Thymoquinone Upregulates microRNA-199a-3p and Downregulates COX-2 Expression and PGE2 Production via Deactivation of p38/ERK/JNK-MAPKs and p65/p50-NF-κB Signaling in Human Lung Cancer Cells
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Cell Culture and Treatment Protocols
2.2. MicroRNA Transfection and Gene Expression Analysis
2.3. Luciferase Reporter Assays
2.4. Cell Transfection and Signaling Inhibitor Experiments
2.5. Nuclear Protein Isolation and NF-κB Activity Detection
2.6. Western Blot Analysis
2.7. Prostaglandin E2 Quantification
2.8. Statistical Analysis
3. Results
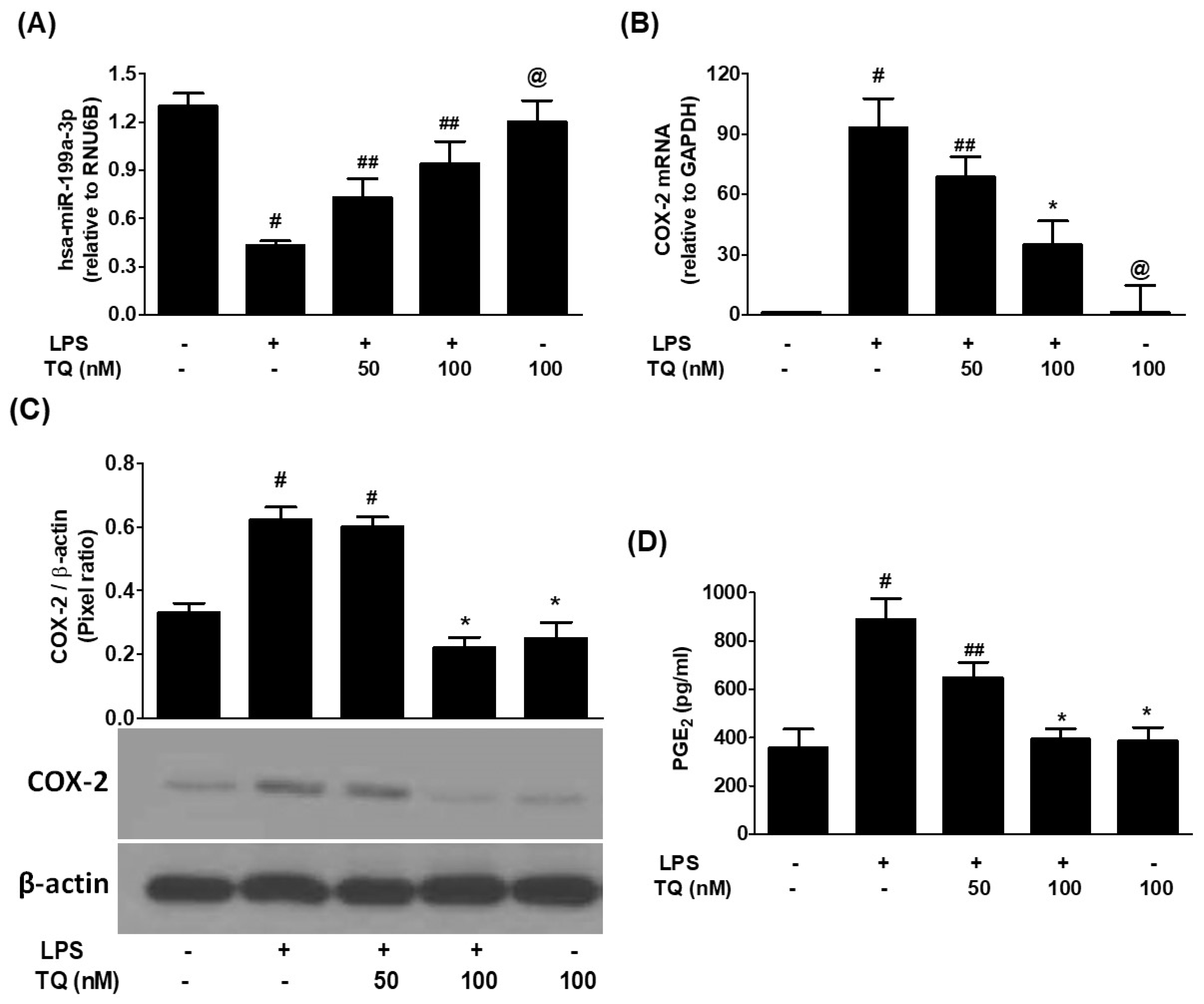
3.1. Inverse Correlation Between hsa-miR-199a-3p Expression and COX-2/PGE2 Induction in LPS-Stimulated A549 Lung Cancer Cells
3.2. Thymoquinone Reverses LPS-Induced COX-2 and PGE2 Upregulation via hsa-miR-199a-3p Induction
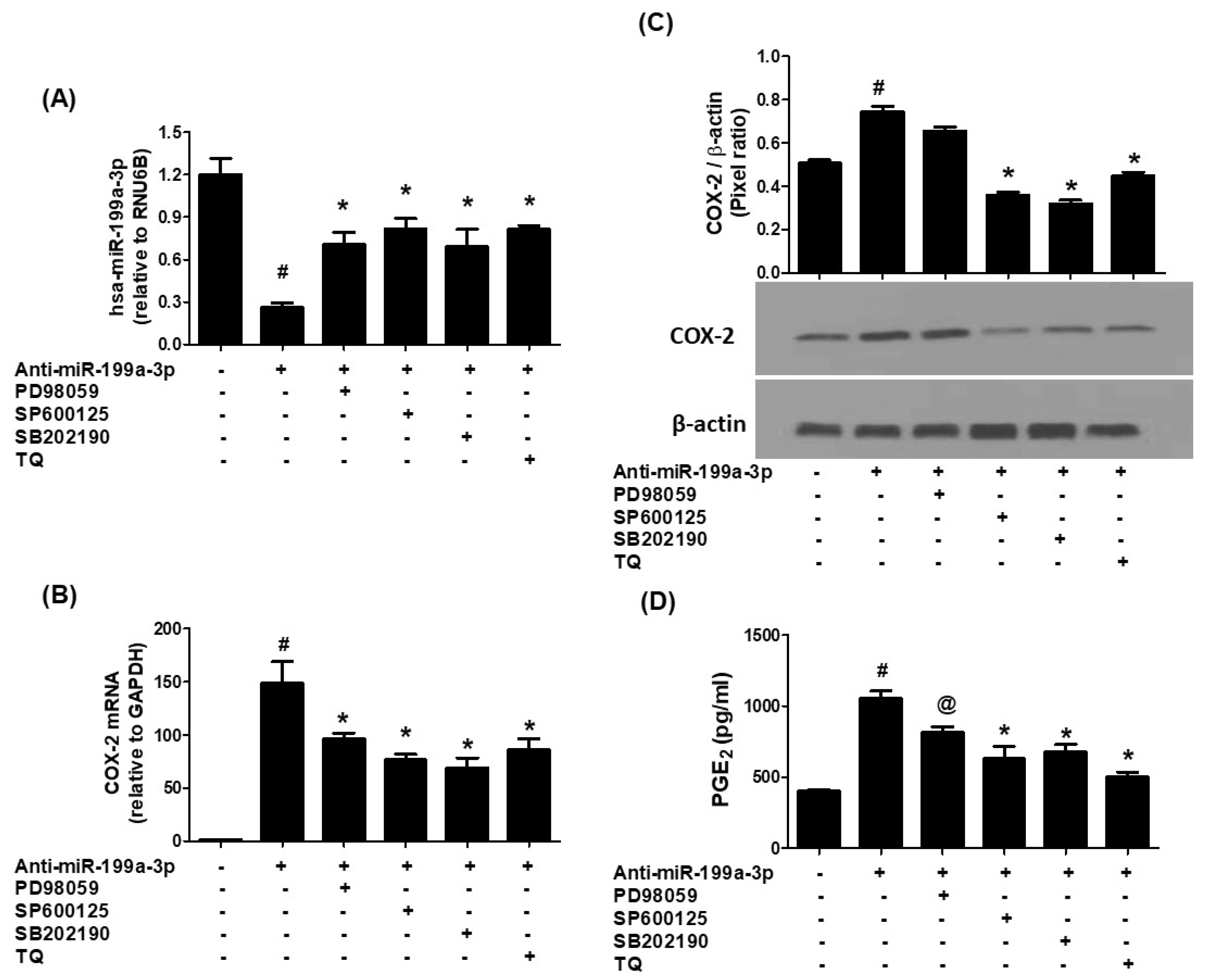
3.3. Thymoquinone-Mediated Suppression of COX-2 Is Dependent on hsa-miR-199a-3p Activity
3.4. MAPK Pathway Involvement in Thymoquinone-Driven hsa-miR-199a-3p Modulation and COX-2/PGE2 Suppression
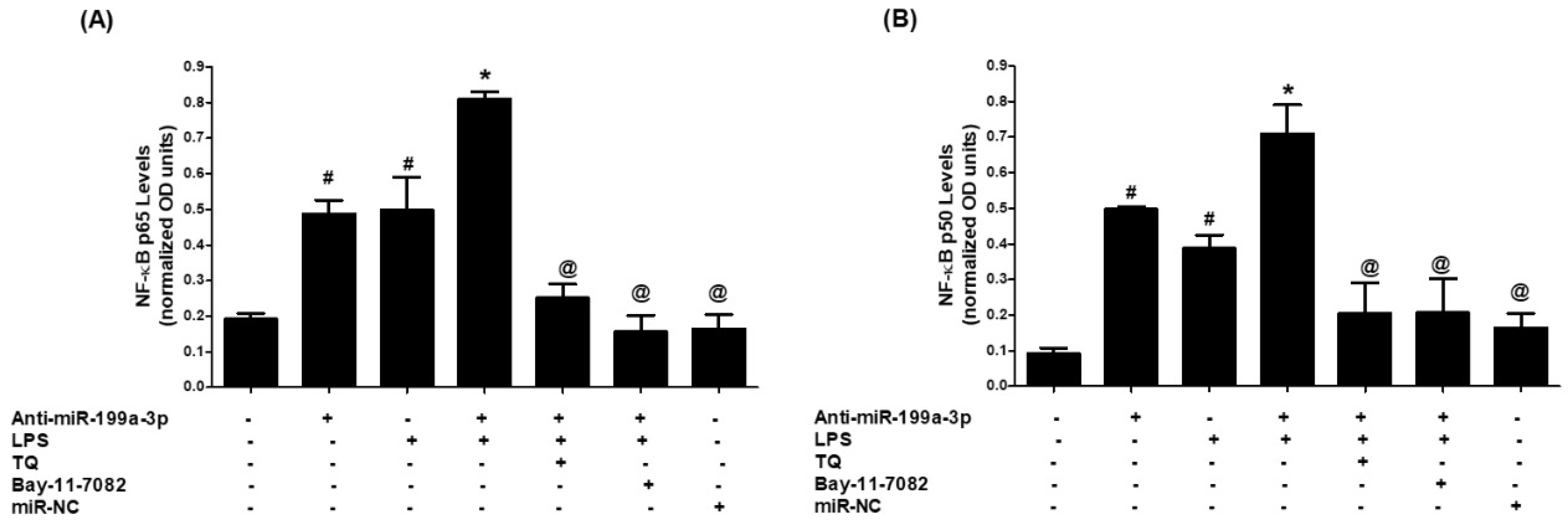
3.5. Thymoquinone Inhibits NF-κB Activation via hsa-miR-199a-3p Induction
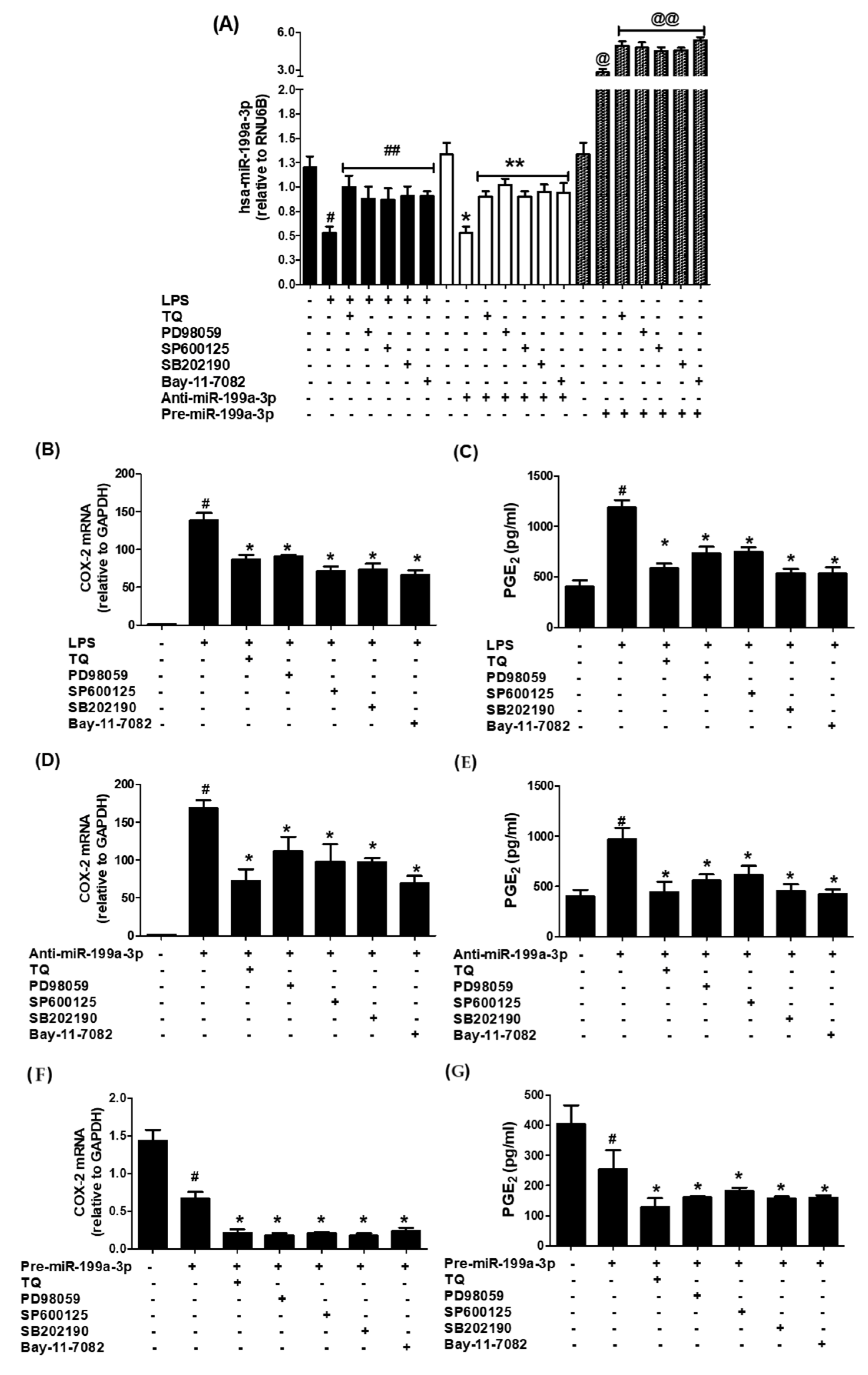
3.6. Validation of Data Using an Additional Human Lung Cancer Cell Line (SHP-77)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashrafi, A.; Akter, Z.; Modareszadeh, P.; Modareszadeh, P.; Berisha, E.; Alemi, P.S.; Chacon Castro, M.D.C.; Deese, A.R.; Zhang, L. Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance. Cancers 2022, 14, 4562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, Y.; Wakelee, H.A. Adjuvant chemotherapy of completely resected early-stage non-small cell lung cancer (NSCLC). Transl. Lung Cancer Res. 2013, 2, 403–410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Konopa, K. Do we have markers to select patients for adjuvant therapies of non-small-cell lung cancer? Ann. Oncol. 