Development, Molecular Docking, and Anti-Anemia Potential of Polyherbal Formulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Procurement
2.2. Chemicals and Instruments
2.3. Collection and Procurement of Plant Material
2.4. Preliminary Qualitative Phytochemical Analysis
2.5. In Silico Molecular Docking
2.5.1. Ligand Selection and Preparation
2.5.2. Protein Preparation
2.5.3. Docking Simulation and Analysis
2.6. Preparation of Polyherbal Formulation
2.7. Thin-Layer Chromatography Analysis
2.8. High-Performance Thin-Layer Chromatography
2.9. Estimation of Iron Content
2.10. Phytoconstituent Analysis
2.10.1. Total Phenolic Content
2.10.2. Total Flavonoid Content
2.10.3. Total Tannin Content
2.11. Antioxidant Activity
2.11.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.11.2. Ferric Reducing Antioxidant Power Assay
2.12. Acute Toxicity Study
2.13. Phenylhydrazine-Induced Anemia in Sprague Dawley Rats
2.13.1. Experimental Induction of Hemolytic Anemia
2.13.2. Experimental Design
2.13.3. Blood Withdrawal and Histopathology Study
2.14. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Screening
3.2. Thin-Layer Chromatography
3.3. High-Performance Thin-Layer Chromatography
3.4. Estimation of Iron, Phenol, Flavonoid, and Tannin Content in Polyherbal Formulation
3.5. In Silico Molecular Docking
3.6. Phytochemical Screening
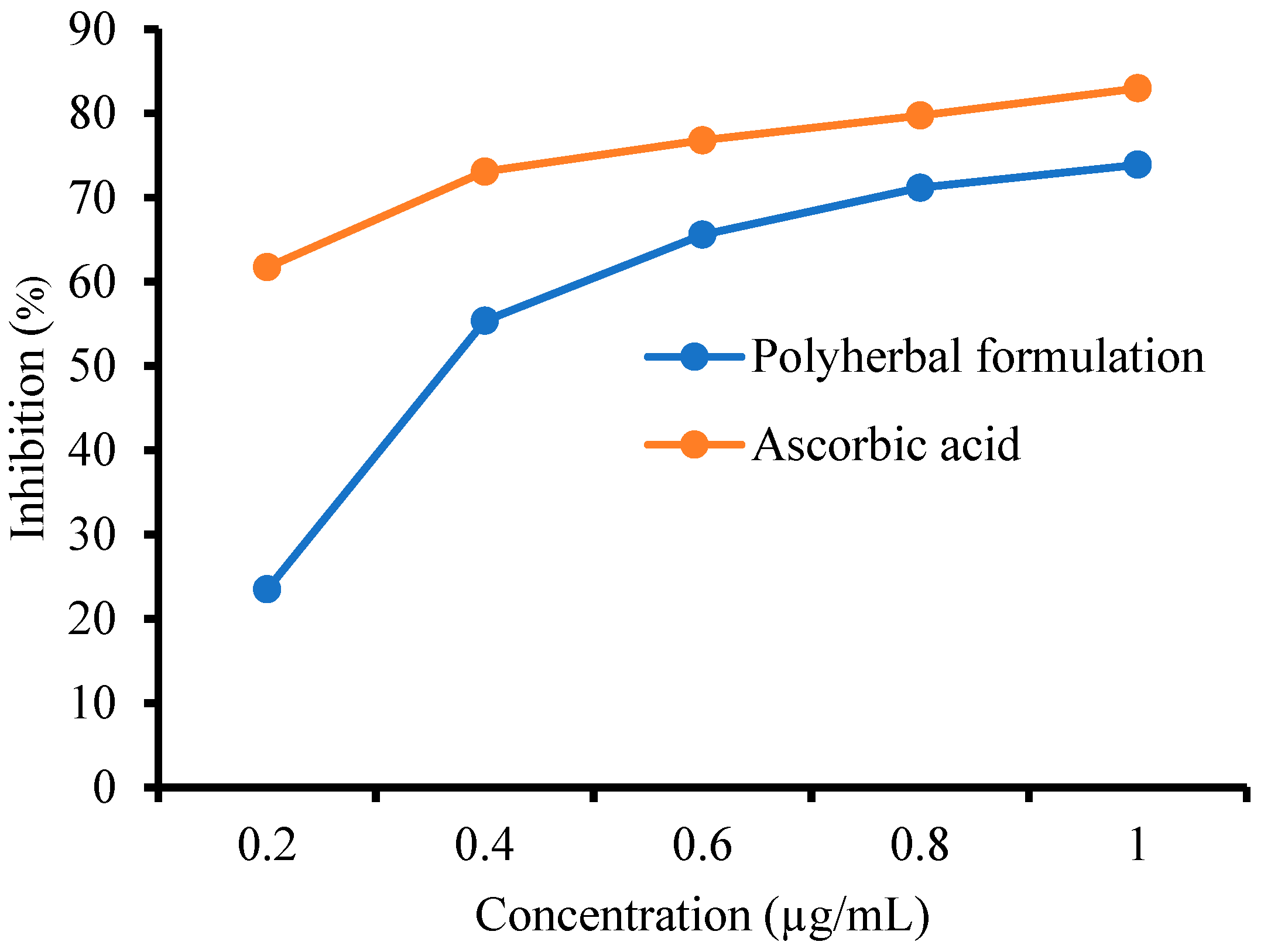
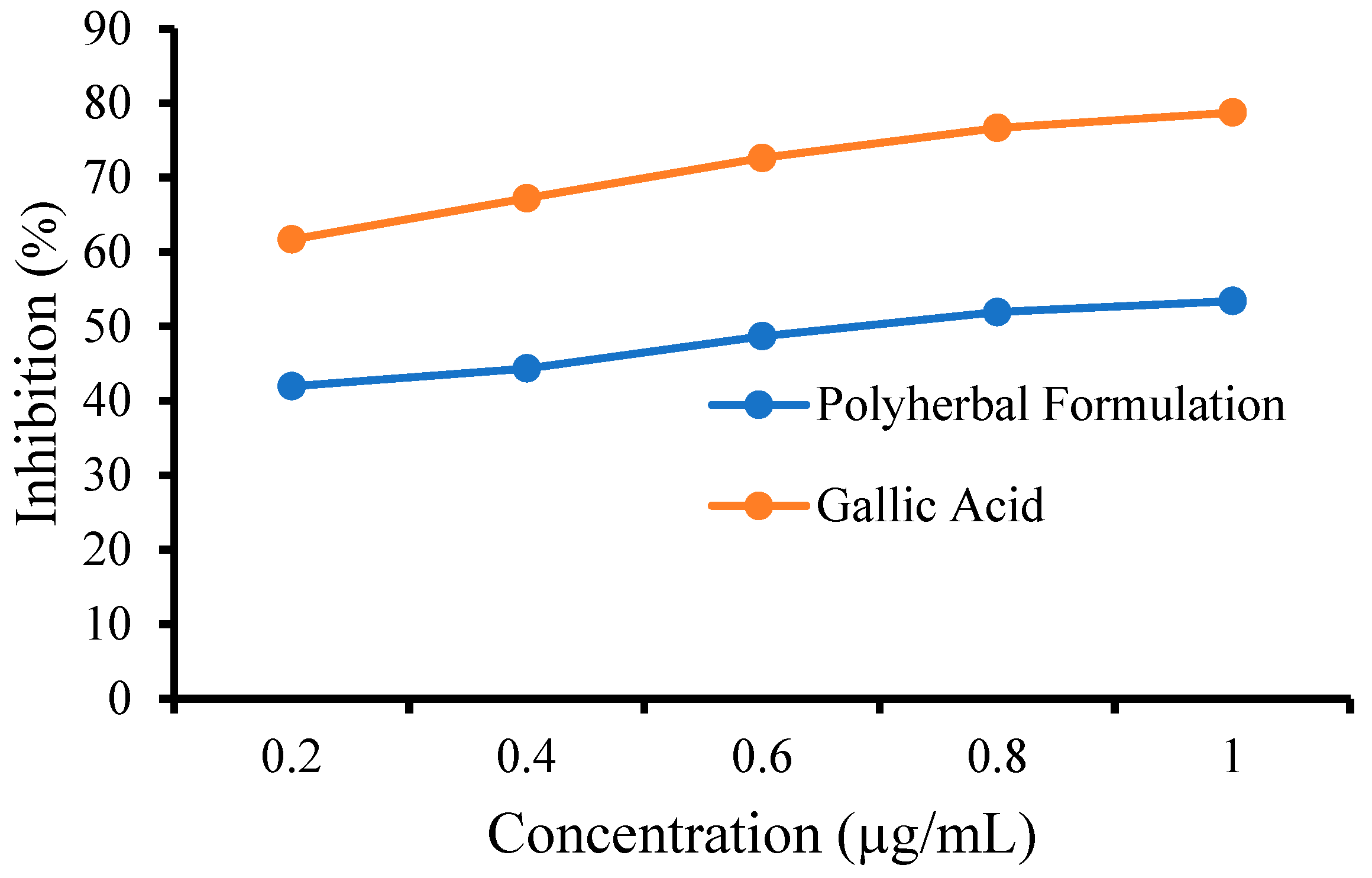
3.6.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
3.6.2. FRAP Assay
3.7. Phenylhydrazine-Induced Anemia in Sprague Dawley Rats
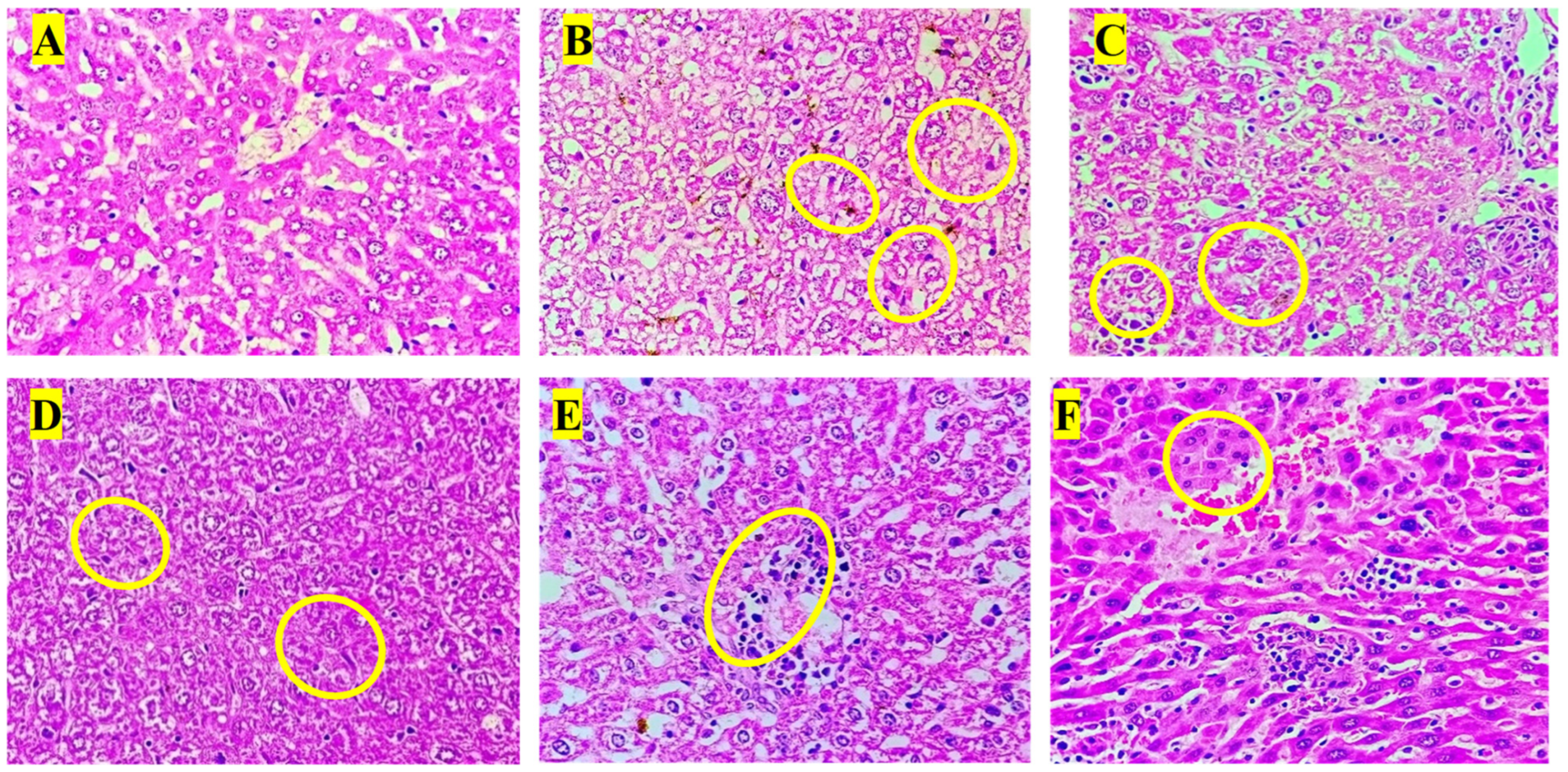
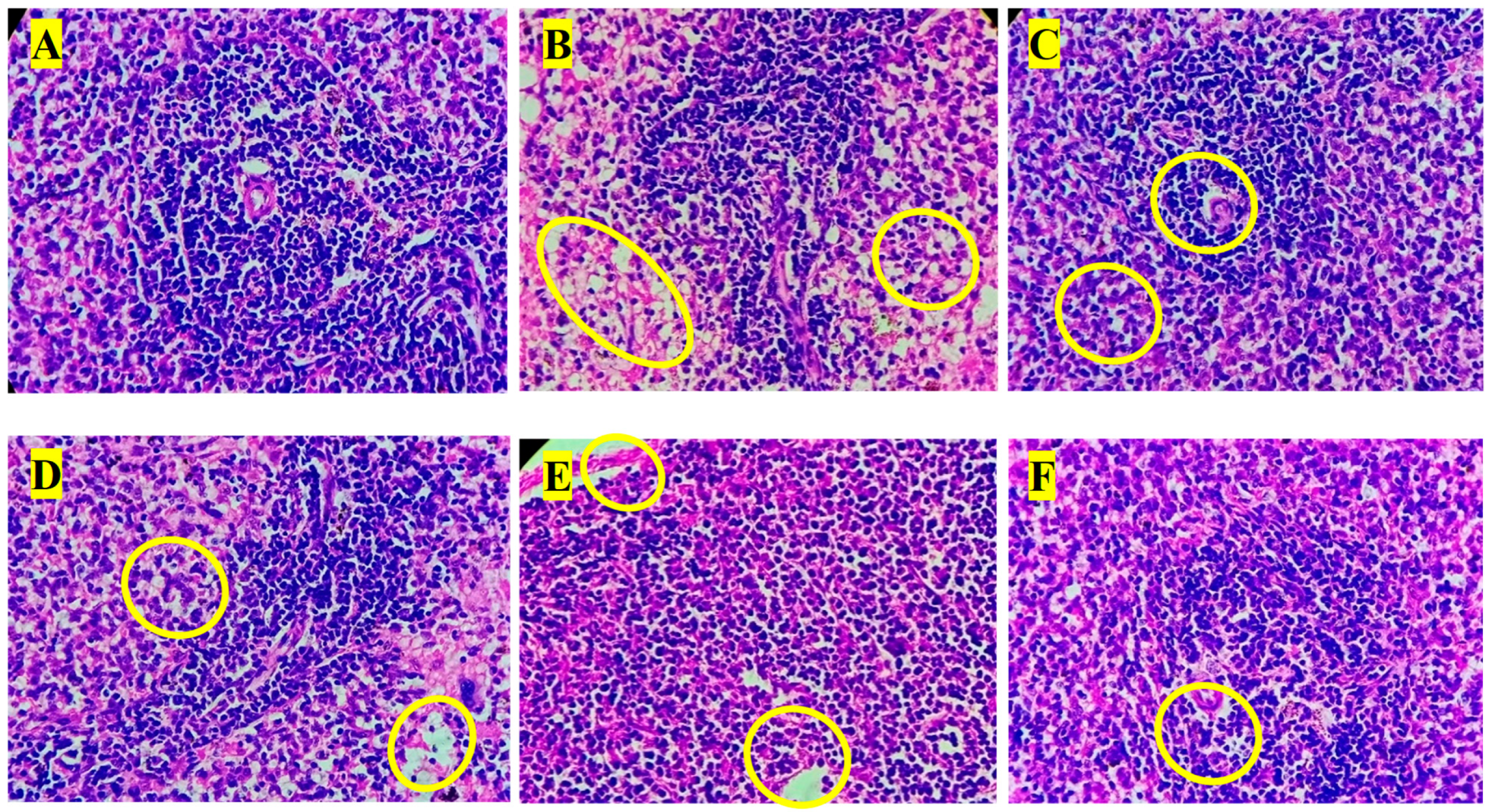
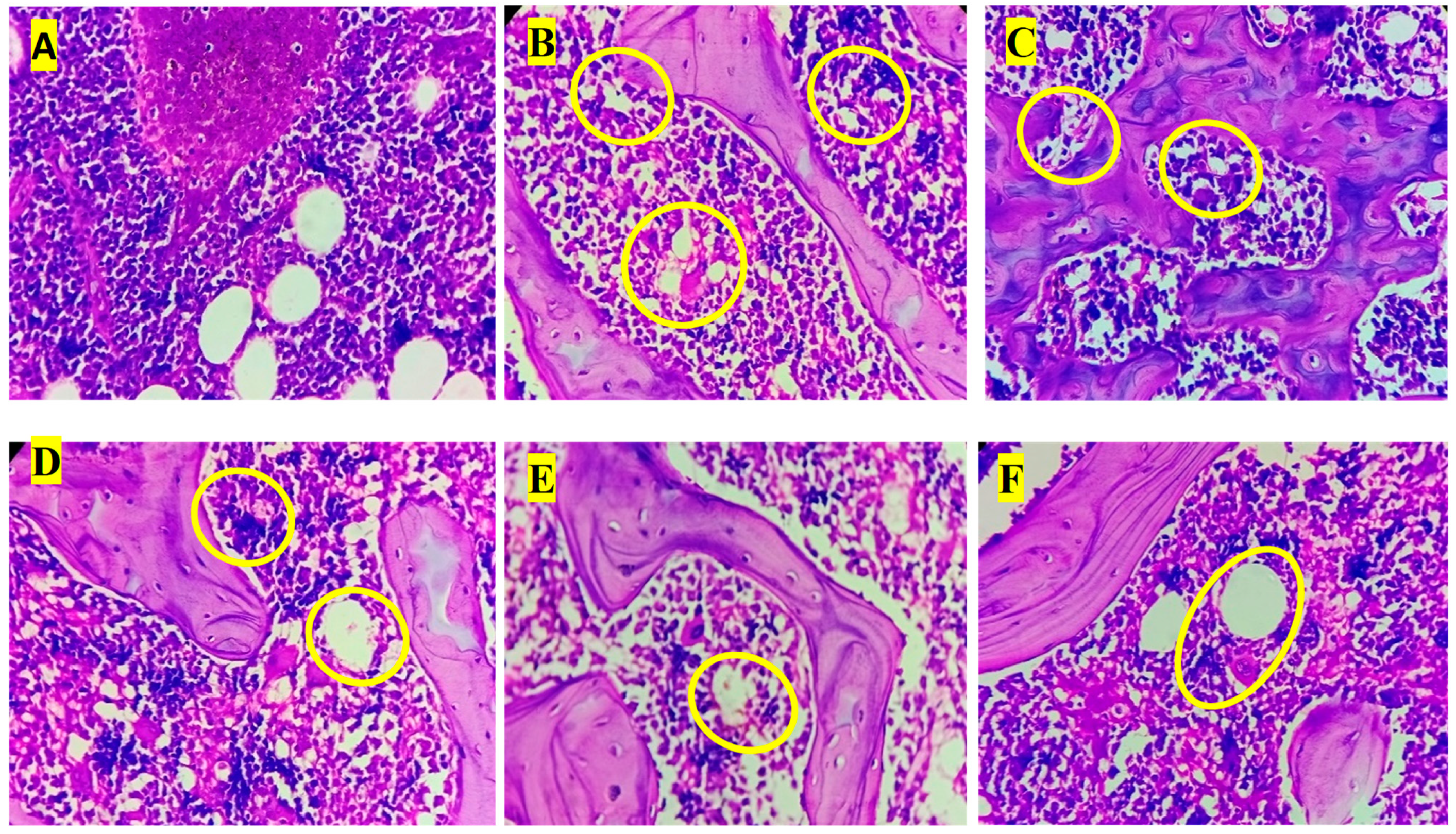
3.8. Histopathology Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brittenham, G.M.; Moir-Meyer, G.; Abuga, K.M.; Datta-Mitra, A.; Cerami, C.; Green, R.; Pasricha, S.-R.; Atkinson, S.H. Biology of Anemia: A Public Health Perspective. J. Nutr. 2023, 153, S7–S28. [Google Scholar] [CrossRef] [PubMed]
- Abdilahi, M.M.; Kiruja, J.; Farah, B.O.; Abdirahman, F.M.; Mohamed, A.I.; Mohamed, J.; Ahmed, A.M. Prevalence of anemia and associated factors among pregnant women at Hargeisa Group Hospital, Somaliland. BMC Pregnancy Childbirth 2024, 24, 332. [Google Scholar] [CrossRef] [PubMed]
