Abstract
Plant polyphenols have been found to be effective in preventing or reducing different skin alterations. A dietary approach based on these compounds could be a safe and effective method to slow down or prevent age-associated deterioration of skin appearance and function. In a previous study, a specific combination of four botanical extracts (pomegranate, sweet orange, herba Cistanche, and Centella asiatica) exhibited potential anti-aging effects in a dermal fibroblast cell model. The present study aims to clinically evaluate the safety and anti-aging efficacy of this new botanical ingredient (eternalyoung®). To this end, a 12-week randomized, double-blind, placebo-controlled study was carried out in 60 Caucasian women with evident signs of both chrono- and photoaging. Product efficacy was measured as follows: skin moisturization (corneometer), transepidermal water loss (tewameter), skin radiance, and color (spectrophotometer), skin elasticity and firmness (cutometer), skin roughness (image analysis), and skin thickness (ultrasound). Both intergroup and intragroup analysis proved that the daily intake of 225 mg of the active ingredient was enough to produce visible and structural improvements to the skin and to the signs of aging without any side effects. Statistically significant improvements compared to the placebo group were observed as early as 4 weeks regarding wrinkle depth, elasticity, firmness, skin thickness, skin moisturization, transepidermal water loss, and dark spots pigmentation. In addition, the subjects who consumed the blend reported better scores on the self-assessment questionnaires. Our results suggest that the intake of the test product can positively affect the appearance, barrier function, and skin density of aged skin after 12 weeks of treatment.
1. Introduction
In general, by the age of 26, people feel younger than their biological age [1] and want their external appearance to reflect their inner youthfulness. This makes people look for solutions that allow them to maintain good-looking skin for longer.
Skin aging is a gradual process that is associated with the loss of some fundamental skin properties, eventually resulting in dryness, wrinkles, laxity, and pigmented spots, among others. Skin aging is a complex, multifactorial process where genetic, environmental (ultraviolet radiation (UVR) and pollution exposure, etc.), and lifestyle factors (diet, habits, stress, etc.) play a crucial role [2]. Therefore, using only topical products is usually insufficient, and a more holistic view of skin care is needed, including a healthy diet and lifestyle as well as physical and mental well-being.
Nutrition has long been associated with skin health and beauty; however, it is only recently that consumers have come to understand this. The correlation between nutrition, skin health, and beauty has been an area of research that has increased in recent years, and various skin conditions such as dryness, acne, aging, or even sun resistance have been shown to be affected by nutritional patterns [3,4,5]. As a result, beauty supplements and nutricosmetics have grown significantly in recent years. However, the nutricosmetics sector’s major challenge is to offer reliable and efficient solutions with proven effectiveness, which is very important for increasing consumer confidence as well as for clinical decision-making.
On the other hand, the demand for botanical dietary supplements has surged in recent years and is expected to continue growing over the next five years. This is partly due to increased environmental concerns and the adoption of vegan diets by consumers. At the same time, several studies have demonstrated the beneficial effects of botanical phenolic compounds to delay or even prevent age-associated deterioration of skin appearance and function [3,6,7,8].
According to various published scientific articles, the consumption of phenolic compounds from Centella asiatica, Punica granatum fruit, herba Cistanche, and sweet orange, with proven antioxidant and anti-inflammatory properties, may benefit the skin due to their ability to modulate or reduce different cellular pathways involved in the skin aging process [9,10,11,12,13,14,15].
It is important that new innovative blends coming onto the market be able to substantiate their claims with proper studies. In the present study, we were interested in investigating the anti-aging efficacy of a specific mixture of four standardized botanical extracts (Punica granatum, Citrus aurantium var. sinensis, herba Cistanche, and Centella asiatica) containing punicalagins, flavones, phenylpropanoids, and triterpenes, on humans [16].
Previous in vitro studies in a cell line of human dermal fibroblasts demonstrated that this specific botanical blend could counteract key mechanisms involved in the skin aging process [16]. This four-extract blend significantly reduced the telomere shortening rate and percentage of critically short telomeres (<3 kpb), which correlates with the risk of cells entering senescence. Additionally, the ingredient prevented the loss of proliferative potential and intracellular radical oxygen species (ROS) formation on stress-induced premature senescence fibroblasts, which may favor the synthesis of dermal structural proteins. Furthermore, this ingredient significantly reduced the levels of advanced glycation end products (AGEs) in fibroblasts exposed to UVR [16]. Based on all the above findings, the present study aimed to explore if the effects previously found in vitro could be translated to the clinic. To this end, a double-blind, placebo-controlled study was performed to evaluate the safety and anti-aging efficacy of this blend in Caucasian women.
2. Materials and Methods
2.1. Study Design Description
A single-center, randomized, double-blind, placebo-controlled study was conducted in Pavia (PV, Italy) from October 2021 to March 2022.
The study was conducted in accordance with the World Medical Association’s (WMA) Helsinki Declaration and its amendments. Both the study protocol and the informed consent form were approved (ref. no. 2021/13 by 15 November 2021) by the “Comitato Etico Indipendente per le Indagini Cliniche Non Farmacologiche” (Società Scientifica Italiana per le Indagini Cliniche Non Farmacologiche. Genova, Italy).
A signed informed consent form was obtained from all the subjects participating in the study before any study-related procedure took place.
2.2. Eligibility Criteria for Participants
The subjects participating in the study were screened and enrolled from Complife’s volunteer database, under the supervision of a board-certified dermatologist, from a panel of healthy subjects, in accordance with the inclusion and non-inclusion criteria described below.
Eligible subjects were all adult (age > 35 years old) female subjects with visible clinical signs of both chrono- and photoaging (including mild-to-moderate wrinkles and dark spots). In particular, the study included healthy subjects with skin phototypes from I to IV who were willing to not expose their skin to both natural and artificial UV, possessed social security or health insurance, and certified the truth of the personal information declared to the investigator.
The study excluded subjects with food allergies or food intolerances, pregnant or breastfeeding women, subjects under any treatment known to interfere with the test product, subjects having an acute, chronic, or progressive illness liable to interfere with the study data or considered hazardous by the researcher for the subject or incompatible with the study requirements, subjects being in the course of a long-term treatment or intending to have one considered by the investigator liable to interfere with the study data or incompatible with the study requirements, and subjects having a personal history of cosmetic, drug, or domestic products with irritative reactions. In addition, subjects are requested to respect the following requirements during the whole study (after inclusion): avoid intaking food supplements or cosmetic products for face care, and do not modify their regular diet, concerning fruit and vegetable intake.
2.3. Settings and Locations
The study was carried out in Complife Italia Srl facilities in Pavia (PV, Italy). Complife Italia is an independent international group of laboratories for in vitro, chemical, microbiological, and clinical testing of cosmetics, medical devices, and nutraceuticals.