2010, 21 (Suppl. S7), vii199–vii202. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Gaspar, L.E.; Chaft, J.E.; Kennedy, E.B.; Azzoli, C.G.; Ellis, P.M.; Lin, S.H.; Pass, H.I.; Seth, R.; Shepherd, F.A.; et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Zhu, Z.R.; Tong, D.; Zhou, R.; Xiao, K.; Peng, L. MicroRNAs and Lung Cancer: A Review Focused on Targeted Genes. Explor. Res. Hypothesis Med. 2021, 6, 67–76. [Google Scholar] [CrossRef]
- Takahashi, T.; Kozaki, K.; Yatabe, Y.; Achiwa, H.; Hida, T. Increased expression of COX-2 in the development of human lung cancers. J. Environ. Pathol. Toxicol. Oncol. 2002, 21, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Parvathareddy, S.K.; Siraj, A.K.; Annaiyappanaidu, P.; Al-Sobhi, S.S.; Al-Dayel, F.; Al-Kuraya, K.S. Prognostic Significance of COX-2 Overexpression in BRAF-Mutated Middle Eastern Papillary Thyroid Carcinoma. Int. J. Mol. Sci. 2020, 21, 9498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gong, Z.; Huang, W.; Wang, B.; Liang, N.; Long, S.; Li, W.; Zhou, Q. Interplay between cyclooxygenase-2 and microRNAs in cancer (Review). Mol. Med. Rep. 2021, 23, 347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cornett, A.L.; Lutz, C.S. Regulation of COX-2 expression by miR-146a in lung cancer cells. RNA 2014, 20, 1419–1430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Cheng, J.; Mao, Y.; Jiang, M.; Fan, X. miR-21 inhibits the effects of cyclooxygenase-2 inhibitor NS398 on apoptosis and invasion in gastric cancer cells. Onco Targets Ther. 2015, 8, 3245–3253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Alharbi, A.; Alrakebeh, A.; Almansour, K.; Almadi, A.; Almuzaini, A.; Salem, M.; Aloboody, B.; Alkobair, A.; Albegami, A.; et al. Thymoquinone provides structural