- Addo, O.Y.; Yu, E.X.; Williams, A.M.; Young, M.F.; Sharma, A.J.; Mei, Z.; Kassebaum, N.J.; Jefferds, M.E.D.; Suchdev, P.S. Evaluation of Hemoglobin Cutoff Levels to Define Anemia Among Healthy Individuals. JAMA Netw. Open 2021, 4, e2119123. [Google Scholar] [CrossRef] [PubMed]
- Gilreath, J.A.; Rodgers, G.M. How I treat cancer-associated anemia. Blood 2020, 136, 801–813. [Google Scholar] [CrossRef]
- Prentice, A.M.; Mendoza, Y.A.; Pereira, D.; Cerami, C.; Wegmuller, R.; Constable, A.; Spieldenner, J. Dietary strategies for improving iron status: Balancing safety and efficacy. Nutr. Rev. 2017, 75, 49–60. [Google Scholar] [CrossRef]
- Cotoraci, C.; Ciceu, A.; Sasu, A.; Hermenean, A. Natural Antioxidants in Anemia Treatment. Int. J. Mol. Sci. 2021, 22, 1883. [Google Scholar] [CrossRef]
- Sheth, P.A.; Pawar, A.T.; Mote, C.S.; More, C. Antianemic activity of polyherbal formulation, Raktavardhak Kadha, against phenylhydrazine-induced anemia in rats. J. Ayurveda Integr. Med. 2021, 12, 340–345. [Google Scholar] [CrossRef]
- Khan, A.A.; Raheem, A.; Parveen, S.; Khatoon, A.; Parveen, S.; Afrin, Z. Safety and efficacy of a polyherbal unani formulation (sharbat-e-faulad) in sū’al-qinya (anaemia)—A multi centric study. Safety 2019, 6, 41–46. [Google Scholar]
- Riaz, M.; Khalid, R.; Afzal, M.; Anjum, F.; Fatima, H.; Zia, S.; Rasool, G.; Egbuna, C.; Mtewa, A.G.; Uche, C.Z. Phytobioactive compounds as therapeutic agents for human diseases: A review. Food Sci. Nutr. 2023, 11, 2500–2529. [Google Scholar] [CrossRef]
- Nwozo, O.S.; Effiong, E.M.; Aja, P.M.; Awuchi, C.G. Antioxidant, phytochemical, and therapeutic properties of medicinal plants: A review. Int. J. Food Prop. 2023, 26, 359–388. [Google Scholar] [CrossRef]
- Ekweogu, C.N.; Ude, V.C.; Nwankpa, P.; Emmanuel, O.; Ugbogu, E.A. Ameliorative effect of aqueous leaf extract of Solanum aethiopicum on phenylhydrazine-induced anaemia and toxicity in rats. Toxicol. Res. 2020, 36, 227–238. [Google Scholar] [CrossRef]
- Ahmad, A.; Alghamdi, S.S.; Mahmood, K.; Afzal, M. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi J. Biol. Sci. 2016, 23, 300–310. [Google Scholar] [CrossRef]
- Mahassni, S.H.; Bukhari, O.A. Beneficial effects of an aqueous ginger extract on the immune system cells and antibodies, hematology, and thyroid hormones in male smokers and non-smokers. J. Nutr. Intermed. Metab. 2019, 15, 10–17. [Google Scholar] [CrossRef]
