Calligonum polygonoides L. Shrubs Provide Species-Specific Facilitation for the Understory Plants in Coastal Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Study Area
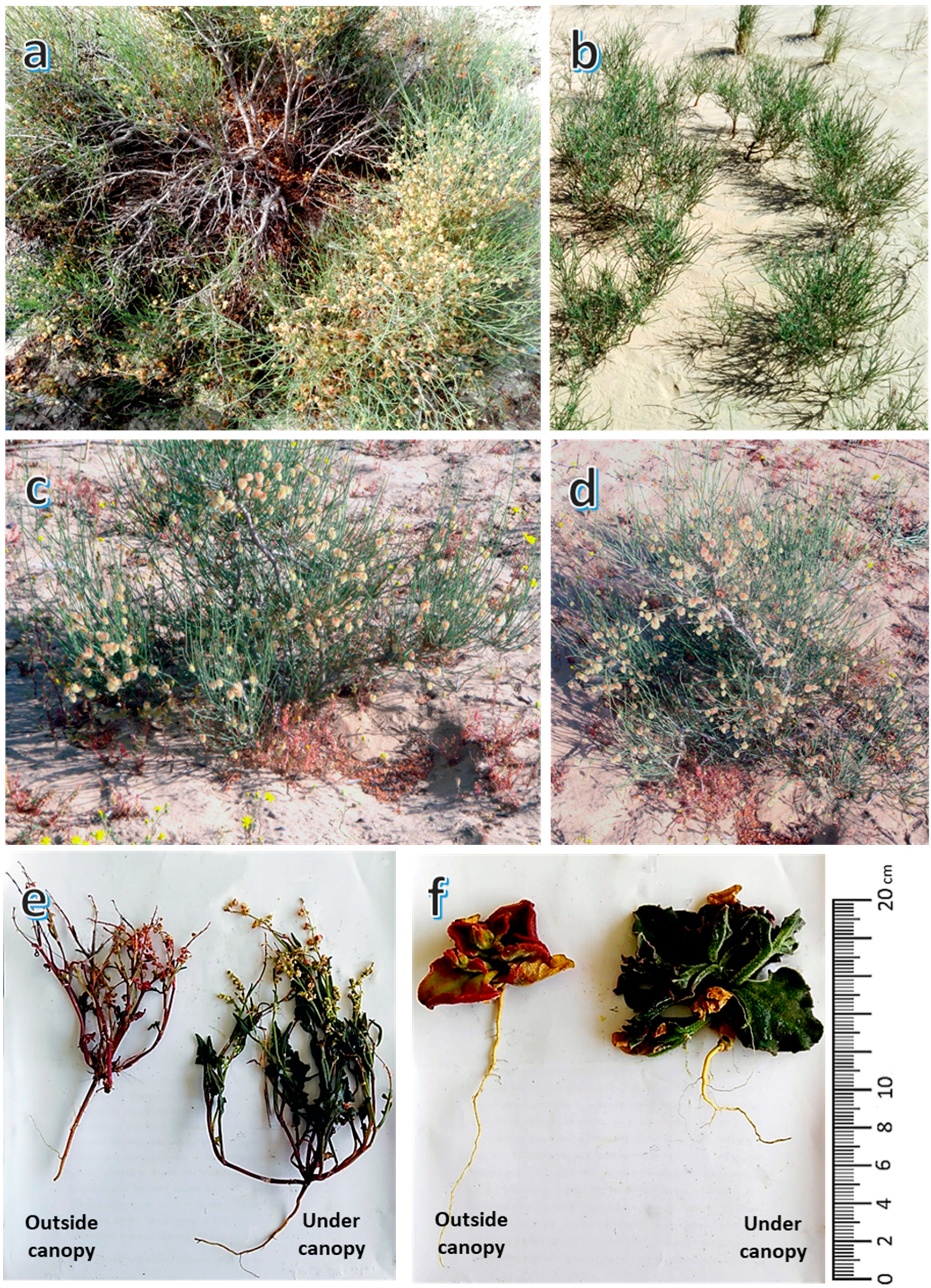
2.2. Study Species
2.3. Field Sampling and Vegetation Survey
2.4. Environmental Measurements and Soil Analysis
2.5. Plant Analyses
2.5.1. Determination of Dry Weight
2.5.2. Estimation of Photosynthetic Pigments
2.5.3. Estimation of Total Anthocyanin Content
2.5.4. Estimation of Proline Content
2.5.5. Assays of Antioxidant Enzymes
2.6. Mycorrhizal Biodiversity Analysis
2.7. Chemical Composition Analysis of C. polygonoides Roots
2.8. Data Analysis
3. Results
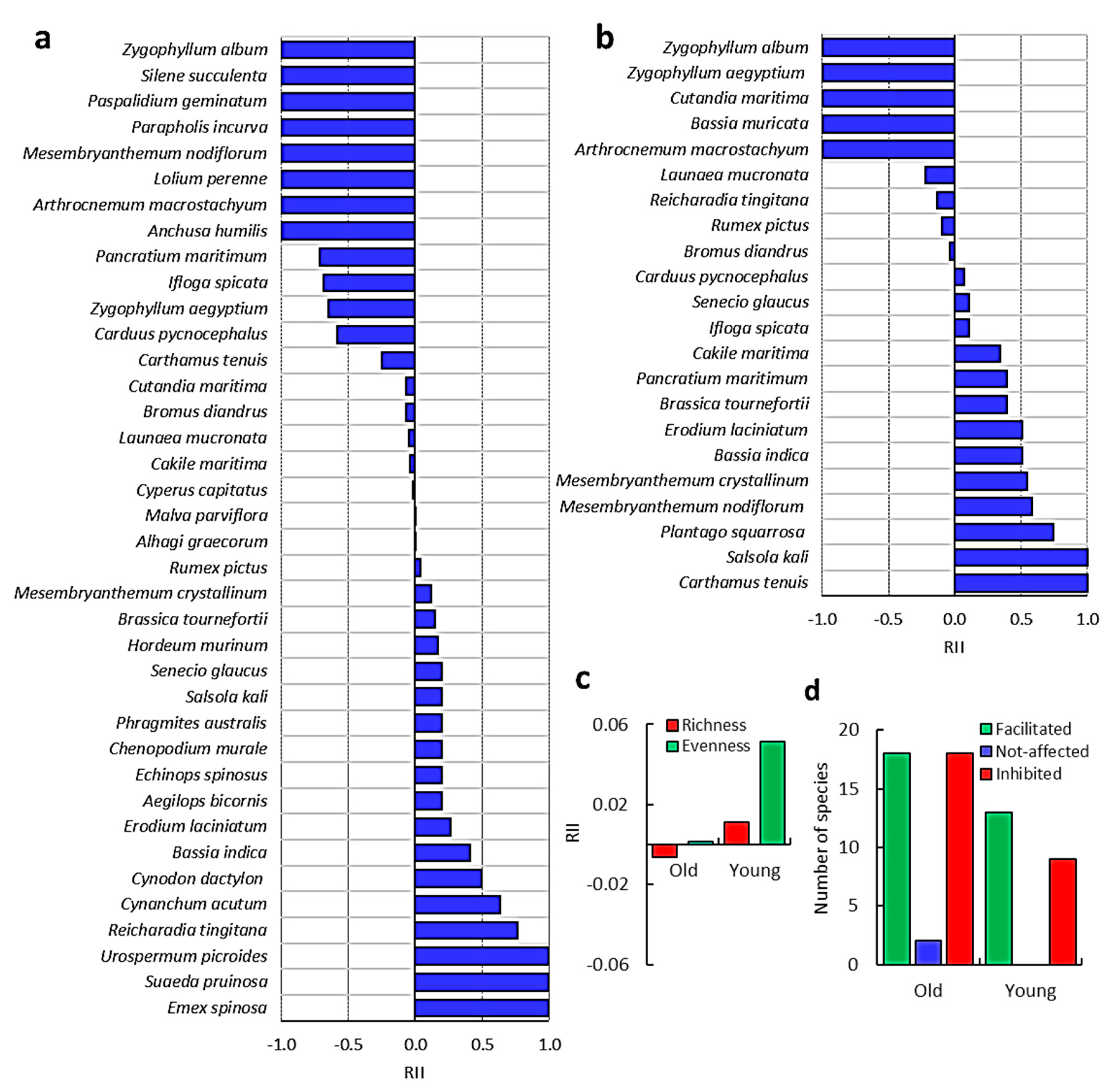
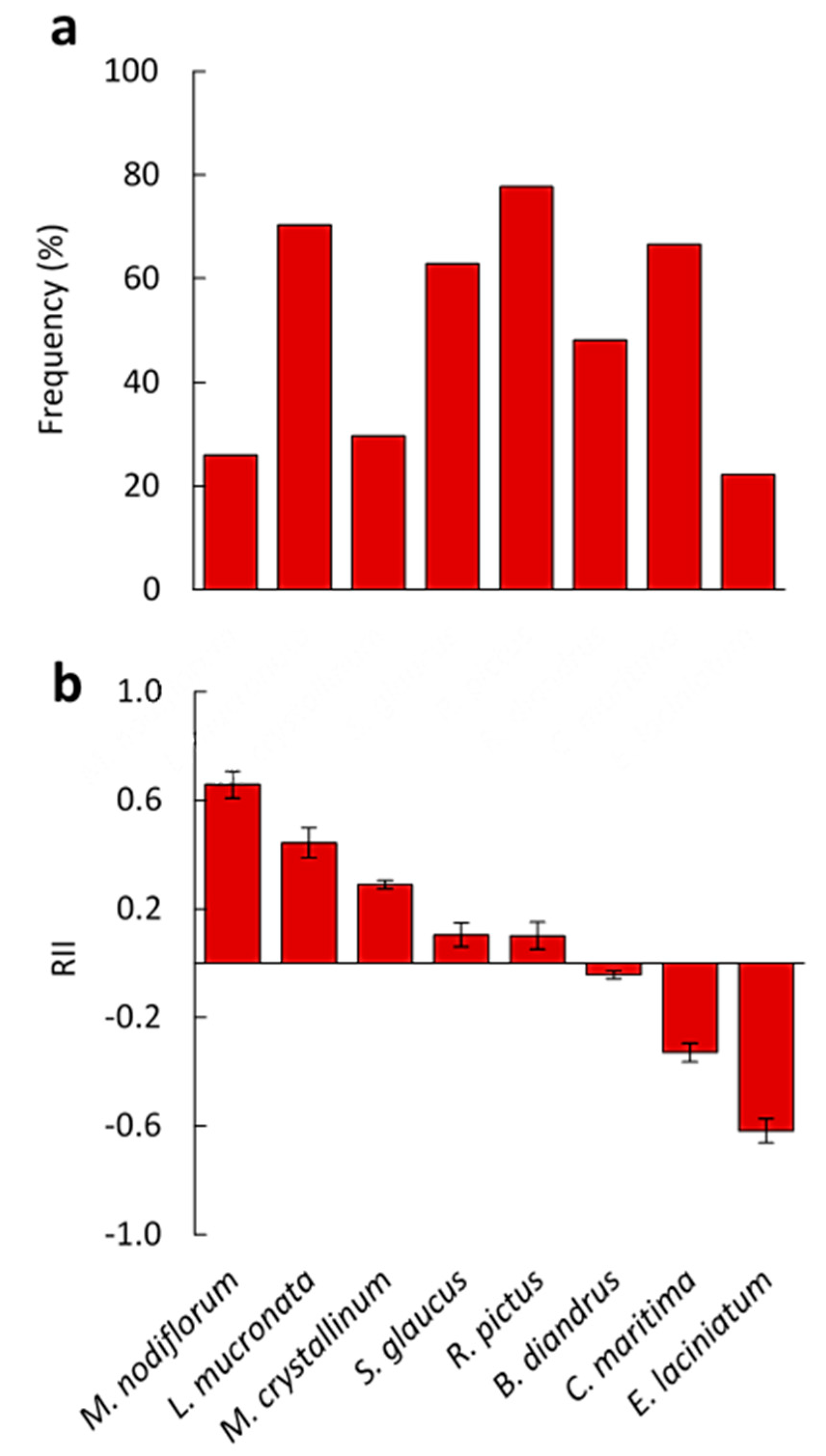
3.1. Vegetation Analysis
3.2. Effect of C. polygonoides Shrub on the Environmental Conditions of the Microhabitat
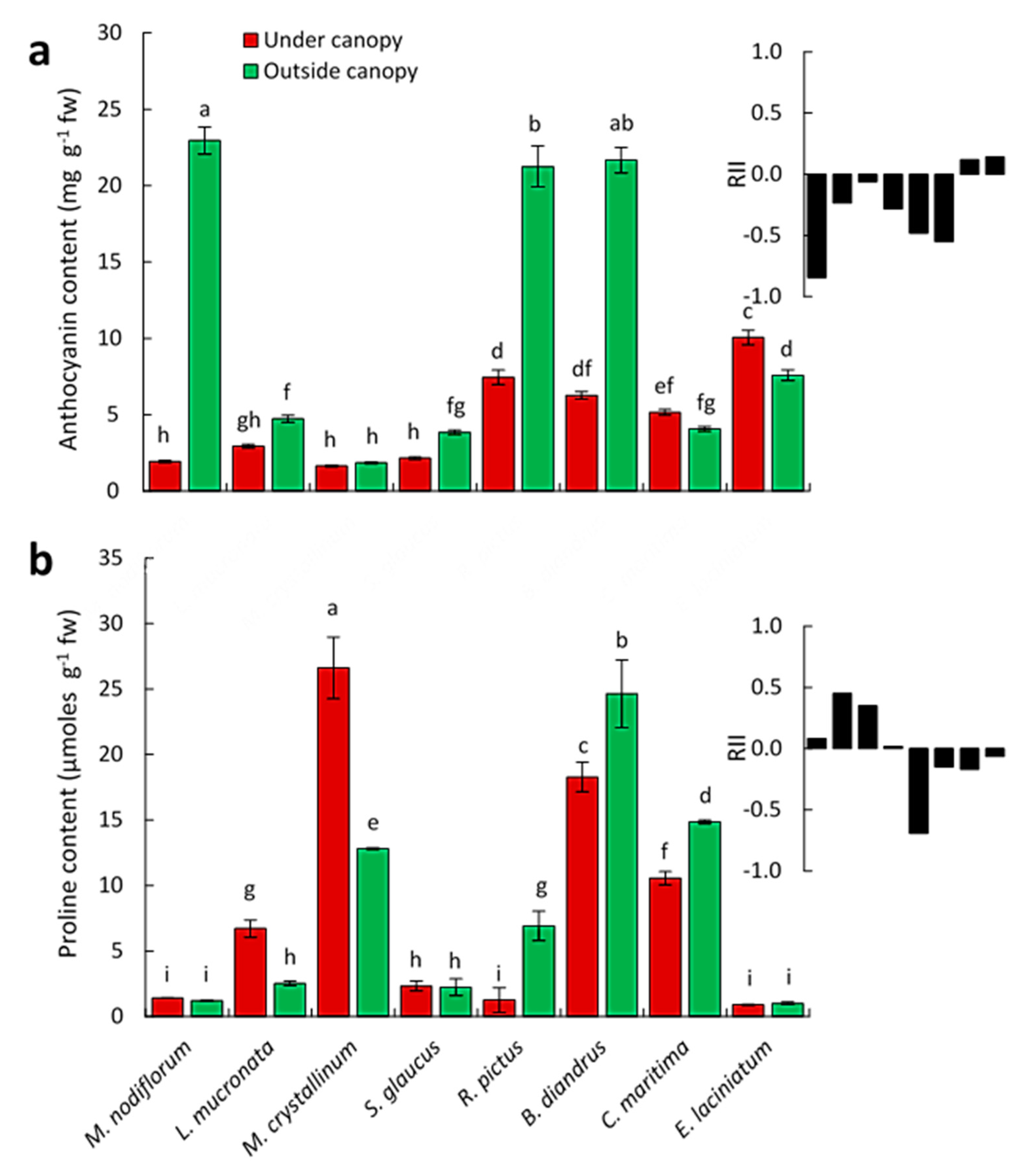
3.3. Effects on the Physiological Responses of Understory Plant Species
3.4. Mycorrhizal Fungi Associated with C. polygonoides Roots
3.5. Chemical Composition of C. polygonoides Root Extract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNEP. World Atlas of Desertification, 2nd ed.; United Nations Environment Programme: Nairobi, Kenya; Arnold: London, UK, 1997. [Google Scholar]
- Callaway, R.M. Positive Interactions and Interdependence in Plant Communities; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Li, M.; Du, Z.; Pan, H.; Yan, C.; Xiao, W.; Lei, J. Effects of neighboring woody plants on target trees with emphasis on effects of understorey shrubs on overstorey physiology in forest communities: A mini-review. Community Ecol. 2012, 13, 117–128. [Google Scholar] [CrossRef]
- Callaway, R.M. Positive interactions among plants. Bot. Rev. 1995, 61, 306–349. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, H. Direct plant–plant facilitation in coastal wetlands: A review. Estuar. Coast. Shelf Sci. 2013, 119, 1–6. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.; Anthelme, F. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Michalet, R.; Pugnaire, F.I. Facilitation in communities: Underlying mechanisms, community and ecosystem implications. Funct. Ecol. 2016, 30, 3–9. [Google Scholar] [CrossRef]
- Yang, L.; Liu, N.; Ren, H.; Wang, J. Facilitation by two exotic Acacia: Acacia auriculiformis and Acacia mangium as nurse plants in South China. For. Ecol. Manag. 2009, 257, 1786–1793. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.; El-Amier, Y. Allelopathy and potential impact of invasive Acacia saligna on plant diversity in Deltaic Mediterranean coast of Egypt. Int. J. Environ. Res. 2015, 9, 923–932. [Google Scholar]