2.4. Intervention
The test item was a commercially available food supplement ingredient (eternalyoung® (EY), supplied by Monteloeder S.L., Miguel Servet 16, Elche, Alicante, Spain) containing a blend of four polyphenolic extracts: Centella asiatica leaf, pomegranate (Punica granatum) fruit, sweet orange fruit (Citrus aurantium var. sinensis), and herba Cistanches stem. In total, w/w, this blend comprises a minimum content of: 2.5% verbascoside; 12.5% hesperidin; 3% punicalagins; and 7% asiaticosides. These main compounds were identified and quantified by HPLC-DAD analysis, comparing the retention time and UV spectra of the peaks in samples with those of authentic standards as previously described [16]. In the experimental product, each jelly capsule contained 225 mg of eternalyoung®, 2 mg of yellow iron oxide, and 98 mg of gelatin. The placebo product was a jelly capsule containing 225 mg of cellulose microcrystalline, 2 mg of yellow iron oxide, and 98 mg of gelatin. The dietary supplement and placebo products were in opaque coloured capsules with identical appearances. They were pre-packed in blisters and consecutively numbered for each subject according to the randomization list. After the initial visit, subjects started taking one capsule of the dietary supplement or the placebo product every day for 12 weeks. The product intake was 30 min before or after eating. To standardize test conditions, two weeks before the baseline evaluations, volunteers were supplied with a neutral cleanser and a base cream to be used in substitution of their usual product for the 14-day washout period and for the whole study period.
2.5. Randomization and Masking
The research coordinator at Complife Italia Srl randomized the eligible subjects. Half of the test subjects received the active product (EY), and the other half received the placebo product according to a randomization list. The restricted randomization list was stratified with a 1:1 allocation using “Efron’s biased coin” algorithm (PASS 2008 software, PASS, LLC., Kaysville, UT, USA). The allocation sequence was then concealed in sequentially numbered, opaque, and sealed envelopes, reporting the unblinded treatment allocation (based on subject entry number in the study). A masked allocation sequence was then prepared to be used by the staff delivering the intervention. An independent technician dispensed either the active or placebo products. Subjects, investigators, and collaborators were blinded.
2.6. Safety
The occurrence of adverse events (AEs) was monitored throughout the study by the investigators and based on subjects’ diary entries. Investigators rated the observed, and reported AEs as being either severe or non-severe based on their potential relationship to study treatment.
2.7. Primary, Secondary Objectives and Outcome Measures
The primary objective of the study was the assessment of anti-aging efficacy. The primary outcome measure was the measurement of the following parameters: skin moisturization, skin elasticity, transepidermal water loss (TEWL), skin thickness, dark spot staining, skin radiance, and skin profilometry.
Skin moisturization was measured using a Corneometer® CM 825 (Courage + Khazaka electronic GmbH, Cologne, Germany). The measurement was taken at five different points on the right cheek. The selected measurement points delineate the vertices and center of a quadrangle virtually drawn across the cheek.
Skin elasticity was measured using a Cutometer® MPA 580 (Courage + Khazaka electronic GmbH, Cologne, Germany). The skin surface of the face (right cheek) was drawn into the aperture (3 mm) of the probe by a negative pressure (450 mbar) for 3 sec and thereafter released for 3 s. The measured parameters were (a) the maximum amplitude of the curve (R0 or skin distensibility), (b) the resistance to the mechanical force versus the ability of the skin to return to its basal state (R2 or gross elasticity), (c) the elastic portion of the suction curve versus the elastic portion of the relaxation curve (R5 or net elasticity), and the portion of the curve between the last maximum amplitude and the maximum amplitude of the curve (R9 or tiring effect).
TEWL was measured using a Tewameter® TM 300 (Courage + Khazaka electronic GmbH, Cologne, Germany). The measurement was taken in the center of the right cheek.
Skin thickness (epidermis + dermis) was measured using an Aloka alfa6 pro-sound ultrasound machine (Hitachi Europe Limited, Buckinghamshire, UK). Skin thickness was measured at five points on the cheek area.
The intensity of dark spots and skin radiance were measured using a colorimeter CM 700D (Konica Minolta, Milano, Italy). The L* and b* parameters measured with the colorimeter were then integrated into the Individual Typology Angle formula (ITA° = Arctan[(L* − 50)/b*] × 180/π). Skin radiance was measured by the 8° gloss parameter. The measurement was taken on the cheek.
Wrinkle depth and skin roughness (Ra parameter) were measured in the periocular area (crow’s feet wrinkles) using a three-dimensional (3-D) microtopography imaging system (PrimosCR, Canfield Scientific, Utrecht, The Netherlands). Ra parameter is related to skin smoothness, and a decrease of Ra can be expressed in absolute values as an increase in skin smoothness.
The secondary endpoint of the study was the assessment of the visual and self-perceived effect of the product on the skin and product tolerability. The subject-based evaluation of the efficacy of the tested product was performed using an 11-item questionnaire adapted to the investigated product and completed by all the study participants. For each item, answers were recorded on a 4-point grading scale (completely agree, agree, disagree, and completely disagree), and results were expressed as the percentage of subjects in agreement.
2.8. Sample Size
The sample size was calculated for the long-term study with a 2-sided 5% significance level and a power of 80%, considering a 20% variation of the primary endpoints due to both inter-individual human variability and error in the measurement techniques. Sample size was calculated using PASS 11 statistical software (version 11.0.8 for Windows) running on Windows Server 2008 R2 Standard SP1 64-bit edition (Microsoft, Redmond, DC, USA). A sample size of 20 subjects per group was necessary given an anticipated dropout rate of 20%.
2.9. Statistical Methods
Statistical analysis was performed using NCSS 10 (version 10.0.7 for Windows; NCSS, Kaysville, UT, USA). Data normality was checked using the Shapiro–Wilk W normality test and data shape. The instrumental data were submitted to Bilateral Student’s t-test for paired data (intragroup analysis); the intergroup statistical analysis is made on the data variations versus the beginning of the study (D0) by means of Bilateral Student’s t-test for unpaired data. A p < 0.05 was considered statistically significant. Age and skin type subgroup statistical analysis was carried out by a two-way ANOVA analysis with a Tukey post hoc adjustment to determine pairwise differences between the groups. A p < 0.05 was considered statistically significant. The statistical analysis output was reported as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. Participants and Product Tolerability
A total of 60 (n = 60) female subjects were successfully randomized between October 2021 and November 2011. Thirty (n = 30) subjects were allocated to the active treatment arm, and thirty (n = 30) were allocated to the placebo treatment arm (Figure 1).
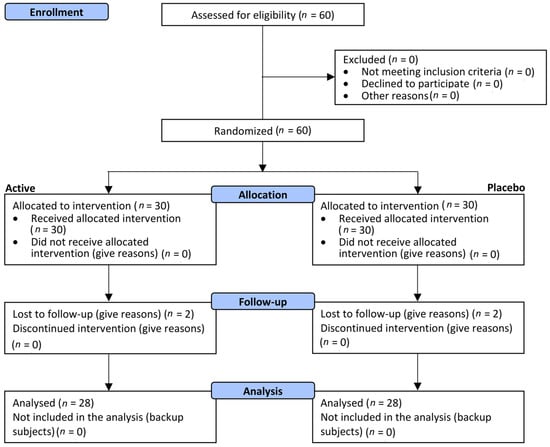
Figure 1.
Participants flow diagram.
Visits to the clinic were performed at baseline and after 28, 56, and 84 days of product intake. A total of 2 subjects per group were lost to follow-up (56- and 84-day visits), not related to product use. These subjects were not included in the statistical analysis. The population were female subjects aged between 35 and 69 years old. Demographics and baseline characteristics were similar across the treatment arms (Table 1). No covariates were identified in relation to demographics or baseline characteristics. Both the active and the placebo products were well-tolerated, and any adverse reactions were reported during the study.

