The Protective Role of Prenatal Alpha Lipoic Acid Supplementation against Pancreatic Oxidative Damage in Offspring of Valproic Acid-Treated Rats: Histological and Molecular Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
- Sodium valproate (VPA): in the form of Depakine ampoule (4 mL) in a concentration of 100 mg/mL, it was purchased from Sanofi Aventis France Company (Paris, France).
- Alpha-lipoic acid (ALA): purchased from Sigma-Aldrich (T5625: Sigma-Aldrich, St. Louis, MO, USA) in the form of a faint yellow powder, 25 g.
2.2. Experimental Protocol
2.3. Sampling and Preparation of the Specimen
2.4. Histological Study
2.5. Electron Microscopic Study
2.6. Immunohistochemical (IHC) Study
2.7. Biochemical Analysis
2.8. Molecular Study
2.9. Statistical Analysis
3. Results
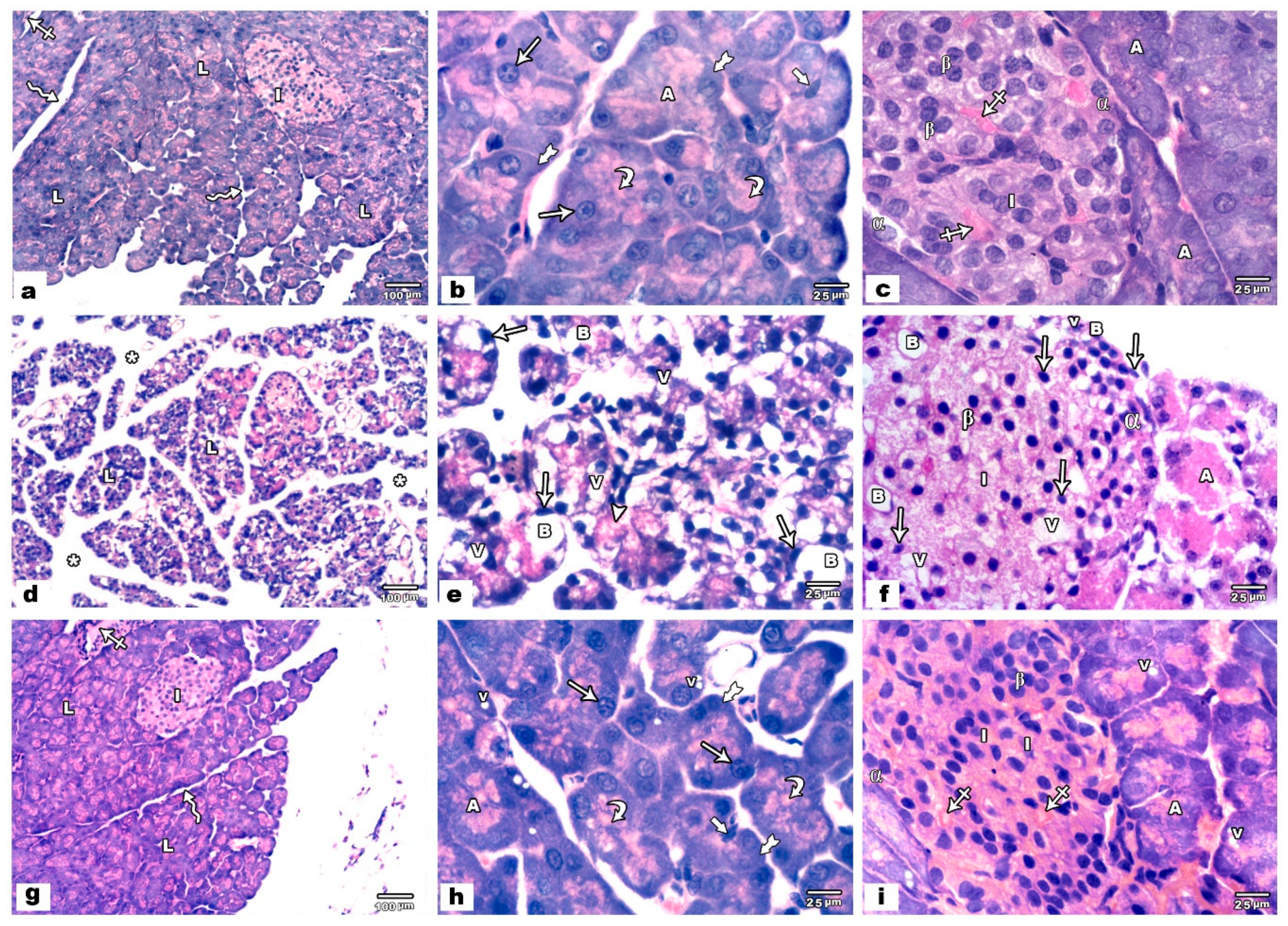
3.1. Histological Results
3.1.1. Light Microscopic Results
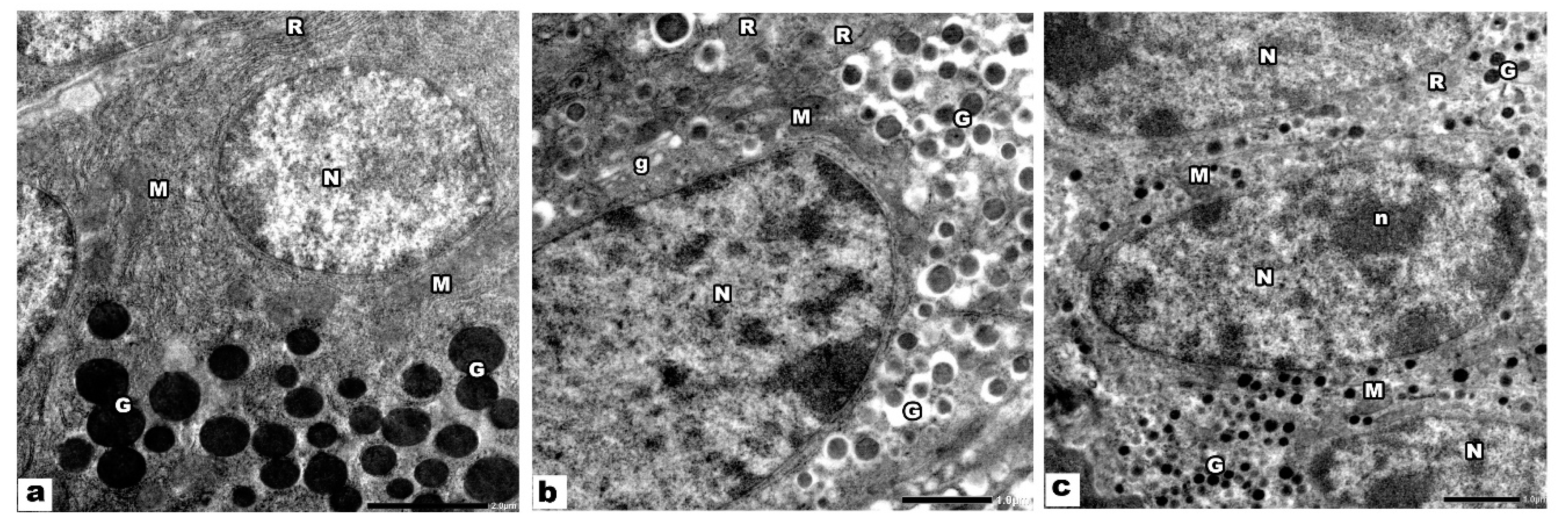
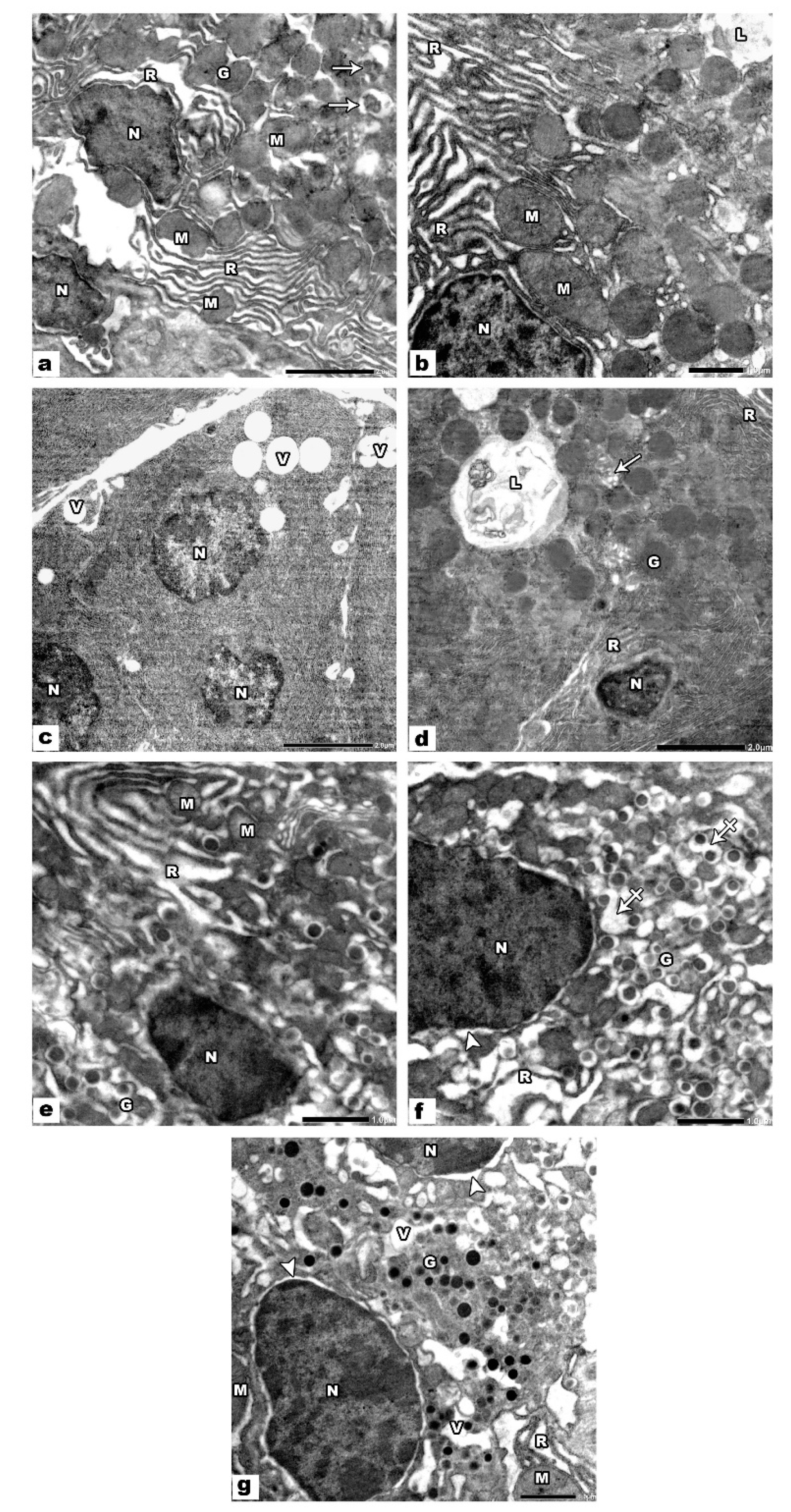
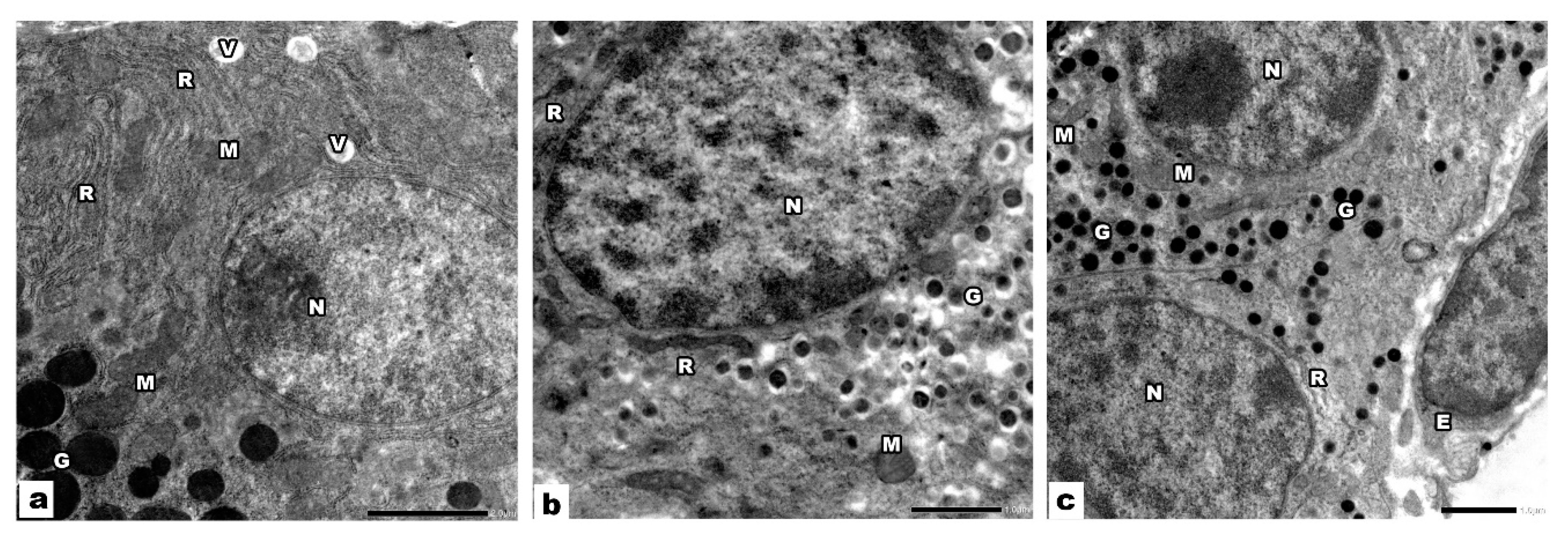
3.1.2. Electron Microscopic Results
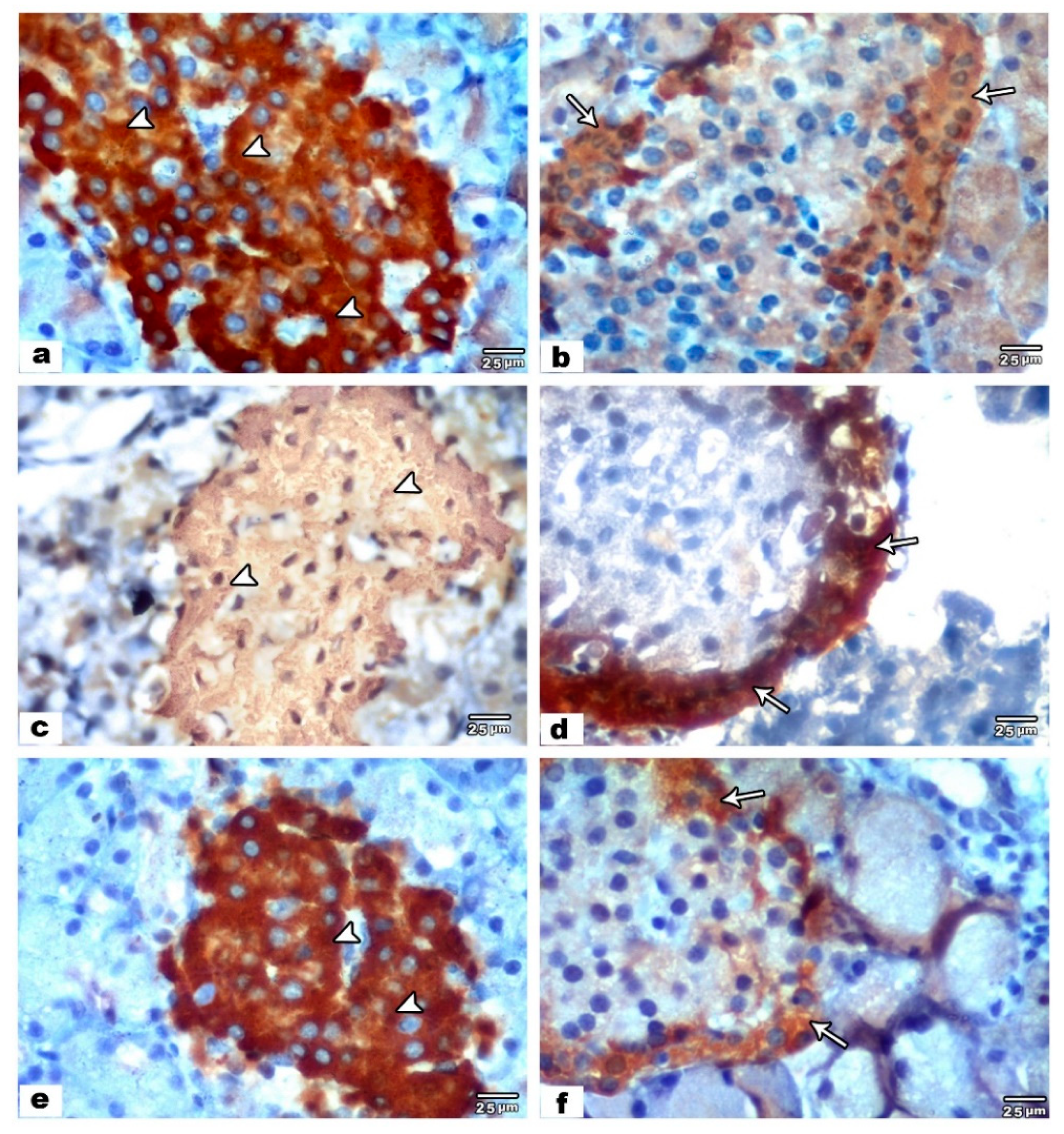
3.2. Immunohistochemical and Morphometric Results
3.3. Biochemical and Molecular Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spina, E.; Perugi, G. Antiepileptic drugs: Indications other than epilepsy. Epileptic Disord. 2004, 6, 57–75. [Google Scholar]
- Veroniki, A.A.; Rios, P.; Cogo, E.; Straus, S.E.; Finkelstein, Y.; Kealey, R.; Reynen, E.; Soobiah, C.; Thavorn, K.; Hutton, B.; et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: A systematic review and network meta-analysis. BMJ Open 2017, 7, e017248. [Google Scholar] [CrossRef]
- Macfarlane, A.; Greenhalgh, T. Sodium valproate in pregnancy: What are the risks and should we use a shared decision-making approach? BMC Pregnancy Childbirth 2018, 18, 200. [Google Scholar] [CrossRef]
- Wieck, A.; Jones, S. Dangers of valproate in pregnancy. BMJ 2018, 361, k1609. [Google Scholar] [CrossRef]
- Harden, C.L.; Pennell, P.B.; Koppel, B.S.; Hovinga, C.A.; Gidal, B.; Meador, K.J.; Hopp, J.; Ting, T.Y.; Hauser, W.A.; Thurman, D.; et al. Management issues for women with epilepsy-Focus on pregnancy (an evidence-based review): III. Vitamin K, folWic acid, blood levels, and breast-feeding. Epilepsia 2009, 50, 1247–1255. [Google Scholar] [CrossRef]
- Petersen, I.; Collings, S.L.; McCrea, R.L.; Nazareth, I.; Osborn, D.P.; Cowen, P.J.; Sammon, C.J. Antiepileptic drugs prescribed in pregnancy and prevalence of major congenital malformations: Comparative prevalence studies. Clin. Epidemiol. 2017, 9, 95–103. [Google Scholar] [CrossRef]