protection of human hemoglobin against oxidative damage: Biochemical studies. Biochimie 2022, 192, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Samarghandian, S.; Shahri, A.M.P.; Samini, F. The Neuroprotective Effects of Thymoquinone: A Review. Dose Response 2018, 16, 1559325818761455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qu, F.; Zheng, J.; Gan, W.; Lian, H.; He, H.; Li, W.; Yuan, T.; Yang, Y.; Li, X.; Ji, C.; et al. MiR-199a-3p suppresses proliferation and invasion of prostate cancer cells by targeting Smad1. Oncotarget 2017, 8, 52465–52473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akhtar, N.; Haqqi, T.M. MicroRNA-199a* regulates the expression of cyclooxygenase-2 in human chondrocytes. Ann. Rheum. Dis. 2012, 71, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rasheed, Z.; Rasheed, N.; Al-Shobaili, H.A. Epigallocatechin-3-O-gallate up-regulates microRNA-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. J. Cell. Mol. Med. 2016, 20, 2241–2248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Wang, X.; Chai, B.; Wu, Z.; Gu, Z.; Zou, H.; Zhang, H.; Li, Y.; Sun, Q.; Fang, W.; et al. miR-199a-3p/5p regulate tumorgenesis via targeting Rheb in non-small cell lung cancer. Int. J. Biol. Sci. 2022, 18, 4187–4202, Erratum in Int. J. Biol. Sci. 2023, 19, 3830. https://doi.org/10.7150/ijbs.86344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, K.C.; Renthal, N.E.; Gerard, R.D.; Mendelson, C.R. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol. Endocrinol. 2012, 26, 1857–1867, Erratum in Mol. Endocrinol. 2013, 27, 188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, W.; Li, Y.; Chai, B.; Liu, X.; Ma, Z. miR-199a: A Tumor Suppressor with Noncoding RNA Network and Therapeutic Candidate in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 