Table 1.
Baseline and demographic characteristics.
3.2. Primary Endpoints
The primary endpoints related to efficacy were measured at baseline and after 28 (D28), 56 (D56), and 84 (D84) days of product intake using non-invasive skin bioengineering techniques. The following parameters were measured: skin moisturization, TEWL, skin profilometry, skin elasticity, dark spot staining, skin radiance, and skin thickness.
3.2.1. Skin Moisturization
A statistically significant increase in the skin moisture content was observed in the subjects taking the dietary supplement from week 2 onward (Figure 2a). In the active treatment arm, the baseline (44.6 ± 1.5) skin moisturization index was significantly increased by 7.1% (47.7 ± 1.6, p = 0.000), 15.3% (51.2 ± 1.7, p = 0.000), and 19.6% (53.0 ± 1.7, p = 0.000) after 28, 56, and 84 days of product intake, respectively (Figure 2a). A small increase of the baseline skin moisturization index by 3.7% (p = 0.000), 3.3% (p = 0.001), and 2.4% (p = 0.039) after 28, 56, and 84 days of product intake, respectively, was also observed in the placebo treatment arm. The skin moisturization variation between the active and the placebo test product was statistically significant at all the checkpoints (p = 0.040 at D28 and p = 0.000 at D56 and D84).
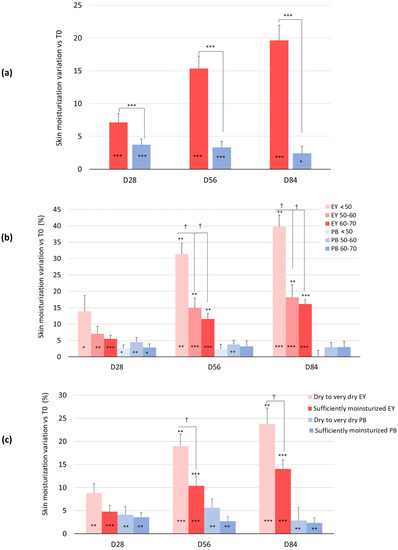
Figure 2.
Change in facial skin hydration. (a) Skin hydration variation versus baseline after 28, 56, and 84 days in the EY treatment (red bars) and placebo (blue bars) groups. Changes in the skin hydration versus baseline in (b) age-related (<50; 50–60 and 60–70 years old) and (c) skin type-related (dry skin type and sufficiently hydrated skin type) subgroups in the EY treatment (red bars) and placebo groups (blue bars). * p < 0.05, ** p < 0.01, and *** p < 0.001. Inter-subgroup statistical analysis is reported with † upon the bars of the histograms as follows: † p < 0.05.
When the data were analyzed by age subgroups (<50, 50–60, and 60–70 years), the study results revealed an inverse correlation between skin moisturization and age, with significant differences detected between the <50-year-old group and the other groups during the second month of this study (Figure 2b). The active product was effective in increasing skin moisturization in all three age groups starting from the first month; however, those who showed the most obvious effect were the youngest group, which experienced an increase of 39.8% compared to the beginning of the study. They were followed by the group aged 50–60, with an increase of 18.2%, and then by the subjects in the segment aged 60–70, who showed an increase in the baseline skin moisturization index of 16.1%. No significant differences were observed between these groups.
Moisturization data were also analyzed in correlation with the clinical classification of skin type. The skin type was categorized as follows: very dry skin was characterized by corneometer units below 30, dry skin between 30 and 45.5, and sufficiently hydrated skin higher than 45.5 corneometric units (c.u.) [17]. Since only one person presented very dry skin, only two subgroups were defined (dry to very dry skin type and sufficiently hydrated). The efficacy of the test product showed a clear dependency on the skin type, where drier skin types reported higher skin moisturization with the ingredient. This effect was observed at the second month of treatment. Thus, at the end of the study, the skin moisturization index was significantly increased by 23.7% in the subjects with dry skin compared to the baseline time. For the sufficiently hydrated subjects, the hydration increase was gradual, but as expected lower than for the former (14.1% compared to the baseline) (Figure 2c).
3.2.2. Transepidermal Water Loss (TEWL)
Changes in the epidermal barrier function were evaluated by measuring the TEWL in g·h−1·m−2 (Table 2). A statistically significant decrease of TEWL was observed from the first month of study in the EY treatment group. In the active treatment arm, the TEWL was decreased by 5.7% (p = 0.012), 10.6% (p = 0.000), and 14.3% (p = 0.000) after 28, 56, and 84 days of product intake, respectively. A small, but statistically significant (p = 0.027) decrease of 1.8% was observed in the placebo treatment arm at D28. The variation between the active and the placebo test product was statistically significant at all the checkpoints (p = 0.012 at D28 and p = 0.000 at D56 and D84). The decrease of the TEWL can be correlated to an improvement of the skin barrier in preserving the water in the deeper layers of the epidermis.

Table 2.
Effect on TEWL and skin elasticity.
3.2.3. Skin Elasticity
Results showed that the overall skin firmness and elasticity improved during the study in the treatment group (Table 2).
- Skin distensibility, measured as R0 (mm), significantly decreased in the dietary supplement group. This parameter is inversely related to skin firmness (a decreased R0 indicates increased skin firmness). In the active treatment arm, skin distensibility was decreased by 5.5% (p = 0.000), 9.9% (p = 0.000), and 11.7% (p = 0.000) after 28, 56, and 84 days of product intake, respectively, with no change observed in the placebo treatment arm. The variation between the active and placebo test products was statistically significant at all the checkpoints (p = 0.002 at D28 and p = 0.000 at D56 and D84);
- The gross elasticity of the skin, including viscous deformation (R2 parameter) was significantly increased in the EY dietary supplement group. In the EY treatment arm, the skin gross elasticity was increased by 6.8% (p = 0.000), 11.8% (p = 0.000), and 14.2% (p = 0.000) after 28, 56, and 84 days of product intake, respectively, while no significant change was observed in the placebo group. The variation between the active and placebo test products was statistically significant at all the checkpoints (p = 0.000 at all the checkpoints);
- Net elasticity, not including viscous deformation (R5 parameter), significantly increased in the EY treatment group. In the active treatment arm, skin net elasticity was increased by 5.8% (p = 0.030), 14.1% (p = 0.000), and 17.3% (p = 0.000) after 28, 56, and 84 days of product intake, respectively, while this parameter was unchanged or even significantly reduced in the placebo treatment arm. The variation between the active and placebo test products was statistically significant at all the checkpoints (p = 0.01 at D28 and p = 0.000 at D56 and D84). R5 increases are an index of reduced skin aging;
- Skin fatigue (R9 parameter) also decreased in the EY supplement group. Thus, in the active treatment arm, the skin tiring effects were decreased by 5.1% (p = 0.032), 9.6% (p = 0.000), and 11.7% (p = 0.000) after 28, 56, and 84 days of product intake, respectively. A small but statistically significant decrease was observed in the placebo group, being 4.0% (p = 0.015) and 4.7% (p = 0.008) at D56 and D84, respectively. The variation between the active and the placebo test products was statistically significant after 56 (p = 0.015) and 84 (p = 0.008) days of product intake;
- When R0, R2, R5, and R9 data were analyzed by age subgroups, no significant differences were found among the different groups (data not shown).