- da Costa, R.F.M.; Kormann, M.L.; Galina, A.; Rehen, S.K. Valproate Disturbs Morphology and Mitochondrial Membrane Potential in Human Neural Cells. Appl. In Vitro Toxicol. 2015, 1, 254–261. [Google Scholar] [CrossRef]
- Komariah, K.; Manalu, W.; Kiranadi, B.; Winarto, A.; Handharyani, E.; Roeslan, M.O. Valproic Acid Exposure of Pregnant Rats During Organogenesis Disturbs Pancreas Development in Insulin Synthesis and Secretion of the Offspring. Toxicol. Res. 2018, 34, 173–182. [Google Scholar] [CrossRef]
- Hamzawy, M.A.; El-Ghandour, Y.B.; Abdel-Aziem, S.H.; Ali, Z.H. Leptin and camel milk abate oxidative stress status, genotoxicity induced in valproic acid rat model of autism. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418785514. [Google Scholar] [CrossRef]
- Jain, A.; Haque, I.; Tayal, V.; Roy, V. Valproic acid-induced acute pancreatitis. Indian J. Psychiatry 2019, 61, 421–422. [Google Scholar] [CrossRef]
- Ma, X.J.; Wang, Y.S.; Gu, W.P.; Zhao, X. The role and possible molecular mechanism of valproic acid in the growth of MCF-7 breast cancer cells. Croat. Med. J. 2017, 58, 349–357. [Google Scholar] [CrossRef]
- Badran, M.; Abuyassin, B.; Golbidi, S.; Ayas, N.; Laher, I. Alpha Lipoic Acid Improves Endothelial Function and Oxidative Stress in Mice Exposed to Chronic Intermittent Hypoxia. Oxid. Med. Cell. Longev. 2019, 2019, 4093018. [Google Scholar] [CrossRef]
- Tian, Y.F.; He, C.T.; Chen, Y.T.; Hsieh, P.S. Lipoic acid suppresses portal endotoxemia-induced steatohepatitis and pancreatic inflammation in rats. World J. Gastroenterol. 2013, 19, 2761–2771. [Google Scholar] [CrossRef]
- Parente, E.; Colannino, G.; Picconi, O.; Monastra, G. Safety of oral alpha-lipoic acid treatment in pregnant women: A retrospective observational study. Eur. Rev. Med. Pharmacol. 2017, 21, 4219–4227. [Google Scholar]
- Al-Matubsi, H.Y.; Oriquat, G.A.; Abu-Samak, M.; al Hanbali, O.A.; Salim, M.D. Corrigendum to “Effects of Lipoic Acid Supplementation on Activities of Cyclooxygenases and Levels of Prostaglandins E2 and F2alpha Metabolites, in the Offspring of Rats with Streptozotocin-Induced Diabetes”. J. Diabetes Res. 2017, 2017, 8135610. [Google Scholar] [CrossRef]