- Gaur, P.K.; Rastogi, S.; Lata, K. Correlation between phytocompounds and pharmacological activities of Boerhavia diffusa Linn with traditional-ethnopharmacological insights. Phytomed. Plus 2022, 2, 100260. [Google Scholar] [CrossRef]
- Dutra, V.d.F.; Biassi, T.P.; Figueiredo, M.S. Sickle cell anemia: Hierarchical cluster analysis and clinical profile in a cohort in Brazil. Hematol. Transfus. Cell Ther. 2023, 45, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, C.-P.; Zhang, Y.; Li, Y.; Sun, J.-R. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int. J. Biol. Macromol. 2019, 137, 504–511. [Google Scholar] [CrossRef]
- Shenoy, S.; Chaskar, U.; Sandhu, J.; Paadhi, M. Effects of eight-week supplementation of Ashwagandha on cardiorespiratory endurance in elite Indian cyclists. J. Ayurveda Integr. Med. 2012, 3, 209–214. [Google Scholar] [CrossRef]
- Mazhar, M.; Faizi, S.; Gul, A.; Kabir, N.; Simjee, S.U. Effects of naturally occurring flavonoids on ferroportin expression in the spleen in iron deficiency anemia in vivo. RSC Adv. 2017, 7, 23238–23245. [Google Scholar] [CrossRef]
- Intharuksa, A.; Kuljarusnont, S.; Sasaki, Y.; Tungmunnithum, D. Flavonoids and Other Polyphenols: Bioactive Molecules from Traditional Medicine Recipes/Medicinal Plants and Their Potential for Phytopharmaceutical and Medical Application. Molecules 2024, 29, 5760. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Phenolics and flavonoids contents of medicinal plants, as natural ingredients for many therapeutic purposes—A review. IOSR J. Pharm. 2020, 10, 42–81. [Google Scholar]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular mechanisms of iron and heme metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Chumpolphant, S.; Suwatronnakorn, M.; Issaravanich, S.; Tencomnao, T.; Prasansuklab, A. Polyherbal formulation exerts wound healing, anti-inflammatory, angiogenic and antimicrobial properties: Potential role in the treatment of diabetic foot ulcers. Saudi J. Biol. Sci. 2022, 29, 103330. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Patil, M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Machado, J.C.B.; Cristina da Silva, J.; Leite, G.V.B.; Dos Santos Dantas, T.; Daniele-Silva, A.; de Freitas Fernandes-Pedrosa, M.; de Oliveira, A.M.; Dantas da Cruz, R.C.; de Souza, I.A.; Weilack, I.; et al. Phytochemical Profile, Acute, and Subacute Toxicity of Spray-Dried Hydroethanolic Extract From Punica granatum Leaves. Chem. Biodivers. 2025, 22, e202402429. [Google Scholar] [CrossRef]
