oral Cavity Probiotic Streptococcus Salivarius K12. Appl. Environ. Microbiol. 2006, 72, 3050–3053. [Google Scholar] [CrossRef]
- Horz, H.P.; Meinelt, A.; Houben, B.; Conrads, G. Distribution and Persistence of Probiotic Streptococcus Salivarius K12 in the Human Oral Cavity as Determined by Real-Time Quantitative Polymerase Chain Reaction. Oral Microbiol. Immunol. 2007, 22, 126–130. [Google Scholar] [CrossRef]
- Francesco, D.P.; Donato, G.; Fomia, F.; Adami, T.; Careddu, D.; Cassandro, C.; Albera, R.A. Preliminary Pediatric Clinical Evaluation of the Oral Probiotic Streptococcus Salivarius K12 in Preventing Recurrent Pharyngitis and/or Tonsillitis Caused by Streptococcus Pyogenes and Recurrent Acute Otitis Media. Int. J. Gen. Med. 2012, 5, 991–997. [Google Scholar] [CrossRef][Green Version]
- Kober, M.-M.; Bowe, W.P. The Effect of Probiotics on Immune Regulation, Acne, and Photoaging. Int. J. Women’s Dermatol. 2015, 1, 85. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.-C.; Liong, M.-T. Bioactives from Probiotics for Dermal Health: Functions and Benefits. J. Appl. Microbiol. 2013, 114, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Silva, J.; Teixeira, P. The Role of Lactobacilli and Probiotics in Maintaining Vaginal Health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Betsi, G.I.; Athanasiou, A.A. Probiotics for the Treatment of Women with Bacterial Vaginosis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Dis. 2007, 13, 657–664. [Google Scholar] [CrossRef]
- Fleming, A.; Allison, V.D. Observations on a Bacteriolytic Substance (“Lysozyme”) Found in Secretions and Tissues. Br. J. Exp. Pathol. 1922, 3, 252–260. [Google Scholar]
- Fleming, A. On a Remarkable Bacteriolytic Element Found in Tissues and Secretions. Proc. R. Soc. London Ser. B Contain. Pap. Biol. Character 1922, 93, 306–317. [Google Scholar] [CrossRef]
- McLoughlin, I.J.; Wright, E.M.; Tagg, J.R.; Jain, R.; Hale, J.D.F. Skin Microbiome-The Next Frontier for Probiotic Intervention. Probiot. Antimicrob. Proteins 2022, 14, 630–647. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food Fermentations: Microorganisms with Technological Beneficial Use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Jackson, T.A.; Pearson, J.F.; Young, S.D.; Armstrong, J.; O’Callaghan, M. Abundance and Distribution of Microbial Populations in Sheep Fleece. N. Z. J. Agric. Res. 2002, 45, 49–55. [Google Scholar] [CrossRef]
- Kloos, W.E.; Musselwhite, M.S. Distribution and Persistence of Staphylococcus and Micrococcus Species and Other Aerobic Bacteria on Human Skin. Appl. Microbiol. 1975, 30, 381–385. [Google Scholar] [CrossRef]
- WO2006104403A1—Skin Treatment Compositions—Google Patents. Available online: https://patents.google.com/patent/WO2006104403A1/en (accessed on 28 July 2021).
- European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters; EUCAST: Växjö, Sweden, 2015.
- Pathogen Safety Data Sheets: Infectious Substances—Micrococcus Spp.—Canada.Ca. Available online: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/micrococcus.html (accessed on 30 July 2021).
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The Role of Topical Probiotics in Skin Conditions: A Systematic Review of Animal and Human Studies and Implications for Future Therapies. Exp. Dermatol. 2020, 29, 15–21. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The Role of Topical Probiotics on Wound Healing: A Review of Animal and Human Studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef]
- Brown, M.M.; Horswill, A.R. Staphylococcus Epidermidis—Skin Friend or Foe? PLoS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).