3.2.4. Skin Profilometry: Wrinkle Depth and Skin Roughness
A significant decrease in wrinkle depth and skin roughness (Ra parameter) was observed in subjects taking the dietary supplement from day 28 onward.
- Regarding wrinkle depth, in the active treatment arm, the baseline (377.1 ± 28.3 μm) wrinkle depth was significantly decreased by 10.2% (337.7 ± 25.2 μm, p = 0.000), 11.2% (332.0 ± 24.1 μm, p = 0.000), and 14.4% (322.1 ± 24.6 μm, p = 0.000) after 28, 56, and 84 days of product intake, respectively. A small decrease in the baseline wrinkle depth of 3.7% (p = 0.018) was also observed in the placebo treatment arm after 84 days of product intake. The wrinkle depth variation observed in the dietary supplement group was statistically significant when compared to the placebo treatment regimen at all the checkpoints. (p = 0.000 at D28, D56, and D84) (Figure 3a). Representative images obtained using PrimosCR are reported in Figure 4. A decrease of periocular wrinkles was detected, as observed by the blue coloring. Specifically, the blue area was less evident and intense, and the color changed from blue to green, indicating less wrinkle depth (the wrinkle is marked by the white arrow);
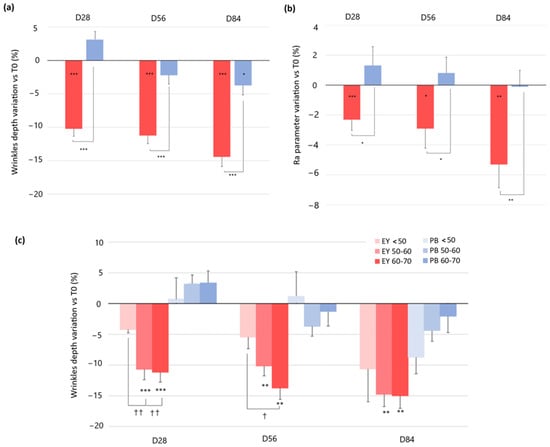 Figure 3. Change in facial skin texture. (a) Wrinkles depth variation and (b) skin roughness (Ra parameter) variation versus baseline after 28, 56, and 84 days in the EY treatment (red bars) and placebo groups (blue bar). (c) Changes in skin hydration versus baseline in the 3 age subgroups (<50, 50–60, and 60–70 years) both in the EY treatment (red bars) and placebo groups (blue bars). Data are means ± SEM. Intragroup (vs. 0) statistical analysis is reported inside the bars of the histograms. Intergroup (vs. placebo) statistical analysis is reported on the bars of the histograms. Statistical analysis is reported as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001. Inter-subgroup statistical analysis is reported with † upon the bars of the histograms as follows: † p < 0.05 and †† p < 0.01.
Figure 3. Change in facial skin texture. (a) Wrinkles depth variation and (b) skin roughness (Ra parameter) variation versus baseline after 28, 56, and 84 days in the EY treatment (red bars) and placebo groups (blue bar). (c) Changes in skin hydration versus baseline in the 3 age subgroups (<50, 50–60, and 60–70 years) both in the EY treatment (red bars) and placebo groups (blue bars). Data are means ± SEM. Intragroup (vs. 0) statistical analysis is reported inside the bars of the histograms. Intergroup (vs. placebo) statistical analysis is reported on the bars of the histograms. Statistical analysis is reported as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001. Inter-subgroup statistical analysis is reported with † upon the bars of the histograms as follows: † p < 0.05 and †† p < 0.01.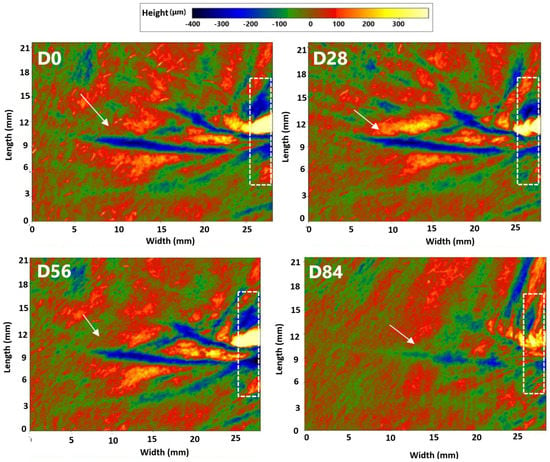 Figure 4. Periocular wrinkles were evaluated with PrimosCR in one volunteer in the EY treatment group. The skin surface is red in color (value approximatively by 0 mm (according to the color scale present in the upper part of the figure). Green and blue colors represent negative values, indicating the presence of wrinkles. Orange and yellow colors represent values higher than zero.. Wrinkle is marked by the white arrow. Eye corner, which is acquired as a reference, is marked by the dotted line box.
Figure 4. Periocular wrinkles were evaluated with PrimosCR in one volunteer in the EY treatment group. The skin surface is red in color (value approximatively by 0 mm (according to the color scale present in the upper part of the figure). Green and blue colors represent negative values, indicating the presence of wrinkles. Orange and yellow colors represent values higher than zero.. Wrinkle is marked by the white arrow. Eye corner, which is acquired as a reference, is marked by the dotted line box. - When the data were analyzed by age subgroups (<50, 50–60, and 60–70 years), the people older than 50 years showed a significantly higher improvement in wrinkle depth when compared to the younger group after 28 and 56 days of treatment. These differences, however, were not statistically significant at the end of the study (Figure 3c).
- Concerning skin roughness, the baseline Ra parameter value in the active treatment arm was significantly decreased by 2.3% (30.4 ± 1.3 μm vs. 29.6 ± 1.3 μm, p = 0.000), 2.9% (29.4 ± 1.3 μm, p = 0.047), and 5.3% (28.7 ± 1.2 μm, p = 0.002) after 28, 56, and 84 days of product intake, respectively. Skin roughness was not changed in the placebo treatment arm. Skin roughness variation between the active and the placebo test products was statistically significant at all the checkpoints (p = 0.018 at D28, p = 0.032 at D56, and p = 0.008 at D84) (Figure 3b). When Ra data were analyzed by age subgroups, no significant differences were found among the different groups (data not shown).
3.2.5. Intensity of Dark Spots: Individual Typology Angle (ITA°)
A significant improvement (decrease in the intensity of pigmentation) of dark spots, determined by ITA° measurement, was observed throughout the study (Table 3 and Figure 5). In the active treatment arm, the intensity of the dark spots decreased by 19.6% (p = 0.012), 23.5% (p = 0.000), and 26.2% (p = 0.000), after 28, 56, and 84 days of product intake, respectively. A small decrease by 11.1% (p = 0.000), 9.2% (p = 0.000), and 13.4% (p = 0.000) after 28, 56, and 84 days of product intake, respectively, was also detected in the placebo group. The differences between the ingredient and the placebo product were statistically significant at all the checkpoints (p = 0.001 at D28 and p = 0.000 at D56 and D84) (Table 3)

Table 3.
Effects on radiance and dark spots colour.
