Comprehensive Identification and Expression Profiling of the VQ Motif-Containing Gene Family in Brassica juncea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Leaf Sampling and Transcriptome Analysis
2.3. Identification of the VQ Gene Family in Mustard
2.4. Gene Structure, Conserved Motif, and Promoter Analysis
2.5. Multiple Sequence Alignment and Phylogenetic Analysis
2.6. Chromosomal Location and Duplication Analysis
2.7. Expression Analysis of BjuVQ Genes Based on Transcriptome Data
2.8. QRT-PCR Expression Analysis
3. Results
3.1. Identification and Characterization of VQ Family Genes in Brassica juncea
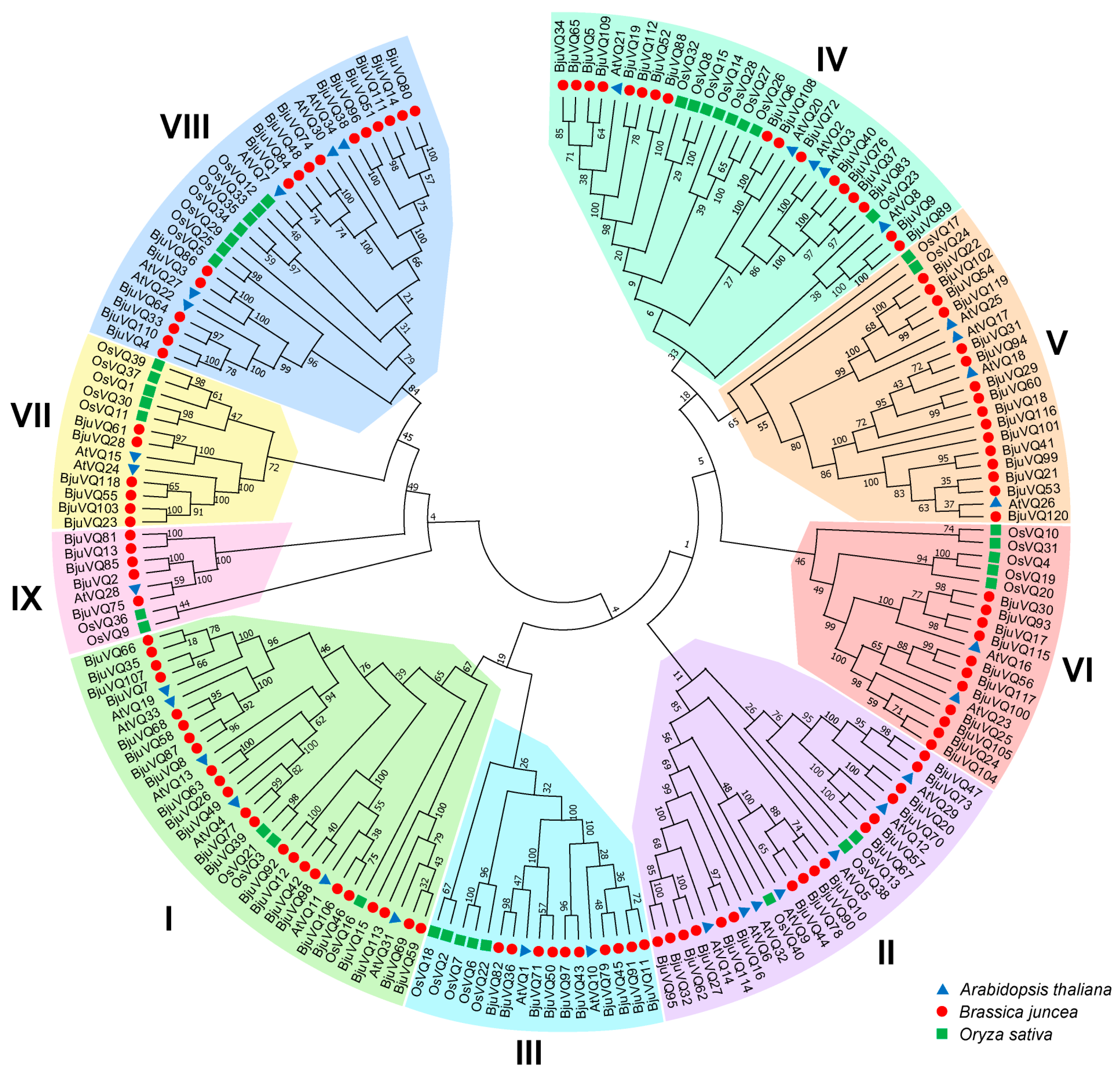
3.2. Phylogenetic Analysis of VQ Proteins from Different Plant Species
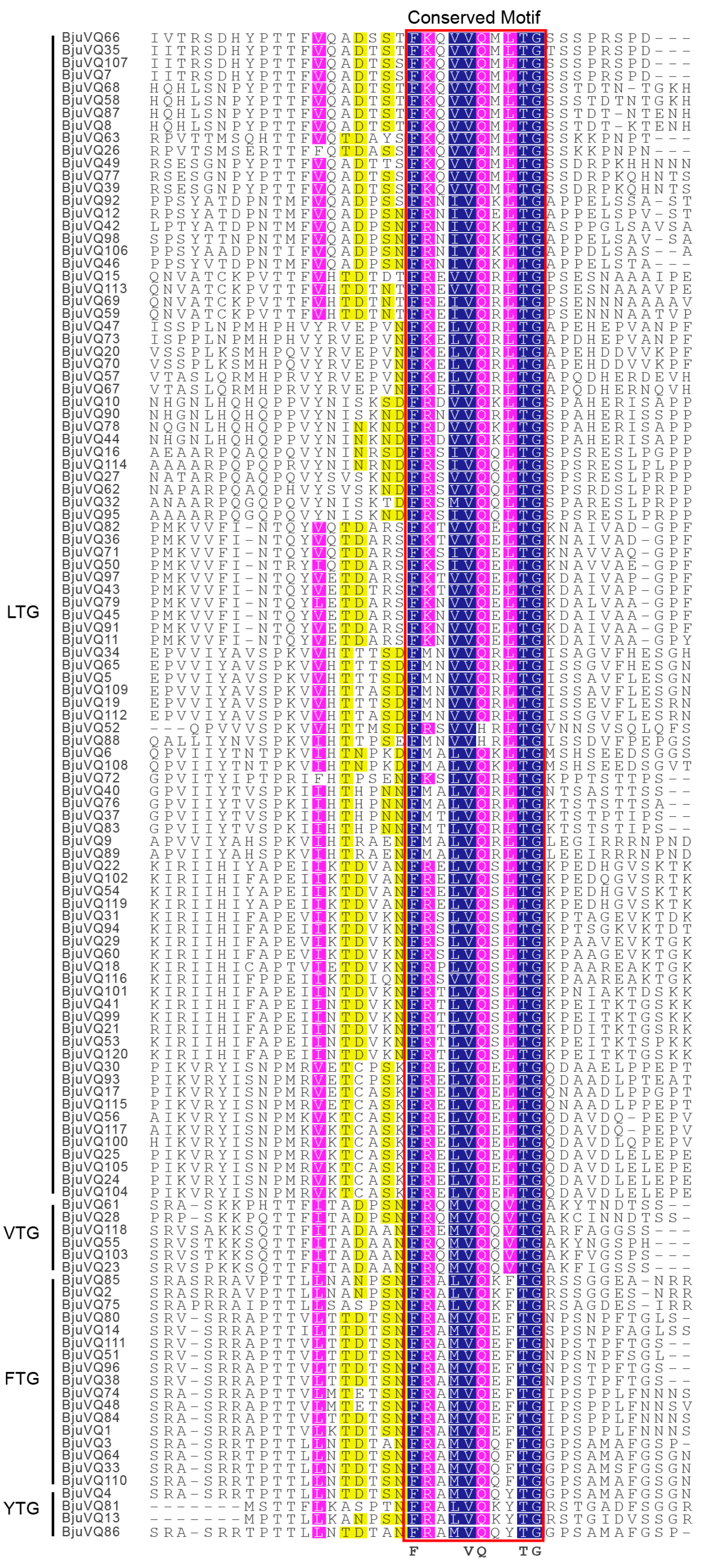
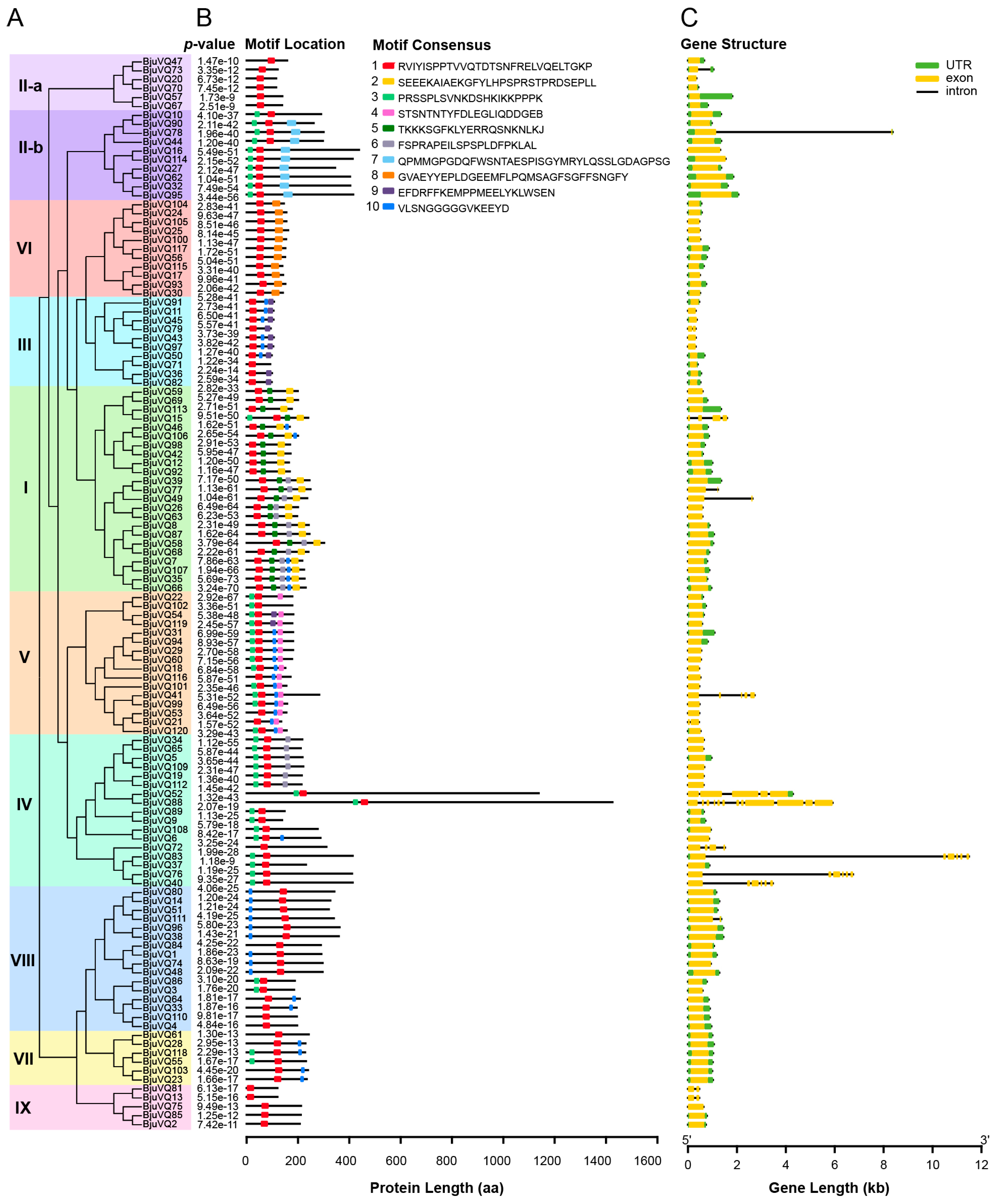
3.3. Conserved Motif and Gene Structural Analysis of BjuVQs
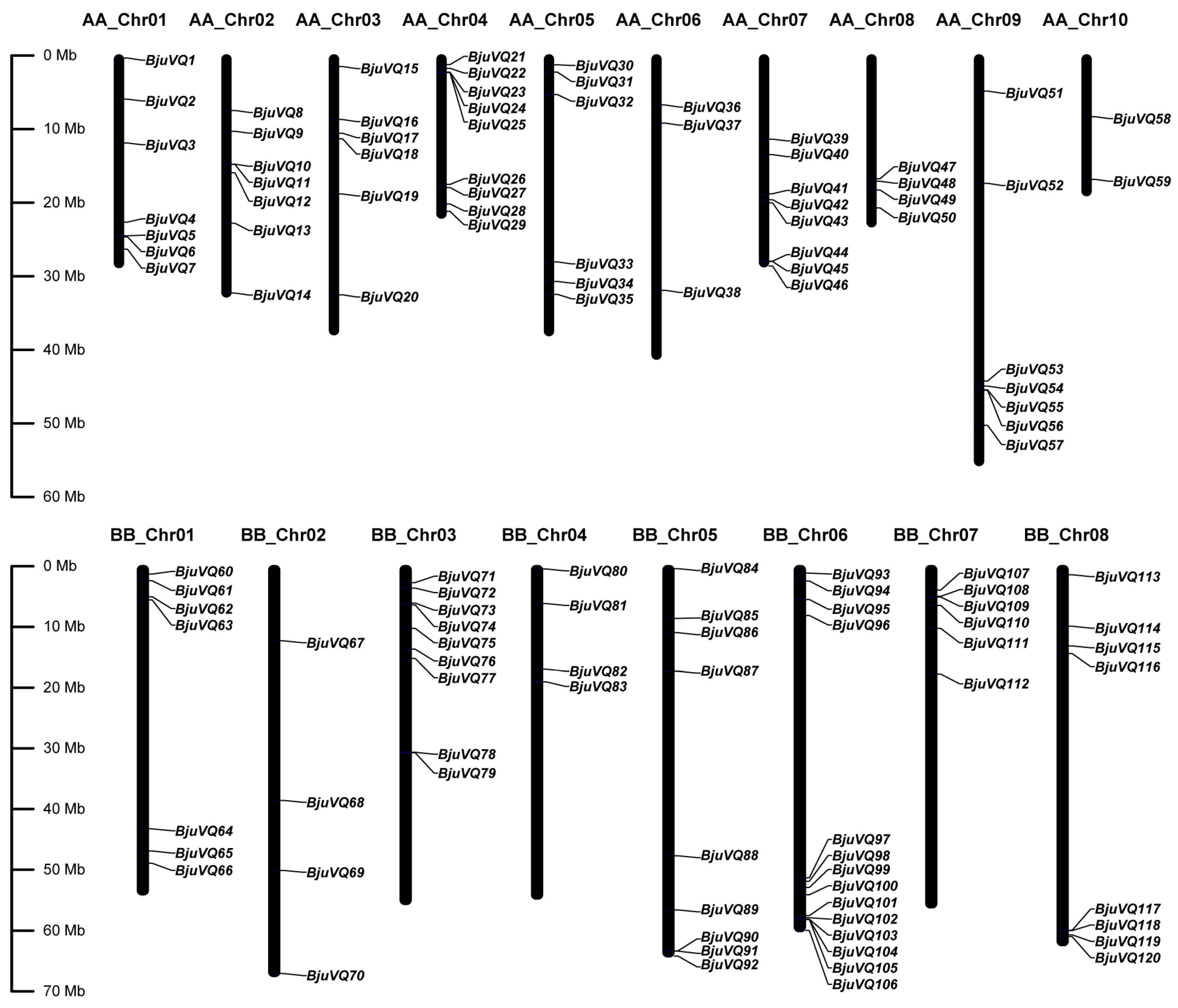
3.4. Genome Distribution and Gene Duplication of BjuVQ Genes
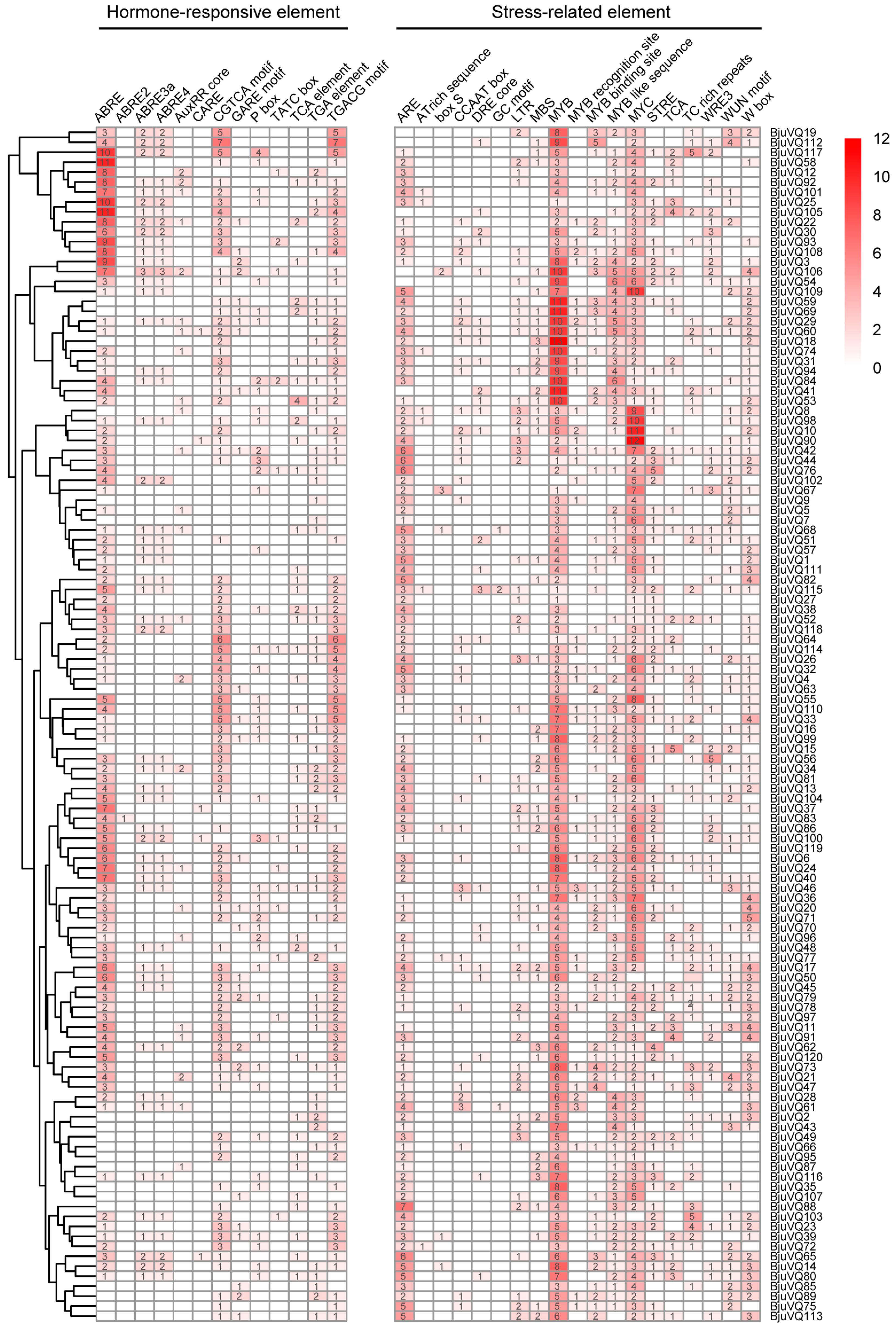
3.5. Analysis of cis-Elements in the Promoters of the BjuVQ Genes
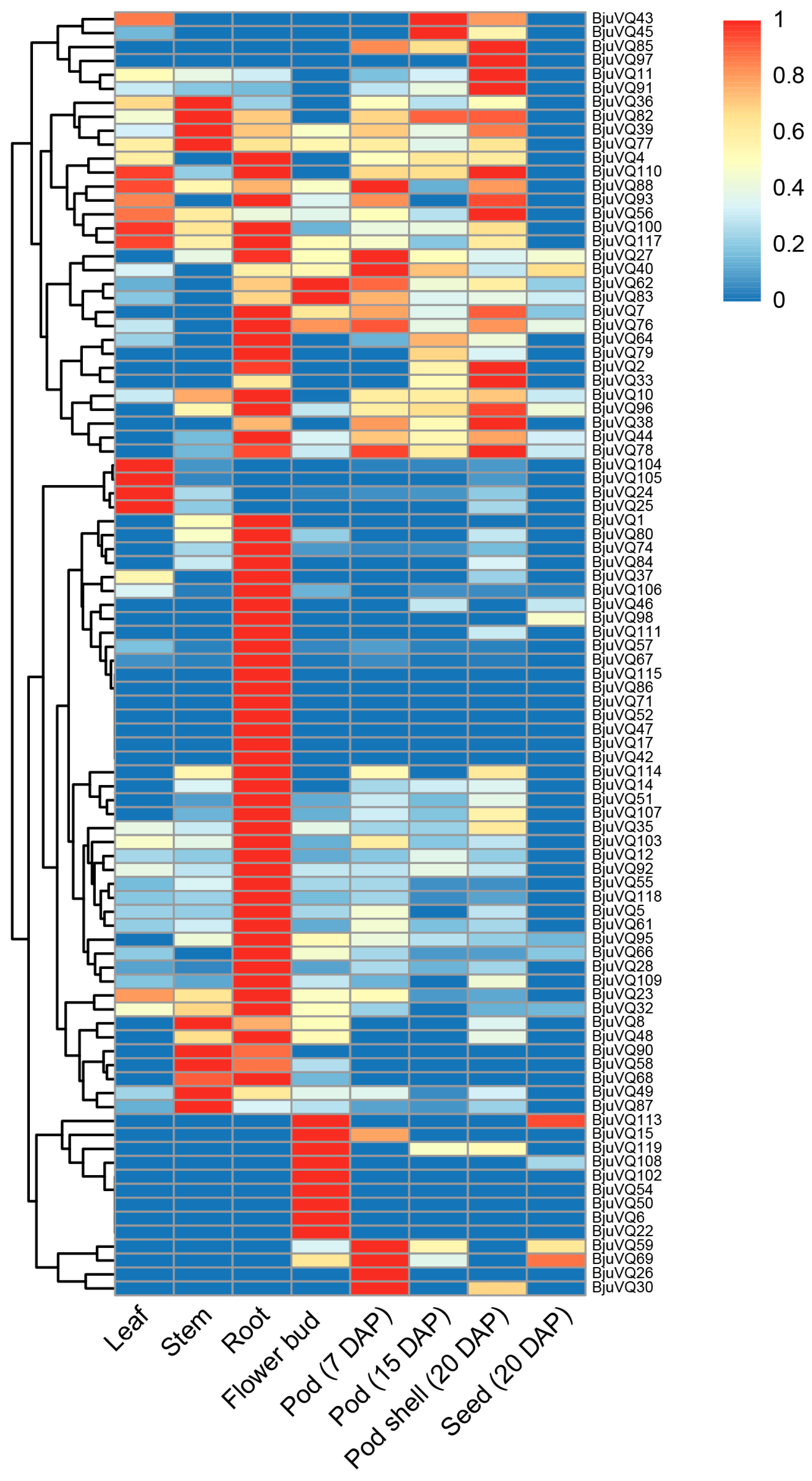
3.6. Organ-Specific Expression Patterns of BjuVQ Genes
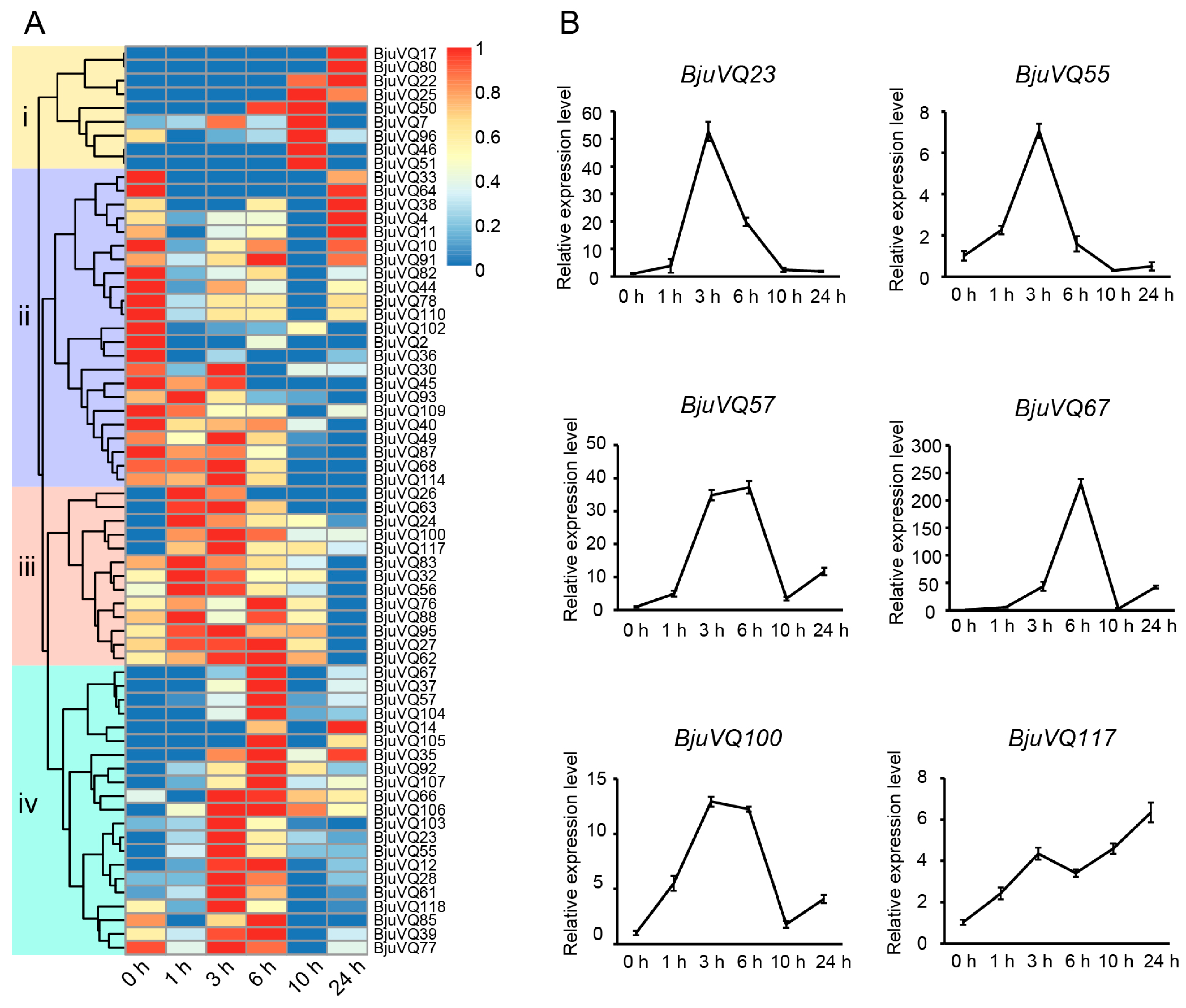
3.7. Expression Patterns of BjuVQ Genes in Response to Cold Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazemi-Shahandashti, S.S.; Maali-Amiri, R.; Zeinali, H.; Khazaei, M.; Talei, A.; Ramezanpour, S.S. Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings. J. Plant Physiol. 2014, 171, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef]
