Quercetin Caused Redox Homeostasis Imbalance and Activated the Kynurenine Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochemical Reagents
2.2. Microbial Culture
Quercetin’s Antibacterial Evaluation and Determination of the Minimum Inhibitory Concentration (MIC)
2.3. Treatments of Cells for Biochemical Assays
2.3.1. Quercetin Treatment Only
2.3.2. Quercetin and Ascorbic Acid Treatment
2.4. Harvesting Cells for Biochemical Analysis
Biochemical Analyses
2.5. Statistical Analysis
3. Results
3.1. Antibacterial Determination
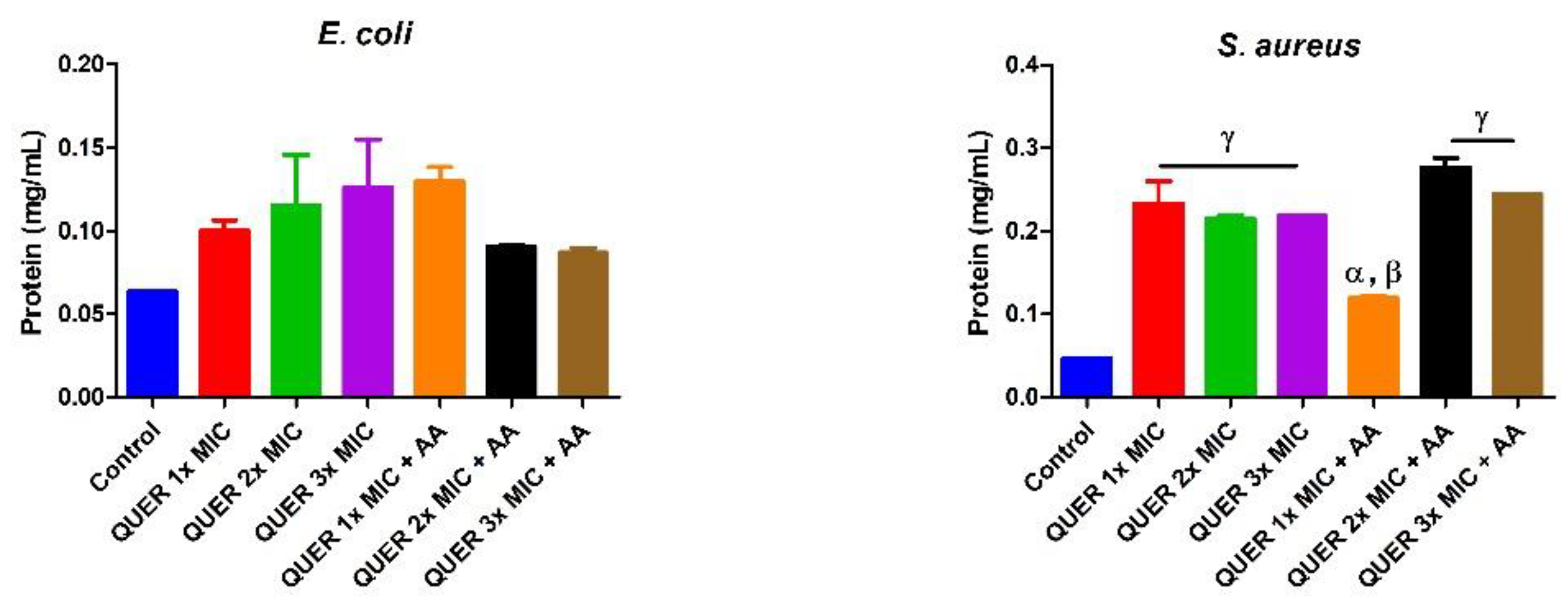
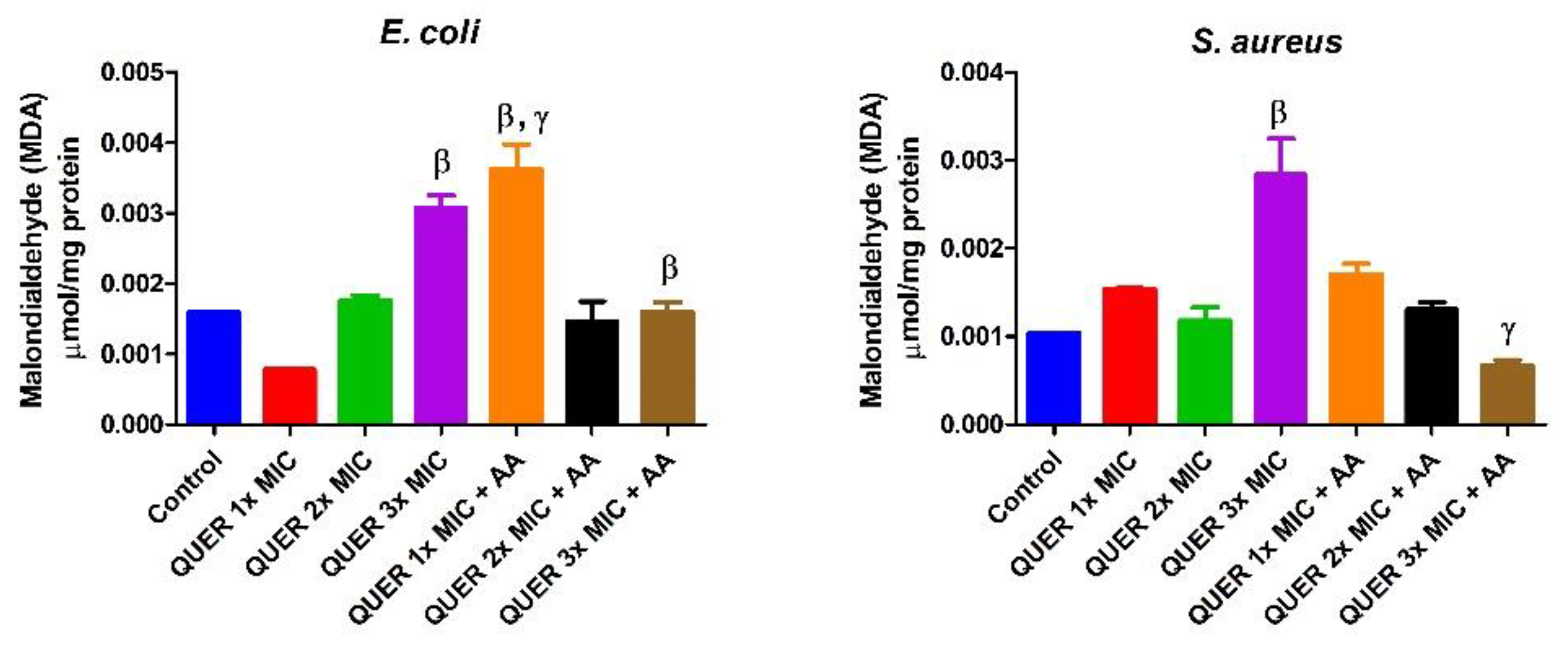
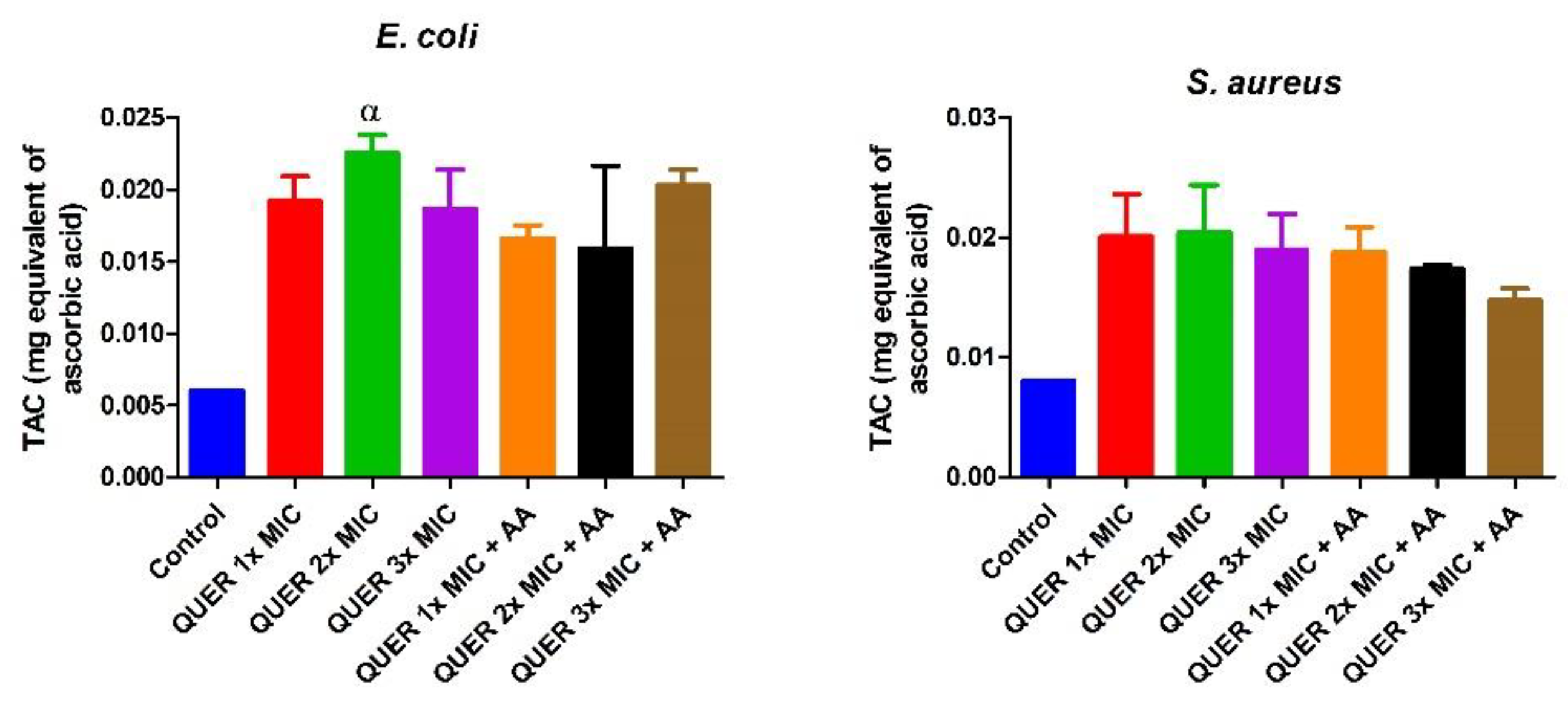
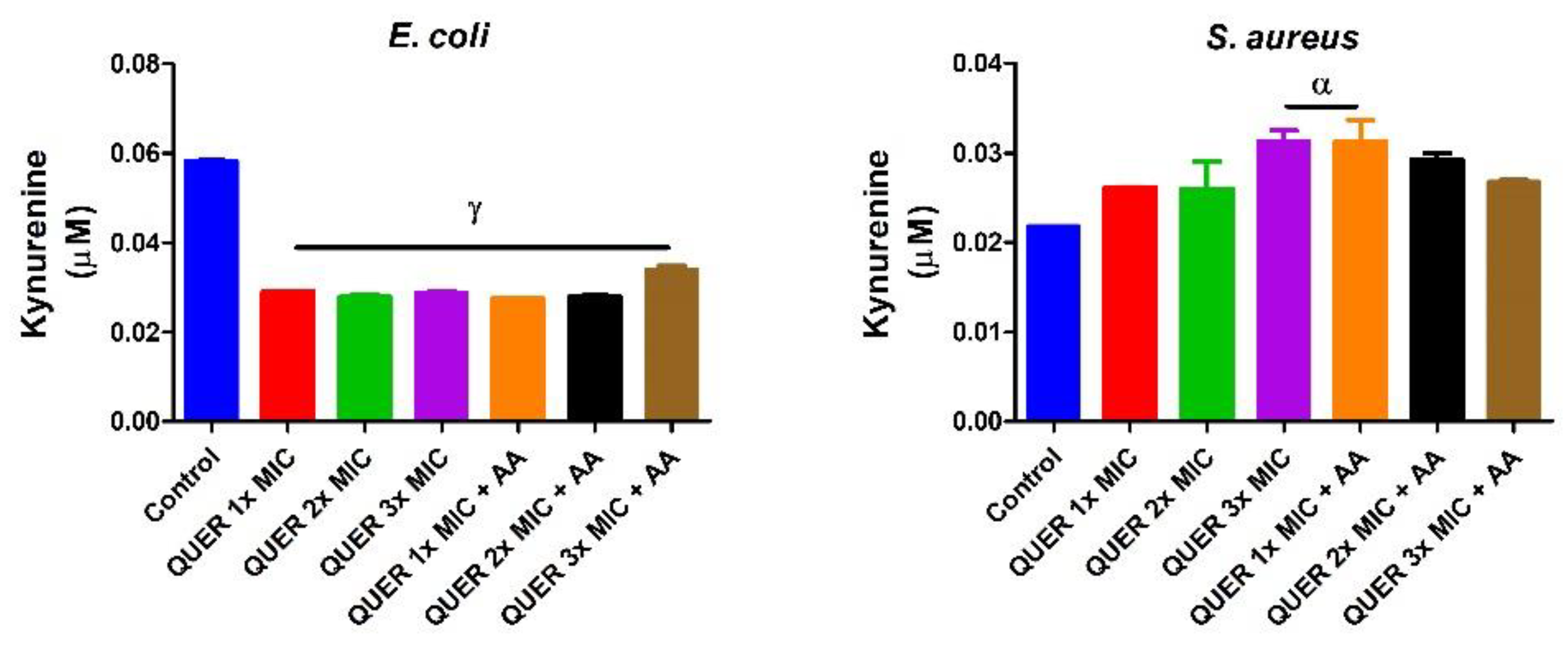
3.2. Biochemical Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Baloch, Z. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Res. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Angiolella, L.; Sacchetti, G.; Efferth, T. Antimicrobial and Antioxidant Activities of Natural Compounds. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Buhian, W.P.C.; Rubio, R.O.; Valle, D.L.; Martin-Puzon, J.J. Bioactive metabolite profiles and antimicrobial activity of ethanolic extracts from Muntingia calabura L. leaves and stems. Asian Pac. J. Trop. Biomed. 2016, 6, 682–685. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Res. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Cox, S.; Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N. Growth inhibition of common food spoilage and pathogenic microorganisms in the presence of brown seaweed extracts. Food Bioprocess Technol. 