- Abeysekera, A.; Gunaherath, K.; Ranasinghe, C. A study of some anthraquinones of Rubia cordifolia L. incorporated into pinda oil: An ayurvedic medicinal oil used for topical application in dermatological and inflammatory conditions. Int. J. Res. Ayurveda Pharm. 2014, 5, 334–338. [Google Scholar] [CrossRef]
- Claeson, U.P.; Malmfors, T.; Wikman, G.; Bruhn, J.G. Adhatoda vasica: A critical review of ethnopharmacological and toxicological data. J. Ethnopharmacol. 2000, 72, 1–20. [Google Scholar] [CrossRef]
- Yadav, P.K.; Singh, S.; Singh, A.K. Pharmacophore-based computational study on inhibitor of TMPRSS6 as hepcidin modulator in an iron overload of beta-thalassaemia. Mol. Simul. 2024, 50, 287–297. [Google Scholar] [CrossRef]
- Wang, Y.; Qinqin, H.; Wang, H.; Zhang, H.; Zhang, X.; Liu, W.; Xiang, Z.; Gu, Y. Network pharmacology and molecular docking to explore the mechanism of Sheng Xue Bao mixture against iron deficiency anemia. Medicine 2023, 102, e35012. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Rafiullah, M.; Shehata, W.A.; Hossain, A.; Ali, M. Comparative phytochemical, thin layer chromatographic profiling and antioxidant activity of extracts from some Indian herbal drugs. J. Bioresour. Bioprod. 2022, 7, 128–134. [Google Scholar] [CrossRef]
- Gupta, M.; Sumaiya, S.; Ali, S.; Naved, T.; Sharma, A.; Ahmad, A.; Sikander, M.; Sarwat, M. Pharmacognostical and Phytochemical Evaluation of a Unani Polyherbal Formulation: Dawa ul Kurkum by HPTLC. Separations 2023, 10, 89. [Google Scholar] [CrossRef]
- Michiu, D.; Socaciu, M.-I.; Fogarasi, M.; Jimborean, A.M.; Ranga, F.; Mureşan, V.; Semeniuc, C.A. Implementation of an Analytical Method for Spectrophotometric Evaluation of Total Phenolic Content in Essential Oils. Molecules 2022, 27, 1345. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Sheth, P.A.; Pawar, A.T.; Choudhari, G.B.; More, C.S. Phytochemical content and antioxidant property of polyherbal formulation, raktavardhak kadha. Res. J. Pharm. Technol. 2022, 15, 4931–4934. [Google Scholar] [CrossRef]
- Deepak, B.; Abhijeet, P. Exploration and Evaluation of In-vitro Antioxidant Activity of ABANA: A Polyherbal Formulation. Curr. Funct. Foods 2023, 1, 2–11. [Google Scholar] [CrossRef]