- Ahmed, H.; Moawad, A.; Owis, A.; AbouZid, S.; Ahmed, O. Flavonoids of Calligonum polygonoides and their cytotoxicity. Pharm. Biol. 2016, 54, 2119–2126. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, C.-M.; Rhee, J.H.; Kim, Y.R. Effects of pyrogallol on growth and cytotoxicity of wild-type and katG mutant strains of Vibrio vulnificus. PLoS ONE 2016, 11, e0167699. [Google Scholar] [CrossRef]
- Koziol, L.; Crews, T.E.; Bever, J.D. Benefits of native mycorrhizal amendments to perennial agroecosystems increases with field inoculation density. Agronomy 2019, 9, 353. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi–from ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Mao, Z.; Corrales, A.; Zhu, K.; Yuan, Z.; Lin, F.; Ye, J.; Hao, Z.; Wang, X. Tree mycorrhizal associations mediate soil fertility effects on forest community structure in a temperate forest. New Phytol. 2019, 223, 475–486. [Google Scholar] [CrossRef]
- Jakobsen, I.; Hammer, E.C. Nutrient dynamics in arbuscular mycorrhizal networks. In Mycorrhizal Networks; Springer: Dordrecht, The Netherlands, 2015; pp. 91–131. [Google Scholar]
- Merrild, M.P.; Ambus, P.; Rosendahl, S.; Jakobsen, I. Common arbuscular mycorrhizal networks amplify competition for phosphorus between seedlings and established plants. New Phytol. 2013, 200, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; Mohammad, M.G.; El-Keblawy, A.A.; Omar, H.; Abouleish, M.; Madkour, M.; Elnaggar, A.; Hosni, R.M. Mechanical and phytochemical protection mechanisms of Calligonum comosum in arid deserts. PLoS ONE 2018, 13, e0192576. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Singh, J.; Bhardwaj, S.; Nathawat, N.; Kumar, M.; Roy, M. Potential of native shrubs Haloxylon salicornicum and Calligonum polygonoides for restoration of degraded lands in arid western Rajasthan, India. Environ. Manag. 2015, 55, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Mashizi, A.K.; Sharafatmandrad, M. Assessing the effects of shrubs on ecosystem functions in arid sand dune ecosystems. Arid Land Res. Manag. 2019, 33, 1–17. [Google Scholar]
- Abd El-Gawad, A.M.; Shehata, H.S. Ecology and development of Mesembryanthemum crystallinum L. in the Deltaic Mediterranean coast of Egypt. Egypt. J. Basic Appl. Sci. 2014, 1, 29–37. [Google Scholar] [CrossRef]
- Shaltout, M.; El Gindy, A.; Omstedt, A. Recent climate trends and future scenarios along the Egyptian Mediterranean coast. Geofizika 2013, 30, 19–41. [Google Scholar]
