Lichen Biomonitoring of Airborne Microplastics in Milan (N Italy)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
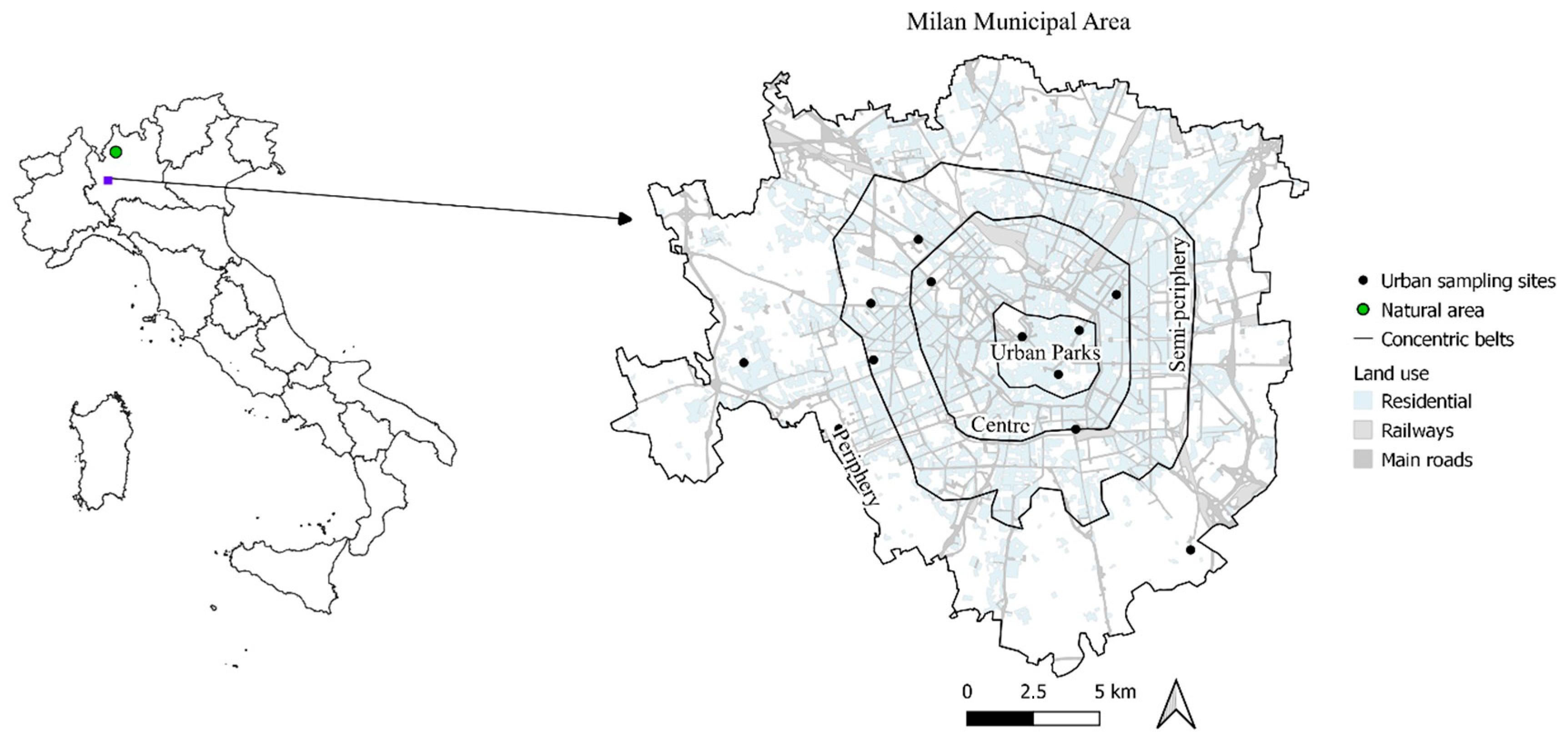
2.1. Study Area
2.2. Experimental
2.3. Microplastic Analysis
2.4. Estimation of MP Deposition Rates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic marine system: An emerging area of research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- GESAMP (IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). In Proceedings of the GESAMP International Workshop on Plastic Particles as a Vector in Transporting Persistent, Bio-Accumulating and Toxic Substances in the Oceans; GESAMP Rep. Stud. No. 82; Bowmer, T., Kershaw, P.J., Eds.; UNESCO-IOC, Paris 2010; 68p. Available online: http://www.gesamp.org/publications/proceedings-of-the-gesamp-workshop-on-microplastic-particles (accessed on 11 July 2022).