2012, 5, 1907–1916. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Yin, Y.; Liu, H. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Mondal, P.; Bose, A. Spectroscopic overview of quercetin and its Cu(II) complex interaction with serum albumins. BioImpacts Tabriz Univ. Med. Sci. 2019, 9, 115–121. [Google Scholar] [CrossRef]
- Russo, G.L. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 2007, 74, 533–544. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. BioMed. Res. Int. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Drent, M.; de Boer, V.C.J.; Bast, A.; Haenen, G.R.M.M. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011, 3, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Bustos, P.S.; Deza-Ponzio, R.; Paez, P.L.; Albesa, I.; Cabrera, J.L.; Virgolini, M.B.; Ortega, M.G. Protective effect of quercetin in gentamicin-induced oxidative stress in vitro and in vivo in blood cells. Effect on gentamicin antimicrobial activity. Environ. Toxicol. Pharmacol. 2016, 48, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Corina, D.; Bojin, F.; Ambrus, R.; Muntean, D.; Soica, C.; Paunescu, V.; Cristea, M.; Pinzaru, I.; Dehelean, C. Physico-chemical and biological evaluation of flavonols: Fisetin, quercetin and kaempferol alone and incorporated in beta cyclodextrins. AntiCancer Agents Med. Chem. 2017, 17, 615–626. [Google Scholar] [CrossRef]