- Danin, A. Plants of Desert Dunes; Springer: Berlin, Germany, 2012. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 2005; Volume 1–4. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plant and Statistical Plant Geography; Clarendon Press: Oxford, UK, 1937. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentic Hall Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Myers, J.; Kratz, W. Relations between pigment content and photosynthetic characteristics in a blue-green alga. J. Gen. Physiol. 1955, 39, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Rahemi, M.; Kholdebarin, B.; Rastegar, S. Biochemical responses in leaves of four fig cultivars subjected to water stress and recovery. Sci. Hortic. 2012, 148, 109–117. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ahmad, P.; Kumar, A.; Ashraf, M.; Akram, N.A. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 2012, 11, 2694–2703. [Google Scholar]
- El-Mashad, A.A.A.; Mohamed, H.I. Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 2012, 249, 625–635. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Furlan, V.; Bartschi, H.; Fortin, J.-A. Media for density gradient extraction of endomycorrhizal spores. Trans. Br. Mycol. Soc. 1980, 75, 336–338. [Google Scholar] [CrossRef]
- Schenck, N.C.; Perez, Y. Manual for The Identification of VA Mycorrhizal Fungi; Synergistic Publications: Gainesville, FL, USA, 1990; Volume 286. [Google Scholar]
- Phillips, J.M.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthode d’estimation ayant une signification fonctionnelle. In Proceedings of the Physiological and Genetical Aspects of Mycorrhizae: Proceedings of the 1st European Symposium on Mycorrhizae, Dijon, France, 1–5 July 1985; pp. 217–221. [Google Scholar]
- Armas, C.; Ordiales, R.; Pugnaire, F.I. Measuring plant interactions: A new comparative index. Ecology 2004, 85, 2682–2686. [Google Scholar] [CrossRef]
- Callaway, R.M.; Walker, L.R. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Reisman-Berman, O. Age-related change in canopy traits shifts conspecific facilitation to interference in a semi-arid shrubland. Ecography 2007, 30, 459–470. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, L.; Yang, Q.; Zhao, W.; Wang, X. Effect of desert shrubs on fine-scale spatial patterns of understory vegetation in a dry-land. Plant Ecol. 2016, 217, 1141–1155. [Google Scholar] [CrossRef]
- Derbel, S.; Chaieb, M. Germination behaviour and seedling establishment of two desert shrubs, Calligonum polygonoides (Polygonaceae) and Spartidium saharae (Fabaceae), under experimental conditions. Acta Bot. Gallica 2007, 154, 533–544. [Google Scholar] [CrossRef]