- Rezaei, M.; Riksen, M.J.; Sirjani, E.; Sameni, A.; Geissen, V. Wind erosion as a driver for transport of light density microplastics. Sci. Total Environ. 2019, 669, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zheng, K.; Zhu, Z.; Chen, G.; Peng, X. Distribution, sedimentary record, and persistence of microplastics in the Pearl River catchment, China. Environ. Pollut. 2019, 251, 862–870. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Wegner, A.; Foekema, E.M. Plastic as a carrier of POPs to aquatic organisms: A model analysis. Environ. Sci. Technol. 2013, 47, 7812–7820. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.A. Adsorption of trace metals by microplastic pellets in fresh water. Environ. Chem. 2015, 12, 600–610. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef]
- Webb, S.; Gaw, S.; Marsden, I.D.; McRae, N.K. Biomarker responses in New Zealand green-lipped mussels Perna canaliculus exposed to microplastics and triclosan. Ecotoxicol. Environ. Saf. 2020, 201, 110871. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää. Atmospheric microplastics: A review on current status and perspectives. Earth-Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Roblin, B.; Ryan, M.; Vreugdenhil, A.; Aherne, J. Ambient atmospheric deposition of anthropogenic microfibers and microplastics on the western periphery of Europe (Ireland). Environ. Sci. Technol. 2020, 54, 11100–11108. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; Van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ambient air pollution: A global assessment of exposure and burden of disease. Clean Air J. 2016, 26, 6. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- Conti, M.E.; Cecchetti, G. Biological monitoring: Lichens as bioindicators of air pollution assessment—A review. Environ. Pollut. 2001, 114, 471–492. [Google Scholar] [CrossRef]
- Loppi, S.; Paoli, L. Comparison of the trace element content in transplants of the lichen Evernia prunastri and in bulk atmospheric deposition: A case study from a low polluted environment (C Italy). Biologia 2015, 70, 460–466. [Google Scholar] [CrossRef]
- Loppi, S.; Ravera, S.; Paoli, L. Coping with uncertainty in the assessment of atmospheric pollution with lichen transplants. Environ. Forensics 2019, 20, 228–233. [Google Scholar] [CrossRef]
- Loppi, S.; Roblin, B.; Paoli, L.; Aherne, J. Accumulation of airborne microplastics in lichens from a landfill dumping site (Italy). Sci. Rep. 2021, 11, 4564. [Google Scholar] [CrossRef] [PubMed]
- Robotto, A.; Barbero, S.; Bracco, P.; Cremonini, R.; Ravina, M.; Brizio, E. Improving Air Quality Standards in Europe: Comparative Analysis of Regional Differences, with a Focus on Northern Italy. Atmosphere 2022, 13, 642. [Google Scholar] [CrossRef]
- Contardo, T.; Vannini, A.; Sharma, K.; Giordani, P.; Loppi, S. Disentangling sources of trace element air pollution in complex urban areas by lichen biomonitoring. A case study in Milan (Italy). Chemosphere 2020, 256, 127155. [Google Scholar] [CrossRef] [PubMed]
- Roblin, B.; Aherne, J. Moss as a biomonitor for the atmospheric deposition of anthropogenic microfibres. Sci. Total Environ. 2020, 715, 136973. [Google Scholar] [CrossRef]
- Windsor, F.M.; Tilley, R.M.; Tyler, C.R.; Ormerod, S.J. Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environ. 2019, 646, 68–74. [Google Scholar] [CrossRef]
- Norén, F. Small plastic particles in coastal Swedish waters. Kimo Swed. 2007, 11, 1–11. [Google Scholar]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 11 July 2022).