- Ye, Y.J.; Xiao, Y.Y.; Han, Y.C.; Shan, W.; Fan, Z.Q.; Xu, Q.G.; Kuang, J.F.; Lu, W.J.; Lakshmanan, P.; Chen, J.Y. Banana fruit VQ motif-containing protein5 represses cold-responsive transcription factor MaWRKY26 involved in the regulation of JA biosynthetic genes. Sci. Rep. 2016, 6, 23632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Yang, D.; Yin, Z.; Jiang, Y.; Ling, H.; Huang, N.; Zhang, D.; Wu, J.; Liu, L.; et al. A comprehensive identification and expression analysis of VQ motif-containing proteins in sugarcane (Saccharum spontaneum L.) under phytohormone treatment and cold stress. Int. J. Mol. Sci. 2022, 23, 6334. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, L.; Wang, H.; Zhang, L.; Wang, F.; Yu, D. Arabidopsis transcription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. Plant J. 2013, 74, 730–745. [Google Scholar] [CrossRef]
- Dong, Q.; Duan, D.; He, J.; Zheng, W.; Huang, D.; Wang, Q.; Yang, J.; Ma, F.; Mao, K. Overexpression of MdVQ37 reduces salt stress tolerance in Malus domestica. Sci. Hortic. 2022, 300, 111077. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Y.; Xiong, R.; Gao, Y.; Yan, H.; Xiang, Y. A Moso bamboo gene VQ28 confers salt tolerance to transgenic Arabidopsis plants. Planta 2020, 251, 99. [Google Scholar] [CrossRef]
- Cheng, X.; Yao, H.; Cheng, Z.; Tian, B.; Gao, C.; Gao, W.; Yan, S.; Cao, J.; Pan, X.; Lu, J.; et al. The wheat gene TaVQ14 confers salt and drought tolerance in transgenic Arabidopsis thaliana plants. Front. Plant Sci. 2022, 13, 870586. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kwon, S.I.; Choi, C.; Lee, H.; Ahn, I.; Park, S.R.; Bae, S.C.; Lee, S.C.; Hwang, D.J. Expression analysis of rice VQ genes in response to biotic and abiotic stresses. Gene 2013, 529, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Sun, G.; Jin, Y.; Qiu, L. Identification of active VQ motif-containing genes and the expression patterns under low nitrogen treatment in soybean. Gene 2014, 543, 237–243. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.; Pan, J.; Yu, D. Arabidopsis VQ motif-containing proteins VQ12 and VQ29 negatively modulate basal defense against Botrytis cinerea. Sci. Rep. 2015, 5, 14185. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Li, Y.; Pan, J.; Hu, Y.; Yu, D. Arabidopsis VQ10 interacts with WRKY8 to modulate basal defense against Botrytis cinerea. J. Integr. Plant Biol. 2018, 60, 956–969. [Google Scholar] [CrossRef]
- Uji, Y.; Kashihara, K.; Kiyama, H.; Mochizuki, S.; Akimitsu, K.; Gomi, K. Jasmonic acid-induced VQ-motif-containing protein OsVQ13 influences the OsWRKY45 signaling pathway and grain size by associating with OsMPK6 in rice. Int. J. Mol. Sci. 2019, 20, 2917. [Google Scholar] [CrossRef]
- Jing, Y.; Lin, R. The VQ motif-containing protein family of plant-specific transcriptional regulators. Plant Physiol. 2015, 169, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Qian, Y.; Ren, Y.; Guan, Y.; Wu, X.; Ge, C.; Ding, H. The role of plant-specific VQ motif-containing proteins: An ever-thickening plot. Plant Physiol. Biochem. 