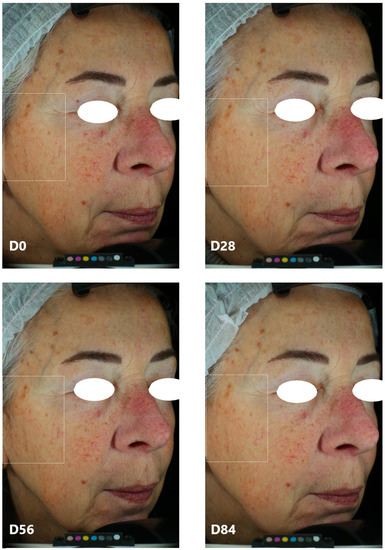
Figure 5.
Representative pictures of one volunteer (vol 41) before and after 28, 56, and 84 days of treatment with the EY ingredient. Digital pictures were acquired by means of VISIA CR (Canfield Imaging Systems, Fairfield, NJ, USA). Starting from D28, dark spots are less evident and the skin’s complexion is more uniform.
3.2.6. Skin Radiance, Gloss Value
Skin gloss, also referred to as skin radiance, was significantly increased during the study (Table 3). In the EY active treatment arm, the skin radiance increased by 13.9% (p = 0.000), 23.7% (p = 0.000), and 27.3% (p = 0.000) after 28, 56, and 84 days of product intake, respectively. A small increase of 7.2% (p = 0.000), 7.8% (p = 0.000), and 9.5% (p = 0.000) after 28, 56, and 84 days of product intake, respectively, was also detected in the placebo treatment arm. The variation between the active and the placebo test products was statistically significant at all the checkpoints (p = 0.000 at all the checkpoints).
3.2.7. Skin Thickness
A statistically significant increase in skin thickness was observed in the subjects taking the dietary EY ingredient from week 4 onward (Figure 6). Thereby, in the active treatment arm, the baseline (1.32 ± 0.04 mm) skin thickness was increased by 0.06 mm (1.38 ± 0.04 mm, p = 0.000), 0.11 mm (1.43 ± 0.04 mm, p = 0.000), and 0.15 mm (1.47 ± 0.04 mm, p = 0.000) after 28, 56, and 84 days of product intake, respectively. A small increase of 0.02 mm (p = 0.006), 0.03 mm (p = 0.031), and 0.05 mm (p = 0.010) after 28, 56, and 84 days of product intake, respectively, was also detected in the placebo treatment arm. The variation between the ingredient and placebo product was statistically significant at all the checkpoints (p = 0.006 at D28, p = 0.031 at D56, and p = 0.010 at D84).
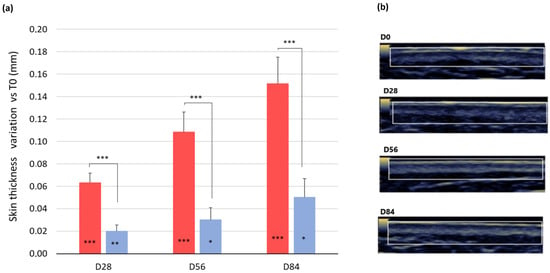
Figure 6.
Effects on skin thickness. (a) Skin thickness variation versus baseline after 28, 56, and 84 days in the treatment (red bars) and in the placebo group (blue bar). Data are means ± SEM. Intragroup (vs. 0) statistical analysis is reported inside the bars. Intergroup (vs. placebo) statistical analysis is reported on the bars. Statistical analysis is reported as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001. (b) Representative skin echography images of one volunteer before and after 28, 56, and 86 days of treatment with the EY ingredient. Pictures were acquired by means of an Aloka Alpha 6 Pro-Sound (Hitachi) ultrasound. The analyzed skin region (dermis + epidermis) is inside the square.
3.3. Secondary Outcome: Self-Assessment Questionnaire
The output of the self-assessment questionnaire is reported in Figure 7. The subjective and qualitative evaluation of the product’s efficacy showed that, according to the study subjects, the use of the dietary supplement was beneficial for their skin. The active test product was scored better by volunteers than the placebo product at all the checkpoints and for all the questionnaire items, the differences between both groups being higher with the time of product intake. In addition, people in the EY treatment group felt their stress and fatigue reduced, while their sense of well-being increased.
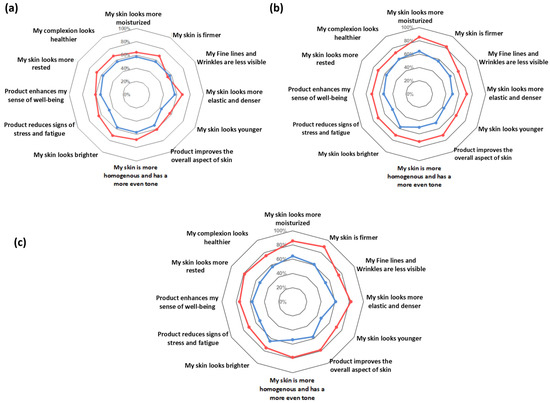
Figure 7.
Self-assessment questionnaire results. (a) Self-assessment questionnaire output at D28. (b) Self-assessment questionnaire output at D56. (c) Self-assessment questionnaire output at D84. Active product EY (red line); placebo product (blue line).
4. Discussion
As the average human life expectancy increases with time, the trend towards healthier lifestyles has become a growing concern, which includes not only physical, emotional, and mental health but also anti-aging and beauty-related characteristics. The demand for natural and plant-based nutricosmetics has been on a constant rise for the last few years due to the growing awareness of the importance of these ingredients for health and beauty [18]. However, despite the substantial availability of natural nutricosmetic ingredients in the market, clinical evidence and tolerance of many of these ingredients are still limited, and longer and better-designed clinical trials are needed to establish their effectiveness.
The most evident and visible symptoms of aging is manifested in the skin appearance changing due to the continuous exposure of endogenous and exogenous factors. Skin aging and photoaging are characterized by a reduction in skin thickness, loss of skin elasticity, collagen degradation, wrinkle formation, and uneven pigmentation, among others [2]. The structural integrity of human skin is largely dependent on the quality of the dermal extracellular matrix (ECM), which is produced, organized, and maintained by dermal fibroblasts. However, as we age, there is an accumulation of senescent fibroblasts. Cellular senescence is defined as an irreversible arrest of cell growth, driven by a variety of mechanisms, including telomere shortening, genotoxic stress, ROS generation, inflammatory cytokines, oncogene overexpression, and DNA damage, such as that induced by UV radiation [19,20]. The accumulation of senescent cells in the skin causes a decline in both the function and the appearance of the skin, leading to a decrease in the skin’s natural self-regenerative potential and a reduction in collagen and elastin fibers [19]. In this scenario, the importance of developing new strategies that can prevent the accumulation of senescent fibroblasts or selectively eliminate them is an evident strategy to counteract skin aging and age-associated skin disorders [21].
In the present study, we evaluated in a clinical scenario the anti-aging efficacy of a four-component plant-based ingredient, eternalyoung® (EY), previously proven to protect fibroblasts from entering senescence by reducing telomere shortening rate, ROS formation, and boosting fibroblast proliferation, which may favor the synthesis of the structural proteins of the dermis, thus preserving its structural organization [16].