- Kauppi, M. Fruticose lichen transplant technique for air pollution experiments. Flora 1976, 165, 407–414. [Google Scholar] [CrossRef]
- Ferry, B.W.; Coppins, B.J. Lichen transplant experiments and air pollution studies. Lichenologist 1979, 11, 63–73. [Google Scholar] [CrossRef]
- Wolterbeek, H. Use of lichen transplants in atmospheric deposition studies. J. Radioanal. Nucl. Chem. 2004, 249, 307–315. [Google Scholar] [CrossRef]
- Ayrault, S.; Clochiatti, R.; Carrot, F.; Daudin, L.; Bennet, J.P. Factors to consider for trace element deposition biomonitoring surveys with lichen transplants. Sci. Total Environ. 2007, 372, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Beaurepaire, M.; Dris, R.; Gasperi, J.; Tassin, B. Microplastics in the atmospheric compartment: A comprehensive review on methods, results on their occurrence and determining factors. Curr. Opin. Food Sci. 2021, 41, 159–168. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, Y.; Jones, T.; Santosh, M.; Liu, P.; Zhang, M.; Xu, L.; Li, W.; Lu, J.; Yang, C.X.; et al. Airborne microplastics: A review of current perspectives and environmental implications. J. Clean. Prod. 2022, 347, 131048. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Gilmour, P.S.; Brown, D.M.; MacNee, W. Ultrafine particles: Mechanisms of lung injury. Philos. Trans. A Math. Phys. Eng. Sci. 2000, 358, 2741–2749. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. 2017, 24, 24928–24935. [Google Scholar] [CrossRef]