8518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, L.N.; Wu, L.N.; Liu, S.W.; Zhang, X.; Luo, X.; Nawaz, S.; Ma, Z.M.; Ding, X.C. miR-199a/b-3p inhibits HCC cell proliferation and invasion through a novel compensatory signaling pathway DJ-1\Ras\PI3K/AKT. Sci Rep. 2024, 14, 224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Y.; Luo, X.; Liao, P.; Luo, Y. Impacts of LncRNA NORAD on the Proliferation, Apoptosis, and Chemosensitivity of Non-small Cell Lung Cancer Cells by Regulating ZNF217 through MiR-199a-3p. Zhongguo Fei Ai Za Zhi 2023, 26, 479–486. (In Chinese) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shatseva, T.; Lee, D.Y.; Deng, Z.; Yang, B.B. MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. J. Cell. Sci. 2011, 124 Pt 16, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chan, W.Y. Flexible and versatile as a chameleon-sophisticated functions of microRNA-199a. Int. J. Mol. Sci. 2012, 13, 8449–8466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, N.; Xia, Y.; He, N.; Zhang, L.; Liu, J. IRGM Inhibits the AKT/mTOR Signaling Pathway by Interacting with TRIM21 to Alleviate Sepsis-Induced Acute Lung Injury. Inflammation 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Makki, M.S.; Haseeb, A.; Haqqi, T.M. MicroRNA-9 promotion of interleukin-6 expression by inhibiting monocyte chemoattractant protein-induced protein 1 expression in interleukin-1β-stimulated human chondrocytes. Arthritis Rheumatol. 2015, 67, 2117–2128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.W. Preparation of Nuclear Extracts from HeLa cells. Cold Spring Harb. Protoc. 2013, 2013, 579–583, Erratum in Cold Spring Harb. Protoc. 2016, 2016, pdb-err094813. https://doi.org/10.1101/pdb.err094813. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Khera, L.; Gaur, N.; Paul, C.; Kaul, R. Role of Modulator of Inflammation Cyclooxygenase-2 in Gammaherpesvirus Mediated Tumorigenesis. Front. Microbiol. 