- Xue, Y.; Du, M.; Zhu, M.-J. Quercetin prevents Escherichia coli O157:H7 adhesion to epithelial cells via suppressing focal adhesions. Front. Microbiol. 2018, 9, 3278. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Obeme-Imom, J.I.; Akpor, B.O.; Rotimi, D.; Batiha, G.E.; Owolabi, A. Altered redox status, DNA damage and modulation of L-tryptophan metabolism contribute to antimicrobial action of curcumin. Heliyon 2020, 6, e03495. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Shittu, E.O.; Akpor, B.O.; Rotimi, D.; Batiha, G.E. Silver nanoparticles restrict microbial growth by promoting oxidative stress and DNA damage. EXCLI J. 2020, 19, 492–500. [Google Scholar]
- Dong, Z.; Cmarik, J.L. Harvesting cells under anchorage-independent cell transformation conditions for biochemical analyses. Sci. STKE: Signal Transduct. Knowl. Environ. 2002, 130, pl7. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Uloko, R.A.; Awakan, O.J.; Adeyanju, A.A.; Otohinoyi, D.A. The oral administration of silver nanoparticles activates the kynurenine pathway in rat brain independently of oxidative stress. Chem.-Biol. Interact. 2019, 302, 22–27. [Google Scholar] [CrossRef]
- Sulaiman, F.A.; Akanji, M.A.; Oloyede, H.O.B.; Sulaiman, A.A.; Olatunde, A.; Joel, E.B.; Adeyemi, O.S. Oral exposure to silver/gold nanoparticles: Status of rat lipid profile, serum metabolites and tissue morphology. J. Med. Sci. 2015, 15, 71–79. [Google Scholar] [CrossRef][Green Version]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [PubMed]
- Adeyemi, O.S.; Faniyan, T.O. Antioxidant status of rats administered silver nanoparticles orally. J. Taibah Univ. Med. Sci. 2014, 9, 182–186. [Google Scholar] [CrossRef]