2021, 159, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Li, X.; Ma, Z.; Lv, Y.; Hu, Y.; Yu, D. Arabidopsis WRKY2 and WRKY34 transcription factors interact with VQ20 protein to modulate pollen development and function. Plant J. 2017, 91, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ling, L.; Huang, X.; Wang, W.; Wang, Y.; Zhang, M.; Chen, S.; Zhou, F.; Qi, Y.; Liang, C.; et al. Genome-wide identification and expression analysis of the VQ gene family in sunflower (Helianthus annuus L.). J. Plant Biochem. Biotechnol. 2020, 30, 56–66. [Google Scholar] [CrossRef]
- Ding, H.; Yuan, G.; Mo, S.; Qian, Y.; Wu, Y.; Chen, Q.; Xu, X.; Wu, X.; Ge, C. Genome-wide analysis of the plant-specific VQ motif-containing proteins in tomato (Solanum lycopersicum) and characterization of SlVQ6 in thermotolerance. Plant Physiol. Biochem. 2019, 143, 29–39. [Google Scholar] [CrossRef]
- Lai, W.; Zhu, C.; Yang, S.; Hu, Z.; Liu, S.; Zhou, Y. Comprehensive identification of the VQ family genes in cucumber and their roles in response to abiotic and biotic stresses. Sci. Hortic. 2022, 295, 110874. [Google Scholar] [CrossRef]
- Li, Y.; Jing, Y.; Li, J.; Xu, G.; Lin, R. Arabidopsis VQ motif-containing protein29 represses seedling deetiolation by interacting with phytochrome-interacting factor1. Plant Physiol. 2014, 164, 2068–2080. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Zhou, C.; Timko, M.P. Genome-wide identification of the VQ protein gene family of tobacco (Nicotiana tabacum L.) and analysis of its expression in response to phytohormones and abiotic and biotic stresses. Genes 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhao, H.; Zhang, X.; Lei, L.; Lai, J. Genome-wide identification of VQ motif-containing proteins and their expression profiles under