2017, 8, 538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.E. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Zhang, J.; Sun, W.; Cao, J.; Ma, Z. COX-2 in lung cancer: Mechanisms, development, and targeted therapies. Chronic. Dis. Transl. Med. 2024, 10, 281–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.I.; Lakshmikanthan, V.; Frilot, N.; Daaka, Y. Prostaglandin E2 promotes lung cancer cell migration via EP4-betaArrestin1-c-Src signalsome. Mol. Cancer Res. 2010, 8, 569–577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, M.; Hu, S.; Liu, C.F.; Jiang, L.; Yu, W.; Li, Z.L.; Guo, W.; Tang, R.; Dong, C.Y.; Wu, T.H.; et al. BPTF cooperates with p50 NF-κB to promote COX-2 expression and tumor cell growth in lung cancer. Am. J. Transl. Res. 2019, 11, 7398–7409. [Google Scholar] [PubMed] [PubMed Central]
- Xiao, Y.; Wang, J.; Qin, Y.; Xuan, Y.; Jia, Y.; Hu, W.; Yu, W.; Dai, M.; Li, Z.; Yi, C.; et al. Ku80 cooperates with CBP to promote COX-2 expression and tumor growth. Oncotarget 2015, 6, 8046–8061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duan, Z.; Choy, E.; Harmon, D.; Liu, X.; Susa, M.; Mankin, H.; Hornicek, F. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol. Cancer Ther. 2011, 10, 1337–1345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Y.; Feng, Y.; Shen, J.K.; Lin, M.; Choy, E.; Cote, G.M.; Harmon, D.C.; Mankin, H.J.; Hornicek, F.J.; Duan, Z. CD44 is a direct target of miR-199a-3p and contributes to aggressive progression in osteosarcoma. Sci. Rep. 2015, 5, 11365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, R.; Liu, C.; Zhang, D.; Liu, B.; Chen, X.; Rycaj, K.; Jeter, C.; Calhoun-Davis, T.; Li, Y.; Yang, T.; et al. miR-199a-3p targets stemness-related and mitogenic signaling pathways to suppress the expansion and tumorigenic capabilities of prostate cancer stem cells. Oncotarget 