The results of the present study demonstrated that a daily intake of 225 mg of EY was enough to produce visible and structural improvements in the skin and to decrease the signs of aging. In fact, the ingredient positively influenced all the clinically monitored parameters, both compared to baseline and the placebo group, as early as 4 weeks of product consumption. The anti-aging efficacy of the test product was demonstrated by the increase in skin moisturization, elasticity, thickness, and radiance and by the decrease in TEWL, the intensity of melanin staining inside dark spots, the wrinkle depth, and the roughness of the skin. It should also be noted that positive results were obtained in almost all of the subjects at the end of the study.
Skin moisturization is one of the most important points to consider in maintaining proper skin condition. Hydration of the skin comes from the internal tissues and is influenced by the physiological conditions of the barrier function [22]. The measurement of transepidermal water loss is a good indicator of the integrity of the skin barrier function, which inherently refers to the skin’s ability to retain moisture. An increase in TEWL indicates impaired barrier function [23]. Altered skin barrier and TEWL have been shown to correlate with skin aging, and improvements in epidermal function can be a useful alternative to prevent and ameliorate disorders that are linked to epidermal dysfunction in the elderly, including eczematous dermatitis, pruritus, and xerosis [24]. The results of the present study indicate that the oral intake of the EY ingredient significantly improved skin hydration (Figure 2a) and decreased TEWL compared to the placebo (Table 2), improving the skin barrier function. Moreover, the skin of the volunteers at the beginning of the study was scored to be in a “healthy condition” (TEWL values of 10–15 g/m2h) and this condition improved to a “very healthy condition” (TEWL values 0–10 g/m2h) only in the EY treatment group. Prior studies have shown that exposure to oxidative stress can induce epidermal permeability barrier disruption, suggesting that antioxidant compounds could improve barrier function [25].Thus, the antioxidant properties of the phenolic compounds present in Centella asiatica [12], Punica granatum fruit [11,26], Herba Cistanche [13], and sweet orange extract [27] could favor an improvement in the skin barrier function.
Regarding skin moisturization, a clear correlation between skin physiology (skin type) and product efficacy became evident, and the moisturizing effect was more evident for the subjects with drier skin, where skin hydration improvement is more needed and appreciated (Figure 2c). Since there was only one subject with very dry skin in the study, it would be interesting to conduct additional studies to explore whether the ingredient can further increase hydration in this group. Additionally, despite having a limited number of individuals, the study showed that the degree of skin hydration in subjects taking the EY ingredient was inversely proportional to their age, and those who showed the most obvious effect were the women under 50 years (Figure 2b). A similar pattern was previously shown in other studies with other moisturizing ingredients [17]. Although the mechanism by which the ingredient increases skin hydration is unknown, it may be due in part to the ability of one of the tested ingredient’s components, asiaticosides, to increase the expression of Aquaporin-3 (AQP3) [28]. AQP3 is an important protein that plays a key role in skin hydration [29]. Moreover, another active compound present in EY, hesperidin, has been shown to increase epidermal filaggrin expression [30]. Filaggrin is important for the formation of the corneocyte, and the generation of its intracellular metabolites contributes to stratum corneum hydration [31].
The skin’s connective tissue is comprised primarily of fibrillar collagen and elastic fibers, along with a complex array of proteoglycans and other extracellular matrix molecules. Dermal fibroblasts are embedded within the matrix, where collagen and elastin give strength and resilience to the skin [32]. The metabolism of ECM proteins is strongly affected by the aging process, which causes an increase in protein degradation as well as collagen degradation and loss of elastin function. This phenomenon results in a reduction of the skin’s elasticity and firmness as well as an increase in its laxity [2,32]. In our study, the observed improvement in all the measured mechanical properties of the facial skin (R0, R2, R5, and R9) and the increase in dermis’ and epidermis’ thickness (Table 2 and Figure 6) indirectly demonstrate the effect of the test product on the ECM components. R5, which represents the net elasticity, is solely affected by the solid components of the skin such as elastic fibers and collagen, and its increase is an index of reduced skin aging [33]; R2 refers to the elasticity of the skin including the viscous deformation, and its increase is associated with the function of the elastic fibers of the skin [34]; R0 is linked with the stretching of both collagen and elastic fibers and is inversely proportional to their thickness and rigidity [35]; and the decrease of the R9 parameter, which represents the tiring effects of the skin after repeated suctioning, provides the resilience of the skin [33].
Also, the improvement in elasticity and firmness, along with the decline of skin fatigue, results in the visual decrease of the wrinkle number and depth (Figure 3 and Figure 4). Wrinkles are a major symptom of skin aging and are caused by several factors. Usually, ROS is one of the important factors involved in the formation of wrinkles. Oxidative stress induced by ROS causes skin inflammation and consequently activates matrix metalloproteinases (MMPs). Activated MMPs, in turn, degrade collagen and promote skin wrinkles and a loss of elasticity and firmness [4,19].
The positive effects observed in this study in the subjects taking EY could be partly due to the capacity of this ingredient to boost fibroblast proliferation and reduce ROS and telomere shortening in aged fibroblasts, allowing cells to escape from the cell senescence pathway [16]. During senescence, cells begin to secrete proinflammatory cytokines, catabolic modulators such as MMPs, and release reactive oxygen species [36]. Additionally, EY significantly reduced the levels of AGEs in fibroblasts [16]. Proteins in the dermal matrix, such as collagen and elastin, and the cytoskeleton are particularly susceptible to glycation and become rigid, lose elasticity, and have reduced regenerative capabilities, leading to skin laxity, cracking, and thinning. [37]. Glycation also causes changes in the behavior of dermal fibroblasts, reducing their proliferation and migration, increasing the production of MMPs, while simultaneously disrupting collagen I maturation and preventing collagen deposition in the extracellular matrix [38].
Furthermore, the botanical extracts that comprise EY have been shown to protect the ECM proteins. Centella asiatica extracts are capable of inhibiting three major skin enzymes implicated in the catabolism of the extracellular matrix: MMP-1(collagenase), hyaluronidase, and elastase [39], while the asiaticosides molecules promote fibroblast proliferation and increase collagen synthesis and intracellular fibronectin content [40]. Citrus fruits and hesperidin have also been shown to have anti-aging and photoprotective properties by preventing the expression of MMPs on skin cells [10,27]. Pomegranate extract also stimulates type I procollagen synthesis and inhibits MMP-1 production in dermal fibroblasts [15]. Verbascoside, the phenylpropanoid present in Cistanche herba, has been proven to effectively decrease the expression of MMP-1 by activating TGFβ/Smad signaling and inhibiting mitogen-activated protein kinases (MAPKs) and the activator protein-1 (AP-1) signaling pathway [41] and to activate the expression of the precursor of MMP-2 in human dermal fibroblasts [42]. In a follow-up clinical study, it could be interesting to investigate the effect of EY supplementation on dermal matrix macromolecule synthesis.
The daily consumption of this botanical blend also provided a more uniform skin complexion. Specifically, the daily consumption of EY has a progressive brightening effect (almost three times more than the placebo group) and significantly lightened the hyperpigmented spots on the cheekbone area (two times more than the placebo group). These improvements were statistically significant compared to the placebo group after only four weeks of taking the dietary supplement ingredient. In addition, at the end of the study, the product’s effect on lightening the dark spots could be correlated with its ability to inhibit melanin production, as it was previously shown in a melanocyte cell model exposed to UVA radiation [16]. The mechanism by which this ingredient is capable of inhibiting melanin production could be due to its antioxidant activity or its effect on the α-Melanocyte stimulating hormone (α-MSH)-melanocortin 1 receptor (MC1R) signal pathway since it has been shown that some of the active compounds present in the ingredient have inhibitory effects on this pathway [43,44,45,46,47,48]. However, additional studies would be necessary to elucidate the mechanism.