- Gercel-Taylor, C. Diphenylamine assay of DNA fragmentation for chemosensitivity testing. Methods Mol. Med. 2005, 111, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Yi, S. Antibacterial activity of quercetin on oral infectious pathogens. Afr. J. Microbiol. Res. 2011, 5, 5358–5361. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative In Vivo and its antibacterial mechanism In Vitro. J. Food Protect. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Chen, W.; Sun, S.; Cao, W.; Liang, Y.; Song, J. Antioxidant property of quercetin-Cr(III) complex: The role of Cr(III) ion. J. Mol. Struct. 2009, 918, 194–197. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Han, Y.; Kato, K. Modulation of host HIF-1α activity and the tryptophan pathway contributes to the anti-Toxoplasma gondii potential of nanoparticles. Biochem. Biophys. Rep. 2017, 11, 84–92. [Google Scholar] [CrossRef]
- Karu, N.; McKercher, C.; Nichols, D.S.; Davies, N.; Shellie, R.A.; Hilder, E.F.; Jose, M.D. Tryptophan metabolism, its relation to inflammation and stress markers and association with psychological and cognitive functioning: Tasmanian Chronic Kidney Disease pilot study. BMC Nephrol. 2016, 17, 171. [Google Scholar] [CrossRef]
- Schwarcz, R. The kynurenine pathway of tryptophan degradation as a drug target. Curr. Opin. Pharmacol. 2004, 4, 12–17. [Google Scholar] [CrossRef]



| Microbial Isolates | Quercetin (mm ± Standard Deviation) | Amoxicillin (mm ± Standard Deviation) |