- Aloke, C.; Uche Emelike, C.; Ajuka Obasi, N.; Nkemjika Ogbu, P.; Oswald Edeogu, C.; Godwin Uzomba, C.; Ekakitie, O.; Adewale Iyaniwura, A.; Okoro, C.C.; Peter Okey, B.; et al. HPLC profiling and studies on Copaifera salikounda methanol leaf extract on phenylhydrazine-induced hematotoxicity and oxidative stress in rats. Arab. J. Chem. 2021, 14, 103428. [Google Scholar] [CrossRef]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Aguilera-Alcala, N.; Morales-Reyes, Z.; Martin-Lopez, B.; Moleon, M.; Sanchez-Zapata, J.A. Role of scavengers in providing non-material contributions to people. Ecol. Indic. 2020, 117, 106643. [Google Scholar] [CrossRef]
- Cui, C.; Wu, Y.; Guo, X.; Hong, Z.; Xiao, J.; Wan, X.; Zong, J.; Hou, R. Accurate and rapid quantification of tea saponins using a thin-layer chromatography (TLC) method based on hemolysis and machine vision. LWT 2024, 199, 116139. [Google Scholar] [CrossRef]
- Dubale, S.; Kebebe, D.; Zeynudin, A.; Abdissa, N.; Suleman, S. Phytochemical Screening and Antimicrobial Activity Evaluation of Selected Medicinal Plants in Ethiopia. J. Exp. Pharmacol. 2023, 15, 51–62. [Google Scholar] [CrossRef]
- Checkouri, E.; Reignier, F.; Robert-Da Silva, C.; Meilhac, O. Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes. Antioxidants 2020, 9, 959. [Google Scholar] [CrossRef]
- Molino, S.; Pilar Francino, M.; Ángel Rufián Henares, J. Why is it important to understand the nature and chemistry of tannins to exploit their potential as nutraceuticals? Food Res. Int. 2023, 173, 113329. [Google Scholar] [CrossRef]
- Yap, V.L.; Tan, L.F.; Rajagopal, M.; Wiart, C.; Selvaraja, M.; Leong, M.Y.; Tan, P.L. Evaluation of phytochemicals and antioxidant potential of a new polyherbal formulation TC-16: Additive, synergistic or antagonistic? BMC Complement. Med. Ther. 2023, 23, 93. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Fernandes, R.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.; Munekata, P.E.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef]
- Rajendran, K.; Chellappan, D.R.; Ramakrishnan, V.; Krishnan, U.M. Therapeutic efficacy of Punarnavadi mandura against phenylhydrazine-induced hemolytic anemia in rats. J. Tradit. Complement. Med. 2025, 15, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.A.Z.; Abdel-Maksoud, F.M.; Abd Elaziz, H.O.; Al-Brakati, A.; Elmahallawy, E.K. Descriptive Histopathological and Ultrastructural Study of Hepatocellular Alterations Induced by Aflatoxin B1 in Rats. Animals 