- Valladares, F.; Pearcy, R. Drought can be more critical in the shade than in the sun: A field study of carbon gain and photo-inhibition in a Californian shrub during a dry El Niño year. Plant Cell Environ. 2002, 25, 749–759. [Google Scholar] [CrossRef]
- Wright, A.J.; Wardle, D.A.; Callaway, R.; Gaxiola, A. The overlooked role of facilitation in biodiversity experiments. Trends Ecol. Evol. 2017, 32, 383–390. [Google Scholar] [CrossRef]
- Schramm, J.W.; Ehrenfeld, J.G. Leaf litter and understory canopy shade limit the establishment, growth and reproduction of Microstegium vimineum. Biol. Invasions 2010, 12, 3195–3204. [Google Scholar] [CrossRef]
- Madrigal-González, J.; Cea, A.P.; Sánchez-Fernández, L.A.; Martínez-Tillería, K.P.; Calderón, J.E.; Gutiérrez, J.R. Facilitation of the non-native annual plant Mesembryanthemum crystallinum (Aizoaceae) by the endemic cactus Eulychnia acida (Cactaceae) in the Atacama Desert. Biol. Invasions 2013, 15, 1439–1447. [Google Scholar] [CrossRef]
- Armas, C.; Kim, J.H.; Bleby, T.M.; Jackson, R.B. The effect of hydraulic lift on organic matter decomposition, soil nitrogen cycling, and nitrogen acquisition by a grass species. Oecologia 2012, 168, 11–22. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Pugnaire, F.I.; Armas, C.; Rodríguez-Echeverría, S.; Schöb, C. The shift from plant–plant facilitation to competition under severe water deficit is spatially explicit. Ecol. Evol. 2017, 7, 2441–2448. [Google Scholar] [CrossRef]
- Kidron, G.J.; Gutschick, V.P. Soil moisture correlates with shrub–grass association in the Chihuahuan Desert. Catena 2013, 107, 71–79. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, W. Species-specific traits determine shrub-annual interactions during a growing season. J. Arid Land 2015, 7, 403–413. [Google Scholar] [CrossRef]
- Armas, C.; Pugnaire, F.I. Plant interactions govern population dynamics in a semi-arid plant community. J. Ecol. 2005, 93, 978–989. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Bertness, M.D.; Yeh, S.M. Cooperative and competitive interactions in the recruitment of marsh elders. Ecology 1994, 75, 2416–2429. [Google Scholar] [CrossRef]
- Pugnaire, F.; Haase, P.; Puigdefábregas, J.; Cueto, M.; Clark, S.; Incoll, L. Facilitation and succession under the canopy of a leguminous shrub, Retama sphaerocarpa, in a semi-arid environment in south-east Spain. Oikos 1996, 76, 455–464. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Jia, Z.; Zhang, L.; Zhang, Y.; Feng, L.; He, L.; Yang, K.; Dai, J.; Chen, J. Changes in soil organic carbon and total nitrogen stocks along a chronosequence of Caragana intermedia plantations in alpine sandy land. Ecol. Eng. 2019, 133, 53–59. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Abd El-Gawad, A.M.; Cesarano, G.; Sarker, T.C.; Saulino, L.; Lanzotti, V.; Saracino, A.; Rego, F.C.; Mazzoleni, S. Comparing chemistry and bioactivity of burned vs. decomposed plant litter: Different pathways but same result? Ecology 2018, 99, 158–171. [Google Scholar] [CrossRef]