|---|---|---|
| S. aureus | 12 ± 0.9 | 19.5 ± 0.5 |
| E. coli | 14 ± 0.5 | 25.5 ± 3.5 |
| P. aeruginosa | 21.5 ± 1.5 | 33.5 ± 1.5 |
| K. pneumonia | 25 ± 1.0 | - |
| B. subtilis | 20 ± 1.0 | 16 ± 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemi, O.S.; Ebugosi, C.; Akpor, O.B.; Hetta, H.F.; Al-Rashed, S.; Otohinoyi, D.A.; Rotimi, D.; Owolabi, A.; Evbuomwan, I.O.; Batiha, G.E.-S. Quercetin Caused Redox Homeostasis Imbalance and Activated the Kynurenine Pathway. Biology 2020, 9, 219. https://doi.org/10.3390/biology9080219
Adeyemi OS, Ebugosi C, Akpor OB, Hetta HF, Al-Rashed S, Otohinoyi DA, Rotimi D, Owolabi A, Evbuomwan IO, Batiha GE-S. Quercetin Caused Redox Homeostasis Imbalance and Activated the Kynurenine Pathway. Biology. 2020; 9(8):219. https://doi.org/10.3390/biology9080219
Chicago/Turabian StyleAdeyemi, Oluyomi Stephen, Chinemerem Ebugosi, Oghenerobor Benjamin Akpor, Helal F. Hetta, Sarah Al-Rashed, David Adeiza Otohinoyi, Damilare Rotimi, Akinyomade Owolabi, Ikponmwosa Owen Evbuomwan, and Gaber El-Saber Batiha. 2020. "Quercetin Caused Redox Homeostasis Imbalance and Activated the Kynurenine Pathway" Biology 9, no. 8: 219. https://doi.org/10.3390/biology9080219
APA StyleAdeyemi, O. S., Ebugosi, C., Akpor, O. B., Hetta, H. F., Al-Rashed, S., Otohinoyi, D. A., Rotimi, D., Owolabi, A., Evbuomwan, I. O., & Batiha, G. E.-S. (2020). Quercetin Caused Redox Homeostasis Imbalance and Activated the Kynurenine Pathway. Biology, 9(8), 219. https://doi.org/10.3390/biology9080219