As nutricosmetic products help improve the skin’s appearance, they can also improve the consumer’s self-perception. In this clinical trial, the observed functional and structural changes were substantiated by the self-assessment questionnaire, as the treatment product was highly rated by volunteers regarding its efficacy. Of note, at the end of the study, 89% of subjects in the treatment group perceived their skin as firmer, 86% more moisturized, 82% more elastic and denser, and 79% felt that the EY product improved the overall aspects of their skin, as it looked more rested, more homogenous, and with a more even tone (Figure 7). Additionally, 82% of the subjects taking EY highlighted that at the end of the study they would like to continue taking and purchasing the product (data not shown).
Nowadays, beauty consumers are looking for overall wellness products that combine cosmetic, health, and mood benefits. In this sense, dietary supplements such as EY could fulfill this requirement, as it was shown to help reduce stress and fatigue, as well as increase the sense of well-being (Figure 7).
The strengths of this study include the randomized, double-blind, placebo-controlled design; the even distribution of women across skin types, age, and grade of skin aging; and the relatively long duration of the assessment period. Additional strengths include the control of external variables (e.g., diet, dietary supplement use, sun or tanning bed exposure) that could confound the observed results, as well as the utilization of standardized facial cleanser and moisturizer throughout the study. Despite these strengths, some limitations to point out is that, for example, all the subjects in the study were only females with Caucasian skin. Nevertheless, we believe that our results could be extended to the general population since the main molecular, cellular, and tissue-specific events leading to aging and photoaging are shared among genders and races. In any case, it would be interesting to study whether this product would also produce the same benefits in other ethnic skin types or among men.
5. Conclusions
In conclusion, in women with moderate signs of skin aging, this study clearly demonstrated the efficacy of the eternalyoung® ingredient in reducing the effects of skin aging. After only 4 weeks, this blend comprised of four botanical extracts resulted in reduced wrinkle depth and skin roughness, improved skin elasticity (R2 and R5), firmness (R0), and reduced skin fatigue (R9), increased skin moisture, improved the skin barrier function by reducing the TEWL, increased the skin thickness, reduced dark spots pigmentation, and increased skin radiance over time.
It is worth noting that the positive results obtained with a simple daily intake of 225 mg were clinically demonstrated by several instrumental determinations and also corroborated by the highly positive self-assessment questionnaire performed by the subjects. Furthermore, no adverse reactions were reported by any of the subjects enrolled in the study.
Author Contributions
Conceptualization, N.C., V.N. and I.S.; methodology, V.N. and E.C.; software, V.N., L.G., P.N. and J.J; Formulation design, N.C.; validation, V.N., I.S. and E.C.; formal analysis, L.G.; investigation, E.C. and N.C.; data curation, V.N., L.G. and P.N.; writing—original draft preparation, N.C. and V.N.; writing—review and editing, N.C., J.J. and V.N; visualization, V.N. and L.G.; supervision, V.N. and I.S.; project administration, N.C.; funding acquisition, N.C. and J.J. All authors have read and agreed to the published version of the manuscript.
Funding
Monteloeder S.L. funded this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Both the study protocol and the informed consent form were approved (ref. no. 2021/13 by 15 November 2021) by the “Comitato Etico Indipendente per le Indagini Cliniche Non Farmacologiche”.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The protocol and the data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to express their gratitude to the Complife Italia staff, who contributed to the study and recruited the subjects, for their professionalism and support during the study’s development.
Conflicts of Interest
N.C., J.J. and P.N. belong to the Research and Development Department at Monteloeder S.L. This does not alter the authors’ adherence to all the journal’s policies on sharing data and materials. The remaining authors declare no conflict of interest.
References
- Rubin, D.C.; Berntsen, D. People over Forty Feel 20% Younger than Their Age: Subjective Age across the Lifespan. Psychon. Bull. Rev. 2006, 13, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Khavkin, J.; Ellis, D.A.F. Aging Skin: Histology, Physiology, and Pathology. Facial Plast. Surg. Clin. N. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-Derived Bioactive Compounds with Anti-Aging Potential for Nutricosmetic and Cosmeceutical Products. Crit. Rev. Food Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef] [PubMed]
- Liakou, A.I.; Theodorakis, M.J.; Melnik, B.C.; Pappas, A.; Zouboulis, C.C. Nutritional Clinical Studies in Dermatology. J. Drugs Dermatol. 2013, 12, 1104–1109. [Google Scholar]
- Pappas, A.; Liakou, A.; Zouboulis, C.C. Nutrition and Skin. Rev. Endocr. Metab. Disord. 2016, 17, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Lucius, K. Botanical Medicine and Phytochemicals in Healthy Aging and Longevity—Part 1. Altern. Complement. Ther. 2020, 26, 31–37. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Jiang, J.-G.; Yang, L.; Wang, D.-W.; Zhu, W. Anti-Ageing Active Ingredients from Herbs and Nutraceuticals Used in Traditional Chinese Medicine: Pharmacological Mechanisms and Implications for Drug Discovery. Br. J. Pharm. 2017, 174, 1395–1425. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cha, H.J.; Nam, K.H.; Yoon, Y.; Lee, H.; An, S. Centella Asiatica Extracts Modulate Hydrogen Peroxide-Induced Senescence in Human Dermal Fibroblasts. Exp. Dermatol. 2011, 20, 998–1003. [Google Scholar] [CrossRef]
- Man, M.-Q.; Yang, B.; Elias, P.M. Benefits of Hesperidin for Cutaneous Functions. Evid. Based Complement. Altern. Med. 2019, 2019, 2676307. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Noratto, G.; Hingorani, L.; Talcott, S.T.; Mertens-Talcott, S.U. Protective Effects of Standardized Pomegranate (Punica Granatum L.) Polyphenolic Extract in Ultraviolet-Irradiated Human Skin Fibroblasts. J. Agric. Food Chem. 2008, 56, 8434–8441. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella Asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ji, S.; Zhang, H.; Mei, S.; Qiao, L.; Jin, X. Herba Cistanches: Anti-Aging. Aging Dis. 2017, 8, 740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Weng, X.; Chen, L.; Chin, L. Effect of Cistanche Tubulosa (Scheuk) Whight Acteoside on Telomerase Activity and Immunity of Aging Mice. Chin. J. Pharmacol. Toxicol. 2008, 22, 270–273. [Google Scholar] [CrossRef]