- Cockburn, W.; Whitelam, G.; Broad, A.; Smith, J. The participation of phytochrome in the signal transduction pathway of salt stress responses in Mesembryanthemum crystallinum L. J. Exp. Bot. 1996, 47, 647–653. [Google Scholar] [CrossRef][Green Version]
- Mancinelli, A.L. Light-dependent anthocyanin synthesis: A model system for the study of plant photomorphogenesis. Bot. Rev. 1985, 51, 107–157. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Robberecht, R.; Flint, S.D. Internal filters: Prospects for UV-acclimation in higher plants. Physiol. Plant. 1983, 58, 445–450. [Google Scholar] [CrossRef]
- Lenz, T.I.; Facelli, J.M. Shade facilitates an invasive stem succulent in a chenopod shrubland in South Australia. Austral Ecol. 2003, 28, 480–490. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.J.; Zhang, F.J.; Zhang, G.Z.; Jiang, X.Y.; Yu, H.M.; Hou, B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Demiral, T.; Türkan, I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- El-Shora, H.M.; Abd El-Gawad, A.M. Physiological and biochemical responses of Cucurbita pepo L. mediated by Portulaca oleracea L. allelopathy. Fresenius Environ. Bull. 2015, 24, 386–393. [Google Scholar]
- El-Sharkawy, H.H.; Rashad, Y.M.; Ibrahim, S.A. Biocontrol of stem rust disease of wheat using arbuscular mycorrhizal fungi and Trichoderma spp. Physiol. Mol. Plant Pathol. 2018, 103, 84–91. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against tomato mosaic virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Wagg, C.; Veiga, R.; van der Heijden, M.G. Facilitation and antagonism in mycorrhizal networks. In Mycorrhizal Networks; Springer: Dordrecht, The Netherlands, 2015; Volume 224, pp. 203–226. [Google Scholar]
- Jing, G.; Huang, H.; Yang, B.; Li, J.; Zheng, X.; Jiang, Y. Effect of pyrogallol on the physiology and biochemistry of litchi fruit during storage. Chem. Cent. J. 2013, 7, 19. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Echeverría, S.; Lozano, Y.M.; Bardgett, R.D. Influence of soil microbiota in nurse plant systems. Funct. Ecol. 2016, 30, 30–40. [Google Scholar] [CrossRef]
- Hortal, S.; Bastida, F.; Armas, C.; Lozano, Y.; Moreno, J.L.; García, C.; Pugnaire, F.I. Soil microbial community under a nurse-plant species changes in composition, biomass and activity as the nurse grows. Soil Biol. Biochem. 2013, 64, 139–146. [Google Scholar] [CrossRef]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef]