2016, 7, 56628–56642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou, J.; Lin, L.; Zhou, W.; Wang, Z.; Ding, G.; Dong, Q.; Qin, L.; Wu, X.; Zheng, Y.; Yang, Y.; et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011, 19, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Dasgupta, D.; Ghosh, A.; Roychoudhury, S.; Kumar, D.; Gorain, M.; Butti, R.; Datta, S.; Agarwal, S.; Gupta, S.; et al. MiRNA199a-3p suppresses tumor growth, migration, invasion and angiogenesis in hepatocellular carcinoma by targeting VEGFA, VEGFR1, VEGFR2, HGF and MMP2. Cell Death Dis. 2017, 8, e2706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, J.C.; Chen, J.; Cheuk, I.W.; Siu, M.T.; Shin, V.Y.; Kwong, A. MicroRNA-199a-3p promotes drug sensitivity in triple negative breast cancer by down-regulation of BRCA1. Am. J. Transl. Res. 2022, 14, 2021–2036. [Google Scholar] [PubMed] [PubMed Central]
- Pottoo, F.H.; Ibrahim, A.M.; Alammar, A.; Alsinan, R.; Aleid, M.; Alshehhi, A.; Alshehri, M.; Mishra, S.; Alhajri, N. Thymoquinone: Review of Its Potential in the Treatment of Neurological Diseases. Pharmaceuticals 2022, 15, 408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pradhan, R.; Singhvi, G.; Dubey, S.K.; Gupta, G.; Dua, K. MAPK pathway: A potential target for the treatment of non-small-cell lung carcinoma. Future Med. Chem. 2019, 11, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Burotto, M.; Chiou, V.L.; Lee, J.M.; Kohn, E.C. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rasheed, Z.; Akhtar, N.; Haqqi, T.M. Cytokine Networks and MAPKs—Toward New Therapeutic Targets for Rheumatoid Arthritis. Cytokines 2011, 31–43. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/b11037-4/cytokine-networks-mapks%E2%80%94toward-new-therapeutic-targets-rheumatoid-arthritis-zafar-rasheed-nahid-akhtar-tariq-haqqi (accessed on 24 September 2025).