- Schneider, T.; Turczak, J.; Przewlocki, R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: Issues for a therapeutic approach in autism. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2006, 31, 36–46. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. 10—The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Content Repository Only; Elsevier Health Science: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Sciau, P. Chapter Two—Transmission Electron Microscopy: Emerging Investigations for Cultural Heritage Materials. In Advances in Imaging and Electron Physics; Hawkes, P.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 198, pp. 43–67. [Google Scholar]
- Cemek, M.; Kaga, S.; Simsek, N.; Buyukokuroglu, M.E.; Konuk, M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J. Nat. Med. 2008, 62, 284–293. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Koroliuk, M.A.; Ivanova, L.I.; Maiorova, I.G.; Tokarev, V.E. A method of determining catalase activity. Lab. Delo 1988, 16–19. [Google Scholar]
- Wendel, A. Glutathione peroxidase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1981; Volume 77, Chapter 44; pp. 325–333. [Google Scholar]
- Sun, Y.; Oberley, L.W.; Li, Y. A Simple Method for Clinical Assay of Superoxide-Dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ueyama, T.; Nishi, T.; Yamamoto, Y.; Kawakoshi, A.; Sunami, S.; Iguchi, M.; Tamai, H.; Ueda, K.; Ito, T.; et al. Nrf2-inducing anti-oxidation stress response in the rat liver—New beneficial effect of lansoprazole. PLoS ONE 2014, 9, e97419. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Huang, X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med. 2018, 15, 873–877. [Google Scholar] [CrossRef]
- Ornoy, A.; Ergaz, Z. Alcohol Abuse in Pregnant Women: Effects on the Fetus and Newborn, Mode of Action and Maternal Treatment. Int. J. Environ. Res. Public Health 2010, 7, 364–379. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxid. Med. Cell. Longev. 2017, 2017, 1930261. [Google Scholar] [CrossRef]
- Otsuka, T.; Tsukahara, T.; Takeda, H. Development of the pancreas in medaka, Oryzias latipes, from embryo to adult. Dev. Growth Differ. 2015, 57, 557–569. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Pang, Q.; Yi, F. Histone deacetylases and their inhibitors: Molecular mechanisms and therapeutic implications in diabetes mellitus. Acta Pharm. Sin. B 2012, 2, 387–395. [Google Scholar] [CrossRef]
- Sostrup, B.; Gaarn, L.W.; Nalla, A.; Billestrup, N.; Nielsen, J.H. Co-ordinated regulation of neurogenin-3 expression in the maternal and fetal pancreas during pregnancy. Acta Obstet. Gyn. Scan 2014, 93, 1190–1197. [Google Scholar] [CrossRef]