- Teoh, Y.P.; Don, M.M.; Ujang, S. Media selection for mycelia growth, antifungal activity against wood-degrading fungi, and GC-MS study by Pycnoporus sanguineus. BioResources 2011, 6, 2719–2731. [Google Scholar]
- Haichar, E.Z.F.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Yadav, B.K.; Akhtar, M.S.; Panwar, J. Rhizospheric plant-microbe interactions: Key factors to soil fertility and plant nutrition. In Plant Microbes Symbiosis: Applied Facets; Arora, N.K., Ed.; Springer: New Delhi, India, 2015; pp. 127–145. [Google Scholar]
- Li, C.; Zhang, B.; Ertunc, T.; Schaeffer, A.; Ji, R. Birnessite-induced binding of phenolic monomers to soil humic substances and nature of the bound residues. Environ. Sci. Technol. 2012, 46, 8843–8850. [Google Scholar] [CrossRef]
- Min, K.; Freeman, C.; Kang, H.; Choi, S.-U. The regulation by phenolic compounds of soil organic matter dynamics under a changing environment. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
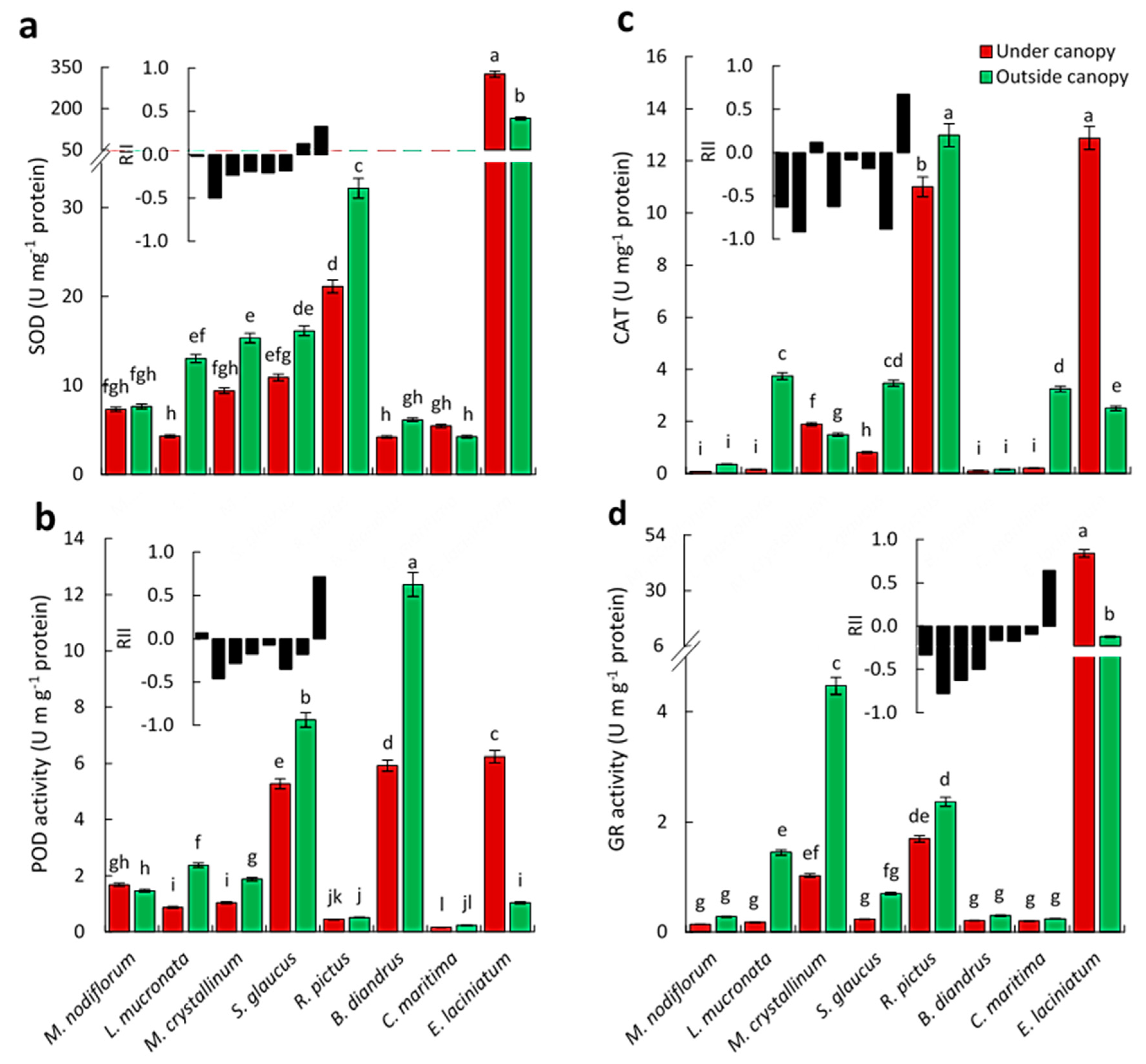
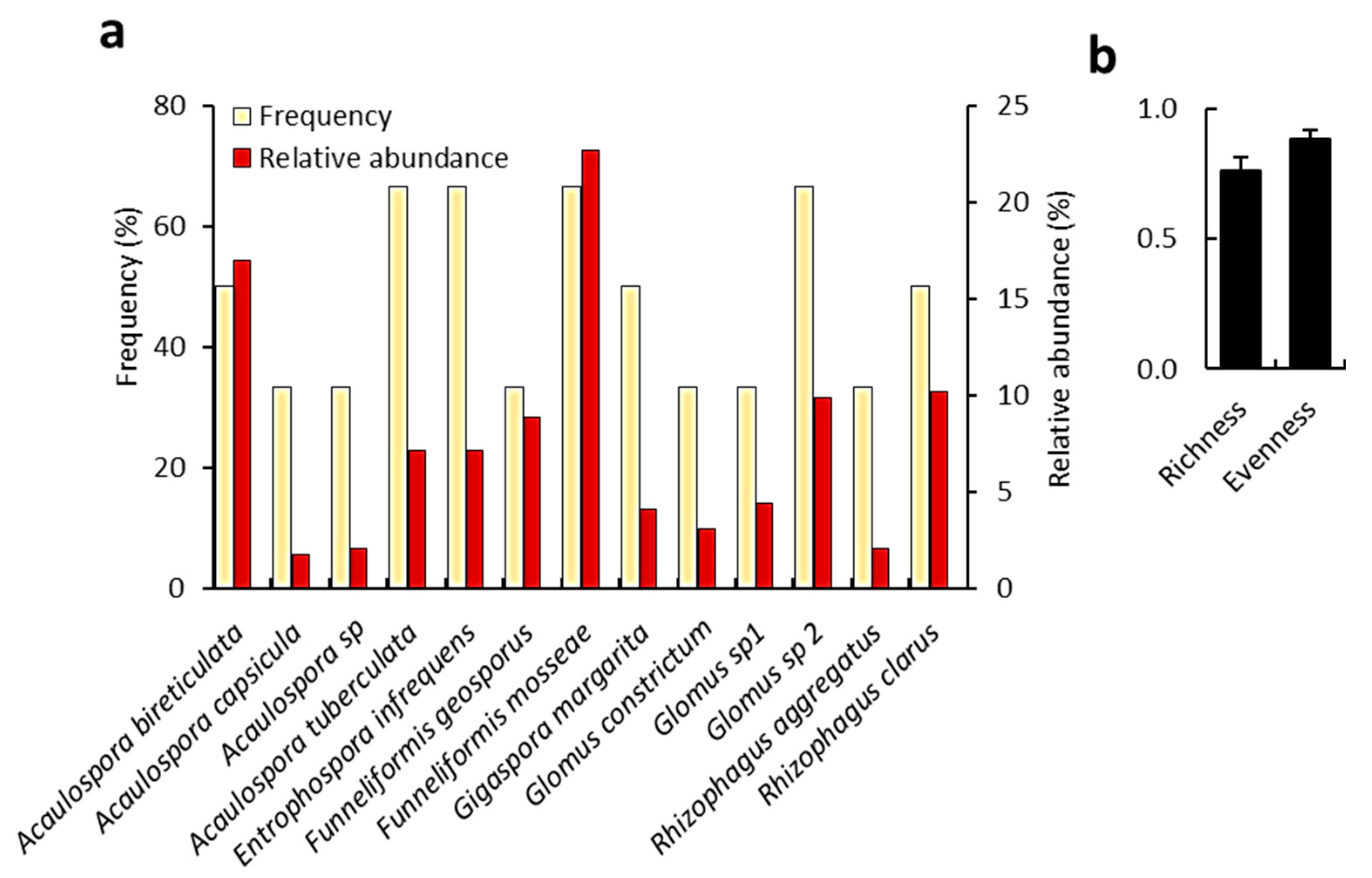
| Parameter | Outside Canopy | Under Canopy | F-Value | |
|---|---|---|---|---|
| Old | Young | |||
| PPFD (μmol m−1 s−1) | 1353.85 ± 58.79 a | 66.20 ± 7.65 b | 147.90 ± 17.13 b | 174.68 *** |
| Temp. (°C) | 29.87 ± 0.70 a | 26.72 ± 0.28 b | 27.53 ± 0.60 b | 5.72 * |
| Moisture content (%) | 0.21 ± 0.01 c | 1.28 ± 0.05 a | 0.39 ± 0.06 b | 1726.17 *** |
| Organic carbon (g kg−1) | 1.59 ± 0.15 b | 2.67 ± 0.34 a | 2.35 ± 0.40 a | 6.26 ** |
| Total nitrogen (g kg−1) | 8.75 ± 0.18 b | 9.52 ± 0.28 a | 9.88 ± 0.46 a | 5.95 ** |
| pH | 8.52 ± 0.12 a | 8.53 ± 0.10 a | 8.36 ± 0.14 a | 0.91 ns |
| EC (mS cm−1) | 0.21 ± 0.01 a | 0.11 ± 0.02 b | 0.16 ± 0.03 a | 7.30 ** |
| Plant Species | Water Content (%) | Chl a (µg g−1 fw) | Chl b (µg g−1 fw) | Total Chls (µg g−1 fw) | Chl a/Chl b | Carotenoids (µg g−1 fw) | |
|---|---|---|---|---|---|---|---|
| Mesembryanthemum nodiflorum | Under | 5.55 ± 1.70 E | 84.53 ± 2.57 G | 102.88 ± 0.80 C | 187.41 ± 3.20 CD | 0.82 ± 0.02 I | 10.16 ± 0.08 D |
| Outside | 3.62 ± 0.37 F | 70.72 ± 0.55 I | 111.01 ± 0.87 A | 181.73 ± 1.42 E | 0.64 ± 0.00 K | 12.38 ± 0.10 B | |
| RII | 0.21 | 0.09 | −0.04 | 0.02 | 0.13 | −0.10 | |
| Launaea mucronata | Under | 5.44 ± 0.18 E | 48.71 ± 1.48 J | 15.92 ± 0.12 N | 64.64 ± 1.57 I | 3.06 ± 0.08 B | 3.18 ± 0.02 L |
| Outside | 6.27 ± 0.08 D | 21.72 ± 0.17 L | 12.95 ± 0.10 O | 34.67 ± 0.27 L | 1.68 ± 0.00 H | 2.66 ± 0.02 N | |
| RII | −0.07 | 0.38 | 0.10 | 0.30 | 0.29 | 0.09 | |