2021, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Ousaaid, D.; Ghouizi, A.E.; Laaroussi, H.; Bakour, M.; Mechchate, H.; Es-Safi, I.; Kamaly, O.A.; Saleh, A.; Conte, R.; Lyoussi, B.; et al. Anti-Anemic Effect of Antioxidant-Rich Apple Vinegar against Phenylhydrazine-Induced Hemolytic Anemia in Rats. Life 2022, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Suh, H.J.; Lee, H.S.; Park, Y.; Park, J.W.; Jung, E.Y. Hematopoietic effect of Bacillus subtilis-fermented antler extract on phenylhydrazine-induced hemolytic anemia in Sprague-Dawley rats. J. Med. Food 2012, 15, 774–780. [Google Scholar] [CrossRef]

| Synonyms | Biological Names | Part | Quantity Sufficient up to 500 mg |
|---|---|---|---|
| Fenugreek | Trigonella foenum-graecum | Seeds | 50 mg |
| Ginger | Zingiber officinale | Rhizomes | 10 mg |
| Vasaka | Adhatoda vasica | Leaves | 50 mg |
| Malabar Kino | Pterocarpus marsupium | Gum | 100 mg |
| Amla | Emblica officinalis | Fruit pericarp | 50 mg |
| Pomegranate | Punica granatum | Peels | 20 mg |
| Ashwagandha | Withania somnifera | Roots | 50 mg |
| Punarnava | Boerhavia diffusa | Roots | 20 mg |
| Shatavari | Asparagus racemosus | Roots | 50 mg |
| Manjishtha | Rubia cordifolia | Roots | 50 mg |
| Phytochemical Constituent | Solvent System | Visualization |
|---|---|---|
| Alkaloids | Dioxane: Ammonia (90:10) | Dragendroff reagent UV light at 254 nm |
| Flavonoids and Tannins | Chloroform: Ethyl Acetate: Formic Acid: Methanol (4:5.2:0.6:0.2) | Anisaldehyde sulphuric acid reagent and heat up to 110 °C UV light at 254 nm |
| Saponins | Toluene: Ethyl Acetate: Formic Acid (5:3.5:0.5) | UV light at 254 nm |
| Active Constituent | Solvent System | Application Volume | Visualization | Derivatization | |
|---|---|---|---|---|---|
| std | phf | ||||
| Gallic acid | Toluene: Ethyl acetate: Formic acid (5:3.5:0.5) | 1 μL | 10 μL | 280 nm | NA |
| Ellagic acid | Toluene: Ethyl acetate: Formic acid (5:3.5:0.5) | 5 μL | 5 μL | 540 nm | Anisaldehyde reagent |
| Group | Treatment |
|---|---|
| Group I—Normal control | No treatment was given |
| Group II—Anemic control | Only PHZ 60 mg/kg |
| Group III—Standard group | PHZ with standard: Livogen XT tablets (9 mg of iron/kg, twice daily, p.o.) |
| Group IV—Test group I | PHZ with PHF 100 mg/kg, once daily, p.o. |
| Group V—Test group II | PHZ with PHF 200 mg/kg, once daily, p.o. |
| Group VI—Test group III | PHZ with PHF 100 mg/kg, twice daily, p.o. |