- Gomez, D.L.; O’Driscoll, M.; Sheets, T.P.; Hruban, R.H.; Oberholzer, J.; McGarrigle, J.J.; Shamblott, M.J. Neurogenin 3 Expressing Cells in the Human Exocrine Pancreas Have the Capacity for Endocrine Cell Fate. PLoS ONE 2015, 10, e0133862. [Google Scholar] [CrossRef]
- Martinez-Sanchez, A.; Rutter, G.A.; Latreille, M. MiRNAs in beta-Cell Development, Identity, and Disease. Front. Genet. 2016, 7, 226. [Google Scholar] [CrossRef]
- Guerrini, R. Valproate as a Mainstay of Therapy for Pediatric Epilepsy. Pediatr. Drugs 2006, 8, 113–129. [Google Scholar] [CrossRef]
- De Felice, A.; Ricceri, L.; Venerosi, A.; Chiarotti, F.; Calamandrei, G. Multifactorial Origin of Neurodevelopmental Disorders: Approaches to Understanding Complex Etiologies. Toxics 2015, 3, 89–129. [Google Scholar] [CrossRef]
- Kokate, P.; Bang, R. Study of congenital malformation in tertiary care centre, Mumbai, Maharashtra, India. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 6, 89–93. [Google Scholar] [CrossRef]
- Wlodarczyk, B.J.; Palacios, A.M.; Chapa, C.J.; Zhu, H.; George, T.M.; Finnell, R.H. Genetic basis of susceptibility to teratogen induced birth defects. Am. J. Med. Genet. Part C Semin. Med. Genet. 2011, 157, 215–226. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Li, Q.; Foote, M.; Chen, J. Effects of histone deacetylase inhibitor valproic acid on skeletal myocyte development. Sci. Rep. 2014, 4, 7207. [Google Scholar] [CrossRef]
- Shkreta, L.; Chabot, B. The RNA Splicing Response to DNA Damage. Biomolecules 2015, 5, 2935–2977. [Google Scholar] [CrossRef]
- Palsamy, P.; Bidasee, K.R.; Shinohara, T. Valproic acid suppresses Nrf2/Keap1 dependent antioxidant protection through induction of endoplasmic reticulum stress and Keap1 promoter DNA demethylation in human lens epithelial cells. Exp. Eye Res. 2014, 121, 26–34. [Google Scholar] [CrossRef]
- Defoort, E.N.; Kim, P.M.; Winn, L.M. Valproic acid increases conservative homologous recombination frequency and reactive oxygen species formation: A potential mechanism for valproic acid-induced neural tube defects. Mol. Pharmacol. 2006, 69, 1304–1310. [Google Scholar] [CrossRef]
- Kawai, Y.; Arinze, I.J. Valproic acid-induced gene expression through production of reactive oxygen species. Cancer Res. 2006, 66, 6563–6569. [Google Scholar] [CrossRef]
- Arafat, E.A.; Shabaan, D.A. The possible neuroprotective role of grape seed extract on the histopathological changes of the cerebellar cortex of rats prenatally exposed to Valproic Acid: Animal model of autism. Acta Histochem. 2019, 121, 841–851. [Google Scholar] [CrossRef]