abiotic stresses in maize. Front. Plant Sci. 2015, 6, 1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, K.; Han, Y.; Yan, L.; Zheng, Y.; Bi, Z.; Zhang, X.; Zhang, X.; Min, D. Genome-wide analysis of the VQ motif-containing gene family and expression profiles during phytohormones and abiotic stresses in wheat (Triticum aestivum L.). BMC Genom. 2022, 23, 292. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wei, F.; Cheng, S.; Ma, L.; Wang, H.; Zhang, M.; Mao, G.; Lu, J.; Hao, P.; Ahmad, A.; et al. A comprehensive analysis of cotton VQ gene superfamily reveals their potential and extensive roles in regulating cotton abiotic stress. BMC Genom. 2020, 21, 795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, F.; Li, J.; Ding, Q.; Zhang, Y.; Li, H.; Zhang, J.; Gao, J. Genome-wide identification and analysis of the VQ motif-containing protein family in Chinese cabbage (Brassica rapa L. ssp. Pekinensis). Int. J. Mol. Sci. 2015, 16, 28683–28704. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, F.; Huang, S.; Fernando, W.G.D. Genome-wide identification and analysis of the valine-glutamine motif-containing gene family in Brassica napus and functional characterization of BnMKS1 in response to Leptosphaeria maculans. Phytopathology 2021, 111, 281–292. [Google Scholar] [CrossRef]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Sun, P.; Jiao, B.; Yang, Y.; Shan, L.; Li, T.; Li, X.; Xi, Z.; Wang, X.; Liu, J. WGDI: A user-friendly toolkit for evolutionary analyses of whole-genome duplications and ancestral karyotypes. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, J.; Yang, J.; Yu, Y.; Yang, Y.; Wang, W. Identification, characterization and expression analysis of the VQ motif-containing gene family in tea plant (Camellia sinensis). BMC Genom. 2018, 19, 710. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhou, Y.; Yang, Y.; Chi, Y.J.; Zhou, J.; Chen, J.Y.; Wang, F.; Fan, B.; Shi, K.; Zhou, Y.H.; et al. Structural and functional analysis of VQ motif-containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiol. 2012, 159, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Vannozzi, A.; Wang, G.; Zhong, Y.; Corso, M.; Cavallini, E.; Cheng, Z.M. A comprehensive survey of the grapevine VQ gene family and its transcriptional correlation with WRKY proteins. Front. Plant Sci. 2015, 6, 417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Zhu, D.; Gao, Y.; Yan, H.; Xiang, Y. Genome-wide analysis of VQ motif-containing proteins in Moso bamboo (Phyllostachys edulis). Planta 2017, 246, 165–181. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Li, H.; Yang, Y.; Guang, Y.; Zhou, Y. The bZIP gene family in watermelon: Genome-wide identification and expression analysis under cold stress and root-knot nematode infection. PeerJ 2019, 7, e7878. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Y.; Sevugan, M.; Ramachandran, S. Valine-glutamine (VQ) motif coding genes are ancient and non-plant-specific with comprehensive expression regulation by various biotic and abiotic stresses. BMC Genom. 2018, 19, 342. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, P. Genome-wide identification and expression analysis of the VQ gene family in Cucurbita pepo L. PeerJ 2022, 10, e12827. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Ji, Y.; Jing, Y.; Li, L.; Chen, Y.; Wang, R.; Zhang, H.; Yu, D.; Chen, L. Arabidopsis SIGMA FACTOR BINDING PROTEIN1 (SIB1) and SIB2 inhibit WRKY75 function in abscisic acid-mediated leaf senescence and seed germination. J. Exp. Bot. 2022, 73, 182–196. [Google Scholar] [CrossRef]
- Lai, Z.; Li, Y.; Wang, F.; Cheng, Y.; Fan, B.; Yu, J.Q.; Chen, Z. Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell 2011, 23, 3824–3841. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, B. Characterization of VQ motif-containing protein family and their expression patterns under phytohormones and abiotic stresses in melon (Cucumis melo L.). Plant Growth Regul. 2019, 89, 273–285. [Google Scholar]
- Yan, H.; Wang, Y.; Hu, B.; Qiu, Z.; Zeng, B.; Fan, C. Genome-wide characterization, evolution, and expression profiling of VQ gene family in response to phytohormone treatments and abiotic stress in Eucalyptus grandis. Int. J. Mol. Sci. 2019, 20, 1765. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Li, Z.; Zhao, Y.; Tan, W.; Liu, Z.; Cui, S.; Yu, X.; Ma, J.; Wang, G.; et al. Genome-wide identification and expression analysis of the VQ gene family in soybean (Glycine max). PeerJ 2019, 7, e7509. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Garcia, D.; Zhang, H.; Feng, K.; Chaudhury, A.; Berger, F.; Peacock, W.J.; Dennis, E.S.; Luo, M. The VQ motif protein IKU1 regulates endosperm growth and seed size in Arabidopsis. Plant J. 2010, 63, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhao, Y.; Jiang, Q.; Yang, J.; Zhao, W.; Taylor, I.A.; Peng, Y.L.; Wang, D.; Liu, J. Structural basis of dimerization and dual W-box DNA recognition by rice WRKY domain. Nucleic Acids Res. 2019, 47, 4308–4318. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhao, S.; Duan, D.; Tian, Y.; Wang, Y.; Mao, K.; Zhou, Z.; Ma, F. Structural and functional analyses of genes encoding VQ proteins in apple. Plant Sci. 2018, 272, 208–219. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Li, H.; Guo, Z.; Zhuang, X.; Huang, W.; Mao, C.; Feng, H.; Zhang, Y.; Wu, H.; Zhou, Y. Comprehensive Identification and Expression Profiling of the VQ Motif-Containing Gene Family in Brassica juncea. Biology 2022, 11, 1814. https://doi.org/10.3390/biology11121814
Zheng J, Li H, Guo Z, Zhuang X, Huang W, Mao C, Feng H, Zhang Y, Wu H, Zhou Y. Comprehensive Identification and Expression Profiling of the VQ Motif-Containing Gene Family in Brassica juncea. Biology. 2022; 11(12):1814. https://doi.org/10.3390/biology11121814
Chicago/Turabian StyleZheng, Jie, Haibo Li, Ziqi Guo, Xiaoman Zhuang, Weifeng Huang, Cui Mao, Huimin Feng, Yang Zhang, Hao Wu, and Yong Zhou. 2022. "Comprehensive Identification and Expression Profiling of the VQ Motif-Containing Gene Family in Brassica juncea" Biology 11, no. 12: 1814. https://doi.org/10.3390/biology11121814
APA StyleZheng, J., Li, H., Guo, Z., Zhuang, X., Huang, W., Mao, C., Feng, H., Zhang, Y., Wu, H., & Zhou, Y. (2022). Comprehensive Identification and Expression Profiling of the VQ Motif-Containing Gene Family in Brassica juncea. Biology, 11(12), 1814. https://doi.org/10.3390/biology11121814