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alghasham, A.; Rasheed, Z. Therapeutic targets for rheumatoid arthritis: Progress and promises. Autoimmunity 2014, 47, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yi, Y.; Han, C.; Shi, B. NF-κB signaling pathway in tumor microenvironment. Front. Immunol. 2024, 15, 1476030. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Q.; Hao, S.; Hong, W.; Tergaonkar, V.; Sethi, G.; Tian, Y.; Duan, C. Versatile function of NF-ĸB in inflammation and cancer. Exp. Hematol. Oncol. 2024, 13, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koros, A.M.; Klein, E.C.; Pan, S.; Atchison, R.W.; Lakomy, R.; Bahnson, A.; Sherer, C. Stability and utility of the unique human small cell carcinoma line SHP-77. Cancer Res. 1985, 45, 2725–2731. [Google Scholar] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Y.S.; Farhana, A.; Mohammed, G.E.Y.; Osman, A.A.; Alsrhani, A.; Shahid, S.M.A.; Kuddus, M.; Rasheed, Z. Thymoquinone Upregulates microRNA-199a-3p and Downregulates COX-2 Expression and PGE2 Production via Deactivation of p38/ERK/JNK-MAPKs and p65/p50-NF-κB Signaling in Human Lung Cancer Cells. Biology 2025, 14, 1348. https://doi.org/10.3390/biology14101348
Khan YS, Farhana A, Mohammed GEY, Osman AA, Alsrhani A, Shahid SMA, Kuddus M, Rasheed Z. Thymoquinone Upregulates microRNA-199a-3p and Downregulates COX-2 Expression and PGE2 Production via Deactivation of p38/ERK/JNK-MAPKs and p65/p50-NF-κB Signaling in Human Lung Cancer Cells. Biology. 2025; 14(10):1348. https://doi.org/10.3390/biology14101348
Chicago/Turabian StyleKhan, Yusuf Saleem, Aisha Farhana, Ghorashy E. Y. Mohammed, Abuzar Abdulwahab Osman, Abdullah Alsrhani, Syed M. A. Shahid, Mohammed Kuddus, and Zafar Rasheed. 2025. "Thymoquinone Upregulates microRNA-199a-3p and Downregulates COX-2 Expression and PGE2 Production via Deactivation of p38/ERK/JNK-MAPKs and p65/p50-NF-κB Signaling in Human Lung Cancer Cells" Biology 14, no. 10: 1348. https://doi.org/10.3390/biology14101348
APA StyleKhan, Y. S., Farhana, A., Mohammed, G. E. Y., Osman, A. A., Alsrhani, A., Shahid, S. M. A., Kuddus, M., & Rasheed, Z. (2025). Thymoquinone Upregulates microRNA-199a-3p and Downregulates COX-2 Expression and PGE2 Production via Deactivation of p38/ERK/JNK-MAPKs and p65/p50-NF-κB Signaling in Human Lung Cancer Cells. Biology, 14(10), 1348. https://doi.org/10.3390/biology14101348