| Phytochemical Constituents | TFG | EO | PM | WS | AR | ZO | RC | BD | AV | PG | PHF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | + | - | - | + | + | - | - | + | + | - | + |
| Flavonoids | + | + | + | - | - | + | + | + | - | + | + |
| Saponins | + | - | - | - | + | - | - | - | - | - | + |
| Tannins | + | + | + | - | - | + | + | + | - | + | + |
| Anthraquinone glycosides | - | - | - | - | - | - | + | - | - | - | + |
| Triterpenes and steroidal glycosides | + | - | - | + | + | + | - | + | - | - | + |
| TLC of Alkaloids | TLC of Flavonoids and Tannins | TLC of Saponins |
|---|---|---|
 |  |  |
| Ligand | Ligand Type | Binding Affinity (kcal/moL) |
|---|---|---|
| Alizarin | Test ligand | −6.3 |
| Catechin | Test ligand | −6.0 |
| Kaempferol | Test ligand | −6.2 |
| Recesmol | Test ligand | −5.2 |
| Rubiadin | Test ligand | −6.3 |
| Rutin | Test ligand | −6.4 |
| Gallic acid | Test ligand | −4.6 |
| Ellagic acid | Test ligand | −6.2 |
| EPE ligand | Native ligand | −5 |
| Fe | Control | −4.8 |
| Comp. | 3D Visualization | 2D Visualization |
|---|---|---|
| Alizarin | 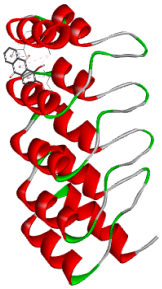 | 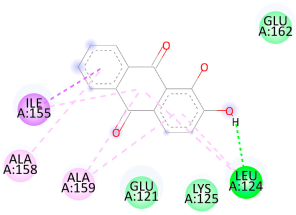 |
| Catechin | 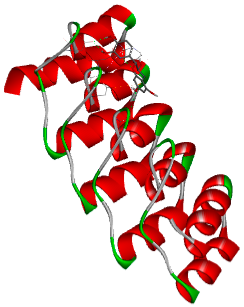 |  |
| Kaempferol | 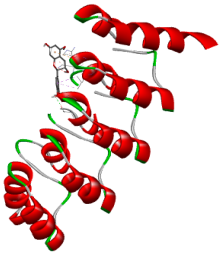 | 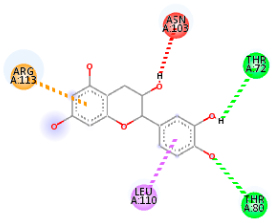 |
| Recesmol | 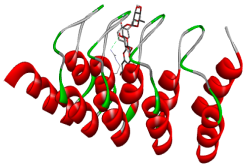 | 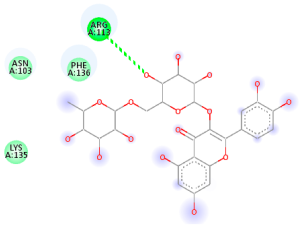 |
| Rubiadin | 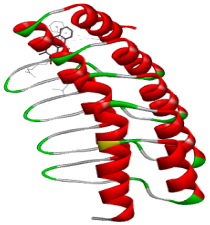 | 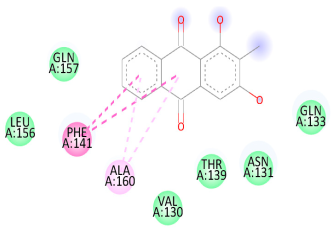 |
| Rutin | 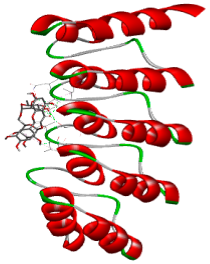 | 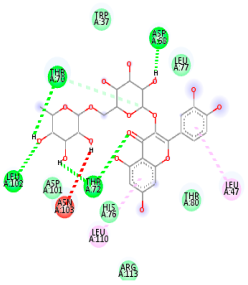 |
| Gallic acid | 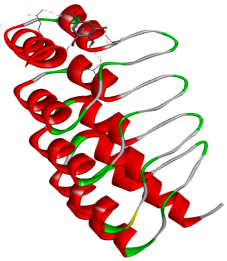 |  |
| Ellagic acid | 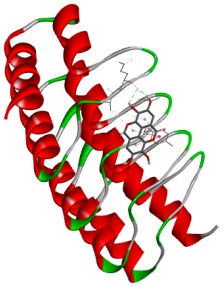 | 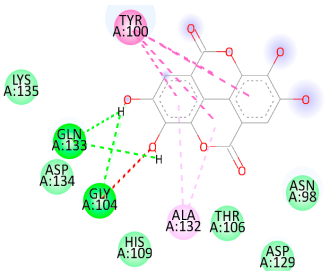 |
 | ||
| Days | Control Group | Anemic Group | Standard Group— Livogen XT Tablet (9 mg iron/kg, Twice Daily) | Test Group I (100 mg/kg, Once Daily) | Test Group II (200 mg/kg, Once Daily) | Test Group III (100 mg/kg, Twice Daily) |
|---|---|---|---|---|---|---|
| RBCs Count (106/μL) | ||||||
| Day 0 | 7.25 ± 0.25 | 6.89 ± 0.24 | 6.83 ± 0.01 | 6.75 ± 0.01 | 6.79 ± 0.02 | 6.78 ± 0.02 |
| Day 3 | 7.01 ± 0.26 | 2.28 ± 0.28 ### | 2.22 ± 0.13 *** | 3.06 ± 0.13 *** | 2.55 ± 0.06 *** | 0.91 ± 0.04 |
| Day 7 | 7.63 ± 0.21 | 3.59 ± 0.22 ### | 5.19 ± 0.23 *** | 3.95 ± 0.03 * | 3.03 ± 0.05 *** | 4.53 ± 0.08 *** |