- Szewc, K.; Graca, B.; Dołęga, A. Atmospheric deposition of microplastics in the coastal zone: Characteristics and relationship with meteorological factors. Sci. Total Environ. 2021, 761, 143272. [Google Scholar] [CrossRef]
| Summary Variable (Unit) | Control | Urban Parks | Centre | Semi-Periphery | Periphery |
|---|---|---|---|---|---|
| Lichen samples (n) | 3 | 9 | 9 | 9 | 9 |
| MPs (nr/gr dw) | 20 ± 4 c | 26 ± 1 c | 44 ± 1 b | 48 ± 3 ab | 56 ± 5 a |
| Fibres (%) | 100 ± 0 | 91 ± 5 | 97 ± 3 | 95 ± 2 | 93 ± 5 |
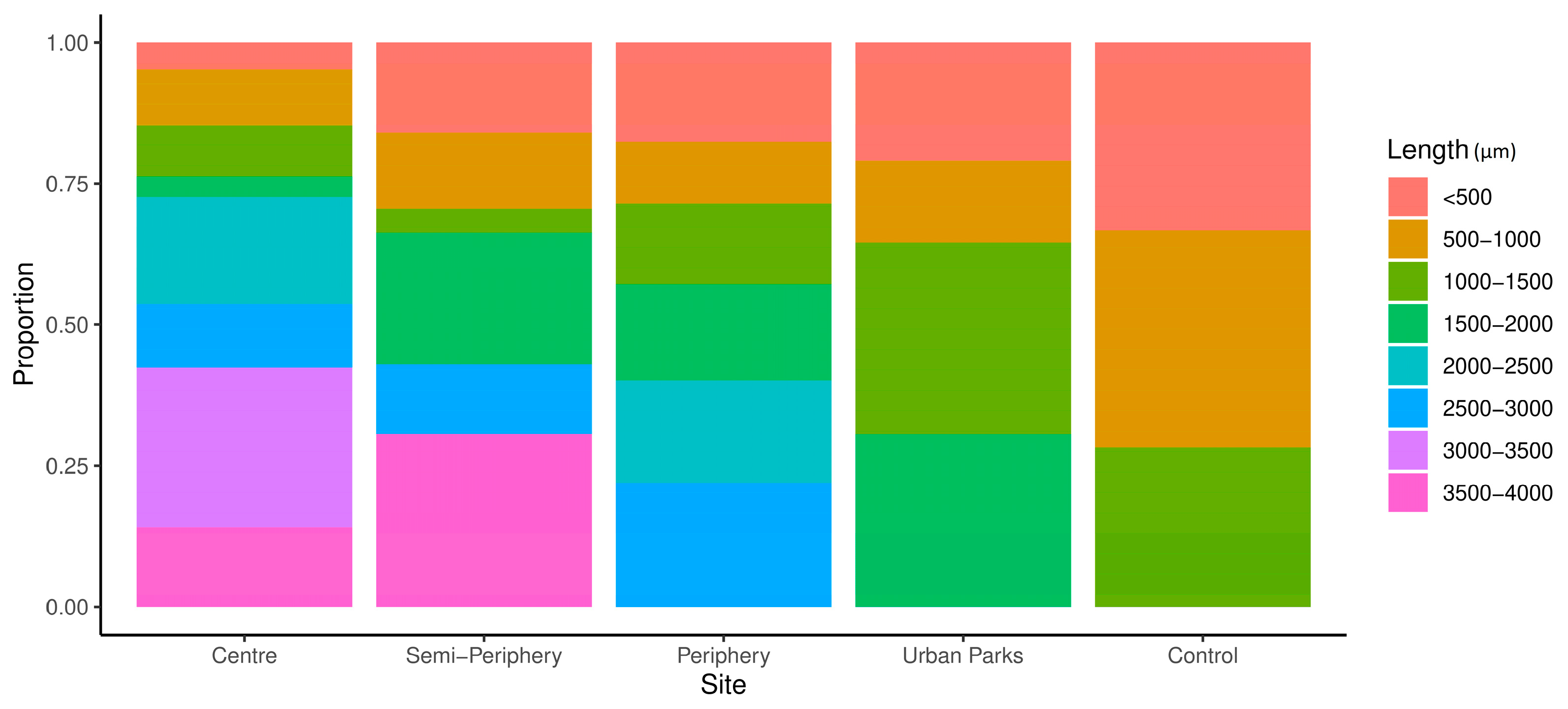
| Fibre length (µm) | 616 ± 92 | 867 ± 146 | 1076 ± 156 | 951 ± 143 | 885 ± 117 |
| Deposition (MPs/m2/d) | 21–43 | 43–50 | 75–82 | 76–91 | 91–119 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafarova, M.; Contardo, T.; Aherne, J.; Loppi, S. Lichen Biomonitoring of Airborne Microplastics in Milan (N Italy). Biology 2022, 11, 1815. https://doi.org/10.3390/biology11121815
Jafarova M, Contardo T, Aherne J, Loppi S. Lichen Biomonitoring of Airborne Microplastics in Milan (N Italy). Biology. 2022; 11(12):1815. https://doi.org/10.3390/biology11121815
Chicago/Turabian StyleJafarova, Mehriban, Tania Contardo, Julian Aherne, and Stefano Loppi. 2022. "Lichen Biomonitoring of Airborne Microplastics in Milan (N Italy)" Biology 11, no. 12: 1815. https://doi.org/10.3390/biology11121815
APA StyleJafarova, M., Contardo, T., Aherne, J., & Loppi, S. (2022). Lichen Biomonitoring of Airborne Microplastics in Milan (N Italy). Biology, 11(12), 1815. https://doi.org/10.3390/biology11121815