| M. crystallinum | Under | 12.25 ± 0.07 A | 79.77 ± 2.42 H | 109.85 ± 0.86 B | 189.62 ± 3.11 C | 0.73 ± 0.02 J | 7.46 ± 0.06 G |
| Outside | 8.10 ± 0.07 B | 44.60 ± 0.35 J | 77.85 ± 0.61 E | 122.45 ± 0.95 H | 0.57 ± 0.00 L | 5.29 ± 0.04 K | |
| RII | 0.20 | 0.28 | 0.17 | 0.22 | 0.12 | 0.17 | |
| Senecio glaucus | Under | 2.70 ± 0.67 G | 36.96 ± 1.12 K | 18.30 ± 0.14 M | 55.26 ± 1.23 J | 2.02 ± 0.05 G | 2.91 ± 0.02 M |
| Outside | 2.85 ± 0.51 G | 33.82 ± 0.026 K | 15.77 ± 0.12 N | 49.58 ± 0.39 K | 2.14 ± 0.00 F | 3.09 ± 0.02 L | |
| RII | −0.03 | 0.04 | 0.07 | 0.05 | −0.03 | −0.03 | |
| Rumex pictus | Under | 7.46 ± 0.58 C | 86.82 ± 2.64 G | 38.32 ± 0.30 K | 125.14 ± 2.86 H | 2.27 ± 0.06 E | 6.82 ± 0.05 I |
| Outside | 2.40 ± 0.48 H | 16.41 ± 0.13 M | 21.02 ± 0.16 L | 37.43 ± 0.29 I | 0.78 ± 0.00 IJ | 2.90 ± 0.02 M | |
| RII | 0.51 | 0.68 | 0.29 | 0.54 | 0.49 | 0.40 | |
| Bromus diandrus | Under | 2.50 ± 0.02 H | 242.63 ± 7.37 A | 87.38 ± 0.68 D | 330.01 ± 7.88 A | 2.78 ± 0.07 C | 14.21 ± 0.11 A |
| Outside | 1.41 ± 0.02 J | 139.24 ± 1.09 C | 42.98 ± 0.33 I | 182.22 ± 1.42 DE | 3.24 ± 0.00 A | 7.30 ± 0.06 H | |
| RII | 0.28 | 0.27 | 0.34 | 0.29 | −0.08 | 0.32 | |
| Cakile maritima | Under | 2.84 ± 0.04 G | 128.81 ± 3.91 D | 57.12 ± 0.45 G | 185.93 ± 4.25 CDE | 2.25 ± 0.06 D | 7.95 ± 0.06 E |
| Outside | 2.86 ± 0.09 G | 109.33 ± 0.85 E | 46.14 ± 0.36 H | 155.47 ± 1.21 F | 2.37 ± 0.00 E | 7.65 ± 0.06 F | |
| RII | 0.00 | 0.08 | 0.11 | 0.09 | −0.02 | 0.02 | |
| Erodium laciniatum | Under | 3.60 ± 0.01 F | 175.70 ± 5.34 B | 75.89 ± 0.59 F | 251.59 ± 5.78 B | 2.32 ± 0.06 D | 11.47 ± 0.09 C |
| Outside | 2.08 ± 0.00 I | 97.75 ± 0.76 F | 41.68 ± 0.32 J | 139.43 ± 1.09 G | 2.35 ± 0.00 DE | 6.63 ± 0.05 J | |
| RII | 0.27 | 0.29 | 0.29 | 0.29 | −0.01 | 0.27 | |
| F-value | 3129.25 | 1427.61 | 14651.38 | 2127.02 | 1545.38 | 10693.12 |
| Plant Species | Under Canopy | Outside Canopy | ||||
|---|---|---|---|---|---|---|
| F | M | A | F | M | A | |
| Bromus diandrus | 95 * | 2.35 | 0 | 75 | 2.15 | 0.17 |
| Cakile maritima | 0 | 0 | 0 | 0 | 0 | 0 |
| Erodium laciniatum | 65 | 2.65 | 0 | 60 | 1.67 | 0 |
| Launaea mucronata | 30 | 0.3 | 0 | 30 | 0.3 | 0 |
| Mesembryanthemum crystallinum | 0 | 0 | 0 | 0 | 0 | 0 |
| M. Nodiflorum | 0 | 0 | 0 | 0 | 0 | 0 |
| Rumex pictus | 0 | 0 | 0 | 0 | 0 | 0 |
| Senecio glaucus | 91 | 6.82 | 1.5 | 26.7 | 0.53 | 0.03 |
| Peak | Rt * | Conc. (%) | Compound Name |
|---|---|---|---|
| 1 | 3.490 | 1.30 | 1,2-Dichloro-tetramethyldisilane |
| 2 | 3.855 | 1.30 | 4-Phenylglutamic acid |
| 3 | 5.980 | 1.04 | 5-(2-Aminopropyl)-2-methylphenol |
| 4 | 6.079 | 12.02 | Palmitic acid |
| 5 | 6.170 | 1.16 | 1,6-Dideoxy-2,4-O-methylenehexitol |