- Ornoy, A.; Weinstein-Fudim, L.; Ergaz, Z. Prevention or Amelioration of Autism-Like Symptoms in Animal Models: Will it Bring Us Closer to Treating Human ASD? Int. J. Mol. Sci. 2019, 20, 1074. [Google Scholar] [CrossRef]
- Beltran-Sarmiento, E.; Arregoitia-Sarabia, C.K.; Floriano-Sanchez, E.; Sandoval-Pacheco, R.; Galvan-Hernandez, D.E.; Coballase-Urrutia, E.; Carmona-Aparicio, L.; Ramos-Reyna, E.; Rodriguez-Silverio, J.; Cardenas-Rodriguez, N. Effects of Valproate Monotherapy on the Oxidant-Antioxidant Status in Mexican Epileptic Children: A Longitudinal Study. Oxid. Med. Cell. Longev. 2018, 2018, 7954371. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Zhou, M.; Li, X.; Fang, Z.; Gao, H.; Li, Y.; Hu, W. Valproic Acid Attenuates Traumatic Brain Injury-Induced Inflammation in Vivo: Involvement of Autophagy and the Nrf2/ARE Signaling Pathway. Front. Mol. Neurosci. 2018, 11, 117. [Google Scholar] [CrossRef]
- Wasik, U.; Milkiewicz, M.; Kempinska-Podhorodecka, A.; Milkiewicz, P. Protection against oxidative stress mediated by the Nrf2/Keap1 axis is impaired in Primary Biliary Cholangitis. Sci. Rep. 2017, 7, 44769. [Google Scholar] [CrossRef]
- Zhang, X.S.; Wu, Q.; Wu, L.Y.; Ye, Z.N.; Jiang, T.W.; Li, W.; Zhuang, Z.; Zhou, M.L.; Zhang, X.; Hang, C.H. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016, 7. [Google Scholar] [CrossRef]
- Aalaei, S.; Mohammadzadeh, M.; Pazhang, Y. Synergistic induction of apoptosis in a cell model of human leukemia K562 by nitroglycerine and valproic acid. EXCLI J. 2019, 18, 619–630. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Shalkami, A.-G.S.; Khalaf, M.M.; Mohamed, W.R.; Hemeida, R.A.M. The impact of Keap1/Nrf2, P38MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar] [CrossRef]
- Olivar, M.P.; Diaz, M.V.; Chicharo, M.A. Tissue effect on RNA:DNA ratios of marine fish larvae. Sci. Mar. 2009, 73, 171–182. [Google Scholar] [CrossRef]
- Oktay, S.; Alev-Tuzuner, B.; Tunali, S.; Ak, E.; Emekli-Alturfan, E.; Tunali-Akbay, T.; Koc-Ozturk, L.; Cetinel, S.; Yanardag, R.; Yarat, A. Investigation of the Effects of Edaravone on Valproic Acid Induced Tissue Damage in Pancreas. Marmara Pharm. J. 2017, 21, 570–577. [Google Scholar] [CrossRef]
- Al-Amoudi, W.M. Protective effects of fennel oil extract against sodium valproate-induced hepatorenal damage in albino rats. Saudi J. Biol. Sci. 2017, 24, 915–924. [Google Scholar] [CrossRef]
- Zaghloul, D.; Gad-El-Rab, W.M.; Bushra, R.R.; Farahat, A.A. The Possible Protective Role of Methionine against Sodium Fluoride-Induced Pancreatic Changes in the Adult Male Albino Rat: A Histological, Immunohistochemical and Morphometric Study. Egypt. J. Histol. 2019, 42, 285–296. [Google Scholar] [CrossRef]