| Day 10 | 7.47 ± 0.12 | 5.06 ± 0.17 ### | 6.16 ± 0.05 *** | 5.29 ± 0.03 *** | 4.51 ± 0.05 *** | 5.62 ± 0.17 * |
| Day 15 | 6.99 ± 0.29 | 6.09 ± 0.28 ## | 6.53 ± 0.04 *** | 4.79 ± 0.04 ** | 6.29 ± 0.07 *** | 6.15 ± 0.04 *** |
| Hemoglobin (g/dL) | ||||||
| Day 0 | 14.52 ± 0.41 | 14.07 ± 0.81 | 13.83 ± 0.46 | 14.28 ± 0.26 | 14.21 ± 0.25 | 14.28 ± 0.22 |
| Day 3 | 14.22 ± 0.23 | 4.07 ± 0.11 ### | 4.29 ± 0.06 | 4.27 ± 0.01 | 4.35 ± 0.10 | 3.83 ± 0.22 |
| Day 7 | 12.14 ± 0.22 | 8.95 ± 0.17 ### | 9.99 ± 0.19 *** | 10.18 ± 0.04 *** | 10.08 ± 0.04 *** | 11.03 ± 0.22 *** |
| Day 10 | 15.1 ± 0.29 | 10.11 ± 0.31 ### | 11.60 ± 0.02 *** | 12.49 ± 0.04 *** | 12.51 ± 0.04 *** | 12.08 ± 0.24 *** |
| Day 15 | 14.36 ± 0.32 | 10.67 ± 0.35 ### | 14.61 ± 0.03 *** | 14.10 ± 0.03 * | 14.71 ± 0.03 *** | 14.82 ± 0.03 *** |
| Hematocrit Count (%) | ||||||
| Day 0 | 43.07 ± 0.23 | 44.80 ± 0.18 | 33.17 ± 0.93 | 35.48 ± 1.19 | 33.43 ± 0.61 | 36.35 ± 0.13 |
| Day 3 | 44.33 ± 0.31 | 22.28 ± 0.34 ### | 21.97 ± 0.28 | 22.60 ± 0.28 | 21.73 ± 0.04 | 8.97 ± 0.14 *** |
| Day 7 | 42.09 ± 0.29 | 27.66 ± 0.59 ### | 23.73 ± 0.04 *** | 32.01 ± 0.99 *** | 35.32 ± 0.05 *** | 36.83 ± 0.20 *** |
| Day 10 | 40.34 ± 0.48 | 30.98 ± 0.45 ### | 44.70 ± 0.21 *** | 36.49 ± 0.28 *** | 38.60 ± 0.60 *** | 42.13 ± 0.24 *** |
| Day 15 | 41.55 ± 0.45 | 36.03 ± 0.67 ### | 44.98 ± 0.50 *** | 38.53 ± 0.34 ** | 41.07 ± 0.34 *** | 43.08 ± 0.28 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharati, D.; Nirhali, S.; Puri, A.; Mohite, P.; Singh, S. Development, Molecular Docking, and Anti-Anemia Potential of Polyherbal Formulation. Biology 2025, 14, 1052. https://doi.org/10.3390/biology14081052
Bharati D, Nirhali S, Puri A, Mohite P, Singh S. Development, Molecular Docking, and Anti-Anemia Potential of Polyherbal Formulation. Biology. 2025; 14(8):1052. https://doi.org/10.3390/biology14081052
Chicago/Turabian StyleBharati, Deepak, Sakshi Nirhali, Abhijeet Puri, Popat Mohite, and Sudarshan Singh. 2025. "Development, Molecular Docking, and Anti-Anemia Potential of Polyherbal Formulation" Biology 14, no. 8: 1052. https://doi.org/10.3390/biology14081052
APA StyleBharati, D., Nirhali, S., Puri, A., Mohite, P., & Singh, S. (2025). Development, Molecular Docking, and Anti-Anemia Potential of Polyherbal Formulation. Biology, 14(8), 1052. https://doi.org/10.3390/biology14081052