- Aslam, M.N.; Lansky, E.P.; Varani, J. Pomegranate as a Cosmeceutical Source: Pomegranate Fractions Promote Proliferation and Procollagen Synthesis and Inhibit Matrix Metalloproteinase-1 Production in Human Skin Cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef]
- Quiles, J.; Cabrera, M.; Jones, J.; Tsapekos, M.; Caturla, N. In Vitro Determination of the Skin Anti-Aging Potential of Four-Component Plant-Based Ingredient. Molecules 2022, 27, 8101. [Google Scholar] [CrossRef]
- Constantin, M.-M.; Poenaru, E.; Poenaru, C.; Constantin, T. Skin Hydration Assessment through Modern Non-Invasive Bioengineering Technologies. Maedica 2014, 9, 33–38. [Google Scholar]
- Nutrition Business Journal (NBC). Condition Specific Report© 2023; market study; Informa PLC: London, UK, 2022. [Google Scholar]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin Senescence: Mechanisms and Impact on Whole-Body Aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of Cellular Senescence. Telomere Shortening as a Marker of Cellular Senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Pham, Q.D.; Topgaard, D.; Sparr, E. Skin Hydration: Interplay between Molecular Dynamics, Structure and Water Uptake in the Stratum Corneum. Sci. Rep. 2017, 7, 15712. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, M.; Gabriel, S.; Lichterfeld-Kottner, A.; Blume-Peytavi, U.; Kottner, J. Transepidermal Water Loss in Healthy Adults: A Systematic Review and Meta-Analysis Update. Br. J. Dermatol. 2018, 179, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Man, M.-Q.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-Associated Alterations in Epidermal Function and Their Clinical Significance. Aging 2020, 12, 5551–5565. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J. Oxidative Targets in the Stratum Corneum. A New Basis for Antioxidative Strategies. Skin Pharmacol. Appl. Skin Physiol. 2001, 14 (Suppl. 1), 87–91. [Google Scholar] [CrossRef] [PubMed]
- Lorzadeh, E.; Heidary, Z.; Mohammadi, M.; Nadjarzadeh, A.; Ramezani-Jolfaie, N.; Salehi-Abargouei, A. Does Pomegranate Consumption Improve Oxidative Stress? A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Clin. Nutr. ESPEN 2022, 47, 117–127. [Google Scholar] [CrossRef]
- Lee, H.J.; Im, A.-R.; Kim, S.-M.; Kang, H.-S.; Lee, J.D.; Chae, S. The Flavonoid Hesperidin Exerts Anti-Photoaging Effect by Downregulating Matrix Metalloproteinase (MMP)-9 Expression via Mitogen Activated Protein Kinase (MAPK)-Dependent Signaling Pathways. BMC Complement. Altern. Med. 2018, 18, 39. [Google Scholar] [CrossRef]
- Wijayadi, L.Y.; Darmawan, H. Asiaticoside Increases Aquaporin-3 Protein Expression in the Cytoplasm of Normal Human Epidermal Keratinocytes. Universa Med. 2017, 36, 25–33. [Google Scholar] [CrossRef]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the Epidermis: More than Skin Deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef]
- Hou, M.; Man, M.; Man, W.; Zhu, W.; Hupe, M.; Park, K.; Crumrine, D.; Elias, P.M.; Man, M.-Q. Topical Hesperidin Improves Epidermal Permeability Barrier Function and Epidermal Differentiation in Normal Murine Skin. Exp. Dermatol. 2012, 21, 337–340. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Kezic, S. Causes of Epidermal Filaggrin Reduction and Their Role in the Pathogenesis of Atopic Dermatitis. J. Allergy Clin. Immunol. 2014, 134, 792–799. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.B.; Sherratt, M.J. Molecular Aspects of Skin Ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of Age and Regional Differences on Skin Elasticity as Measured by the Cutometer®. Skin Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef]
- Koch, R.J.; Cheng, E.T. Quantification of Skin Elasticity Changes Associated with Pulsed Carbon Dioxide Laser Skin Resurfacing. Arch. Facial Plast. Surg. 1999, 1, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, H. In Vivo Study of Skin Mechanical Properties in Raynaud’s Phenomenon. Skin Res. Technol. 2007, 13, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The Senescence-Associated Secretory Phenotype (SASP) in the Challenging Future of Cancer Therapy and Age-Related Diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M. Advanced Glycation End Products in Skin Ageing and Photoageing: What Are the Implications for Epidermal Function? Exp. Dermatol. 2016, 25, 947–948. [Google Scholar] [CrossRef]
- Guillon, C.; Ferraro, S.; Clément, S.; Bouschbacher, M.; Sigaudo-Roussel, D.; Bonod, C. Glycation by Glyoxal Leads to Profound Changes in the Behavior of Dermal Fibroblasts. BMJ Open Diabetes Res. Care 2021, 9, e002091. [Google Scholar] [CrossRef] [PubMed]
- Nema, N.K.; Maity, N.; Sarkar, B.K.; Mukherjee, P.K. Matrix Metalloproteinase, Hyaluronidase and Elastase Inhibitory Potential of Standardized Extract of Centella asiatica. Pharm. Biol. 2013, 51, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Brzezińska, M. Centella Asiatica in Cosmetology. Postepy Dermatol. Alergol. 2013, 30, 46–49. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, S.; Hwang, E.; Yi, T.-H.; Wang, Y.-S. Effects of Phenylethanol Glycosides from Orobanche Cernua Loefling on UVB-Induced Skin Photodamage: A Comparative Study. Photochem. Photobiol. Sci. 2021, 20, 599–614. [Google Scholar] [CrossRef]
- Si, N.; Kanazawa, H.; Okuyama, K.; Imada, K.; Wang, H.; Yang, J.; Zhao, H.; Bian, B.; Ito, A.; Sato, T. Involvement of Catechols in Acteoside in the Activation of Promatrix Metalloproteinase-2 and Membrane Type-1-Matrix Metalloproteinase Expression via a Phosphatidylinositol-3-Kinase Pathway in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2018, 41, 1530–1536. [Google Scholar] [CrossRef]
- Kwon, K.J.; Bae, S.; Kim, K.; An, I.S.; Ahn, K.J.; An, S.; Cha, H.J. Asiaticoside, a Component of Centella Asiatica, Inhibits Melanogenesis in B16F10 Mouse Melanoma. Mol. Med. Rep. 2014, 10, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Y.; Xu, K.; Wang, X.; Wen, Y.-T.; Wang, L.-J.; Huang, D.-Q.; Chen, X.-X.; Chai, W.-M. Punicalagin as a Novel Tyrosinase and Melanin Inhibitor: Inhibitory Activity and Mechanism. LWT 2022, 161, 113318. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, W.J.; Chang, S.E.; Lee, G.-Y. Hesperidin, A Popular Antioxidant Inhibits Melanogenesis via Erk1/2 Mediated MITF Degradation. Int. J. Mol. Sci. 2015, 16, 18384–18395. [Google Scholar] [CrossRef]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Dal Toso, R.; Baldisserotto, A.; Manfredini, S. Activity and Stability Studies of Verbascoside, a Novel Antioxidant, in Dermo-Cosmetic and Pharmaceutical Topical Formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef]
- Yang, W.T.; Kim, K.S.; Kwon, Y.S.; Kim, D.H.; Kim, D.H. Whitening and anti-aging effects of Cistanche deserticola extract. J. Plant Biotechnol. 2016, 43, 492–499. [Google Scholar] [CrossRef]
- Son, Y.-O.; Lee, S.-A.; Kim, S.-S.; Jang, Y.-S.; Chun, J.-C.; Lee, J.-C. Acteoside Inhibits Melanogenesis in B16F10 Cells through ERK Activation and Tyrosinase Down-Regulation. J. Pharm. Pharmacol. 2011, 63, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).