- Antonucci, L.; Fagman, J.B.; Kim, J.Y.; Todoric, J.; Gukovsky, I.; Mackey, M.; Ellisman, M.H.; Karin, M. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc. Natl. Acad. Sci. USA 2015, 112, E6166–E6174. [Google Scholar] [CrossRef]
- Abdul-Hamid, M.; Moustafa, N. Protective effect of curcumin on histopathology and ultrastructure of pancreas in the alloxan treated rats for induction of diabetes. J. Basic Appl. Zool. 2013, 66, 169–179. [Google Scholar] [CrossRef]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef]
- Gorąca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid–biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. BBA-Gen. Subj. 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- Mendoza-Nunez, V.M.; Garcia-Martinez, B.I.; Rosado-Perez, J.; Santiago-Osorio, E.; Pedraza-Chavem, J.; Hernandez-Abad, V.J. The Effect of 600 mg Alpha-lipoic Acid Supplementation on Oxidative Stress, Inflammation, and RAGE in Older Adults with Type 2 Diabetes Mellitus. Oxid. Med. Cell. Longev. 2019, 2019, 3276958. [Google Scholar] [CrossRef]
- Khalili, M.; Azimi, A.; Izadi, V.; Eghtesadi, S.; Mirshafiey, A.; Sahraian, M.A.; Motevalian, A.; Norouzi, A.; Sanoobar, M.; Eskandari, G.; et al. Does Lipoic Acid Consumption Affect the Cytokine Profile in Multiple Sclerosis Patients: A Double-Blind, PlaceboControlled, Randomized Clinical Trial. Neuroimmunomodulation 2014, 21, 291–296. [Google Scholar] [CrossRef]
- Topsakal, S.; Ozmen, O.; Aslankoc, R.; Aydemir, D.H. Pancreatic damage induced by cigarette smoke: The specific pathological effects of cigarette smoke in the rat model. Toxicol. Res. UK 2016, 5, 938–945. [Google Scholar] [CrossRef]
- Grandi, G.; Pignatti, L.; Ferrari, F.; Dante, G.; Neri, I.; Facchinetti, F. Vaginal alpha-lipoic acid shows an anti-inflammatory effect on the cervix, preventing its shortening after primary tocolysis. A pilot, randomized, placebo-controlled study. J. Matern-Fetal Neonatal Med. 2017, 30, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Villagarcia, H.G.; Massa, M.L.; Francini, F. Alpha-lipoic acid and its protective role in fructose induced endocrine-metabolic disturbances. Food Funct. 2019, 10, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhai, X.; Wang, H.; Chen, Z.; Fu, C.; Zhu, M. Alpha lipoic acid inhibits oxidative stress-induced apoptosis by modulating of Nrf2 signalling pathway after traumatic brain injury. J. Cell. Mol. Med. 2019, 23, 4088–4096. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.A.; Pat, B.K.; Gobe, G.C.; Coombes, J.S. Evidence for a non-antioxidant, dose-dependent role of alpha-lipoic acid in caspase-3 and ERK2 activation in endothelial cells. Apoptosis 2005, 10, 657–665. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, H.I.; Kim, D.H.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Alpha-lipoic acid attenuates p-cresyl sulfate-induced renal tubular injury through suppression of apoptosis and autophagy in human proximal tubular epithelial cells. Biomed. Pharmacother. 2019, 112, 108679. [Google Scholar] [CrossRef]
- Go, Y.M.; Jones, D.P. Redox clamp model for study of extracellular thiols and disulfides in redox signaling. Methods Enzymol. 2010, 474, 165–179. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-Lipoic Acid as a Biological Antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Bruin, J.E.; Woynillowicz, A.K.; Hettinga, B.P.; Tarnopolsky, M.A.; Morrison, K.M.; Gerstein, H.C.; Holloway, A.C. Maternal antioxidants prevent beta-cell apoptosis and promote formation of dual hormone-expressing endocrine cells in male offspring following fetal and neonatal nicotine exposure. J. Diabetes 2012, 4, 297–306. [Google Scholar] [CrossRef]
- Cappellani, D.; Sardella, C.; Campopiano, M.C.; Falorni, A.; Marchetti, P.; Macchia, E. Spontaneously remitting insulin autoimmune syndrome in a patient taking alpha-lipoic acid. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Kulakli, F. Effect of Alpha Lipoic Acid in the Treatment of Multiple Sclerosis-Induced Neuropathic Pain: A Case Report. Eurasian J. Med. Oncol. 2018, 2, 179–181. [Google Scholar] [CrossRef]
| Group I (-ve Control) | Group II (+ve Control, ALA) | Group III (VPA Treated) | Group IV (VPA + ALA Treated) | ANOVA p Value | Tukey’s Test p Value | |
|---|---|---|---|---|---|---|
| Insulin positive cells (mean ± SD) | 83.6 ± 7.7 | 85.1 ± 2.1 | 57.3 ± 7.3 | 78.1 ± 4.6 | ˂0.001 * | P1 = 1.00 |
| P2 ˂ 0.001 * | ||||||
| P3 = 0.37 | ||||||
| P4 ˂ 0.001 * | ||||||
| Glucagon positive cells (mean ± SD) | 29.9 ± 4.5 | 30.8 ± 6.9 | 41.2 ± 4.9 | 26.6 ± 7.5 | ˂0.001 * | P1 = 0.99 |
| P2 ˂ 0.001 * | ||||||
| P3 = 0.79 | ||||||
| P4 ˂ 0.001 * |
| Group I (-ve Control) | Group II (+ve Control, ALA) | Group III (VPA Treated) | Group IV (VPA + ALA Treated) | ANOVA p Value | Tukey’s Test p Value | |
|---|---|---|---|---|---|---|