| 6 | 6.379 | 2.48 | β-hydroxyethyl isopropyl ether |
| 7 | 6.810 | 4.46 | 3-methylpentane |
| 8 | 7.475 | 0.02 | Malonodinitrile, 2-(2,4,6-trichlorophenylhydrazono) |
| 9 | 8.372 | 7.66 | Acetic acid |
| 10 | 8.510 | 3.93 | Acetasol |
| 11 | 11.504 | 1.07 | 1,2,4-Butanetriol |
| 12 | 13.975 | 1.04 | 3-[4-(2-Methoxyphenyl)-1-piperazinyl]-1-propanol |
| 13 | 14.940 | 5.46 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl |
| 14 | 15.386 | 3.43 | Catechol |
| 15 | 18.108 | 33.00 | Pyrogallol |
| 16 | 18.265 | 2.06 | Citronellylacetone |
| 17 | 18.300 | 1.05 | 2-Chloro-2-propenyl 4-methylphenyl ether |
| 18 | 18.330 | 1.29 | 3-Methoxy-5-methyl-4-nitrophthalic acid |
| 19 | 18.370 | 1.09 | 6-Acetoxy-4-methyl-hept-4-enoic acid |
| 20 | 18.435 | 1.39 | Methyl 6-methyl-2-oxo-2H-pyran-5-carboxylate |
| 21 | 18.672 | 1.25 | Cyclohexane-1,2-dimethanol, diacetate |
| 22 | 18.918 | 1.03 | 1-(3,6-Dimethyl-2-pyrazinyl)-1-propanone |
| 23 | 21.101 | 1.36 | 5,5′-Diamino-3,3′-bis-1,2,4-triazole |
| 24 | 22.095 | 1.11 | 5-Bromouridine |
| 25 | 22.227 | 1.26 | 1,4-Cyclooctanediol |
| 26 | 23.228 | 1.23 | 1-Methylverbenol |
| 27 | 25.758 | 1.33 | 4-Nitrophenyl hexopyranoside |
| 28 | 25.811 | 1.16 | 1H-Pyrrole-2,5-dione, 3-.beta.-D-ribofuranosyl |
| 29 | 27.380 | 1.09 | 4-Nitrophenyl hexopyranoside |
| 30 | 28.921 | 1.91 | 1-Ethyloctyl methyl sulfide |
| 31 | 31.490 | 1.02 | Trimethylsilyl 12-oxooctadecanoate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ElGawad, A.M.; Rashad, Y.M.; Abdel-Azeem, A.M.; Al-Barati, S.A.; Assaeed, A.M.; Mowafy, A.M. Calligonum polygonoides L. Shrubs Provide Species-Specific Facilitation for the Understory Plants in Coastal Ecosystem. Biology 2020, 9, 232. https://doi.org/10.3390/biology9080232
Abd-ElGawad AM, Rashad YM, Abdel-Azeem AM, Al-Barati SA, Assaeed AM, Mowafy AM. Calligonum polygonoides L. Shrubs Provide Species-Specific Facilitation for the Understory Plants in Coastal Ecosystem. Biology. 2020; 9(8):232. https://doi.org/10.3390/biology9080232
Chicago/Turabian StyleAbd-ElGawad, Ahmed M., Younes M. Rashad, Ahmed M. Abdel-Azeem, Sami A. Al-Barati, Abdulaziz M. Assaeed, and Amr M. Mowafy. 2020. "Calligonum polygonoides L. Shrubs Provide Species-Specific Facilitation for the Understory Plants in Coastal Ecosystem" Biology 9, no. 8: 232. https://doi.org/10.3390/biology9080232
APA StyleAbd-ElGawad, A. M., Rashad, Y. M., Abdel-Azeem, A. M., Al-Barati, S. A., Assaeed, A. M., & Mowafy, A. M. (2020). Calligonum polygonoides L. Shrubs Provide Species-Specific Facilitation for the Understory Plants in Coastal Ecosystem. Biology, 9(8), 232. https://doi.org/10.3390/biology9080232