| MDA (nmol/g tissue) | 9.49 ± 1.04 | 7.78 ± 0.33 | 23.87 ± 3.29 | 10.47 ± 0.54 | ˂0.001 * | P1 = 0.34, P2 ˂ 0.001 *, P3 = 0.76, P4 ˂ 0.001 * |
| TAC (mmol/L) | 978.5 ± 33.3 | 996.8 ± 24.2 | 355.9 ± 33.9 | 1030.3 ± 54.5 | ˂0.001 * | P1 = 0.85, P2 ˂ 0.001 *, P3 = 0.12, P4 ˂ 0.001 * |
| GSH (μmol/mL) | 156.2 ± 20.2 | 174.1 ± 20.2 | 50.7 ± 12.2 | 169.4 ± 11.5 | ˂0.001 * | P1 = 0.27, P2 ˂ 0.001 *, P3 = 0.96, P4 ˂ 0.001 * |
| Catalase (U/mL) | 552.6 ± 46.9 | 595.1 ± 55.6 | 403.2 ± 43.7 | 545.1 ± 25.4 | ˂0.001 * | P1 = 0.37, P2 ˂ 0.001 *, P3 = 0.24, P4 ˂ 0.001 * |
| SOD (U/mL) | 86.8 ± 7.2 | 94.1 ± 5.3 | 68.4 ± 16.7 | 80.4 ± 10.7 | ˂0.001 * | P1 = 0.45, P2 ˂ 0.001 *, P3 = 0.55, P4 ˂ 0.001 * |
| Nrf2 gene expression (2−∆∆CT) Mean ± SD | 1.00 ± 0.10 | 1.00 ± 0.11 | 0.53 ± 0.08 | 0.85 ± 0.13 | ˂0.001 * | P1 = 0.36, P2 ˂ 0.001 *, P3 = 0.16, P4 ˂ 0.001 * |
| Caspase-3 gene expression (2−∆∆CT) Mean ± SD | 1.00 ± 0.09 | 1.1 ± 0.21 | 2.8 ± 0.31 | 2.3 ± 0.16 | ˂0.001 * | P1 = 0.54, P2 ˂ 0.001 *, P3 = 0.05*, P4 ˂ 0.001 * |
| Bcl-2 gene expression (2−∆∆CT) Mean ± SD | 1.00 ± 0.10 | 1.08 ± 0.24 | 0.32 ± 0.07 | 0.87 ± 0.11 | ˂0.001 * | P1 = 0.43, P2 ˂ 0.001 *, P3 = 0.21, P4 ˂ 0.001 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoneim, F.M.; Alrefai, H.; Elsamanoudy, A.Z.; Abo El-khair, S.M.; Khalaf, H.A. The Protective Role of Prenatal Alpha Lipoic Acid Supplementation against Pancreatic Oxidative Damage in Offspring of Valproic Acid-Treated Rats: Histological and Molecular Study. Biology 2020, 9, 239. https://doi.org/10.3390/biology9090239
Ghoneim FM, Alrefai H, Elsamanoudy AZ, Abo El-khair SM, Khalaf HA. The Protective Role of Prenatal Alpha Lipoic Acid Supplementation against Pancreatic Oxidative Damage in Offspring of Valproic Acid-Treated Rats: Histological and Molecular Study. Biology. 2020; 9(9):239. https://doi.org/10.3390/biology9090239
Chicago/Turabian StyleGhoneim, Fatma M., Hani Alrefai, Ayman Z. Elsamanoudy, Salwa M. Abo El-khair, and Hanaa A. Khalaf. 2020. "The Protective Role of Prenatal Alpha Lipoic Acid Supplementation against Pancreatic Oxidative Damage in Offspring of Valproic Acid-Treated Rats: Histological and Molecular Study" Biology 9, no. 9: 239. https://doi.org/10.3390/biology9090239
APA StyleGhoneim, F. M., Alrefai, H., Elsamanoudy, A. Z., Abo El-khair, S. M., & Khalaf, H. A. (2020). The Protective Role of Prenatal Alpha Lipoic Acid Supplementation against Pancreatic Oxidative Damage in Offspring of Valproic Acid-Treated Rats: Histological and Molecular Study. Biology, 9(9), 239. https://doi.org/10.3390/biology9090239