The Evolution of Molybdenum Dependent Nitrogenase in Cyanobacteria
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
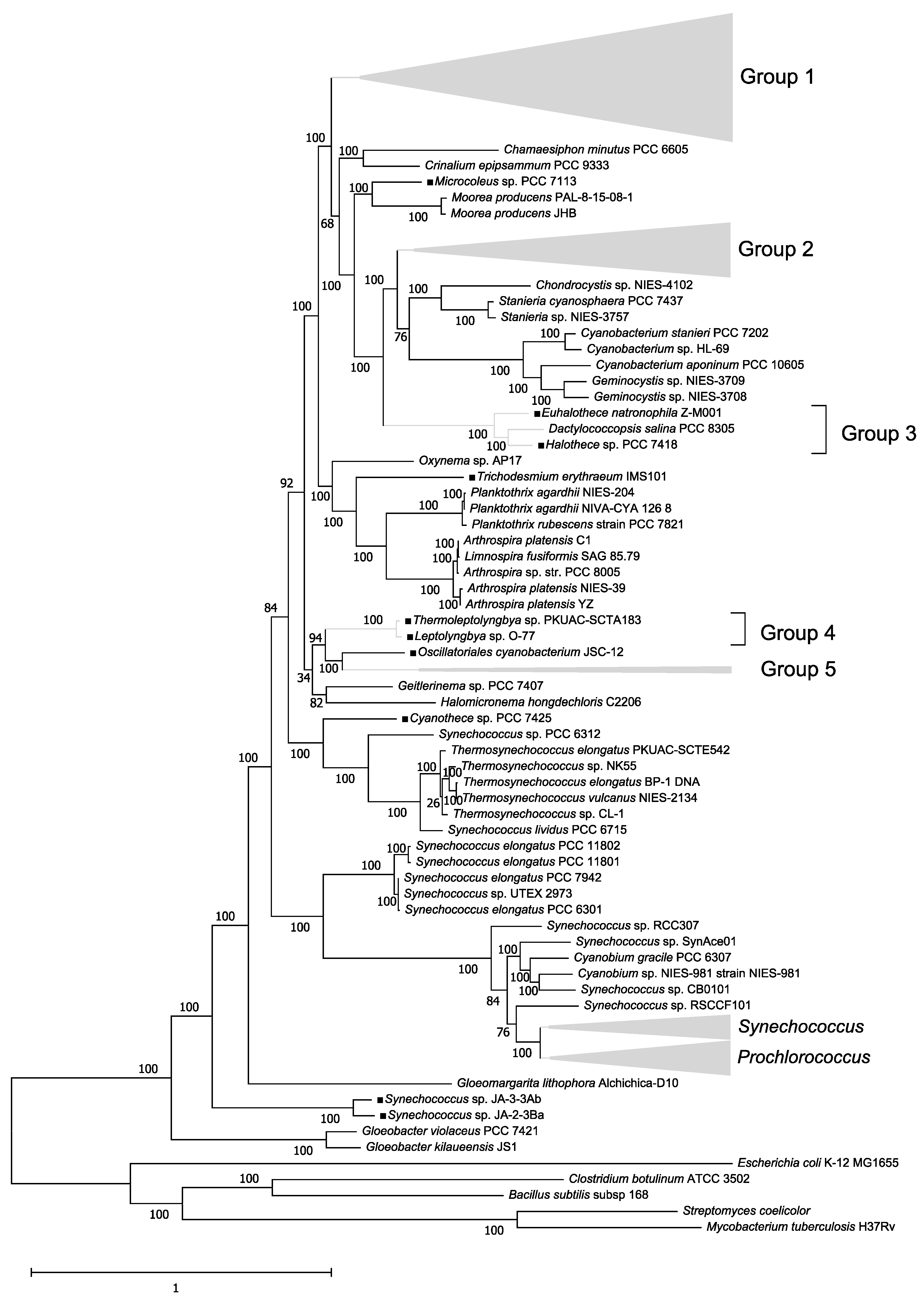
2.1. Construction of a Phylogenetic Tree of 179 Cyanobacteria Species
2.2. Collection of nif Amino Acid Sequence Data
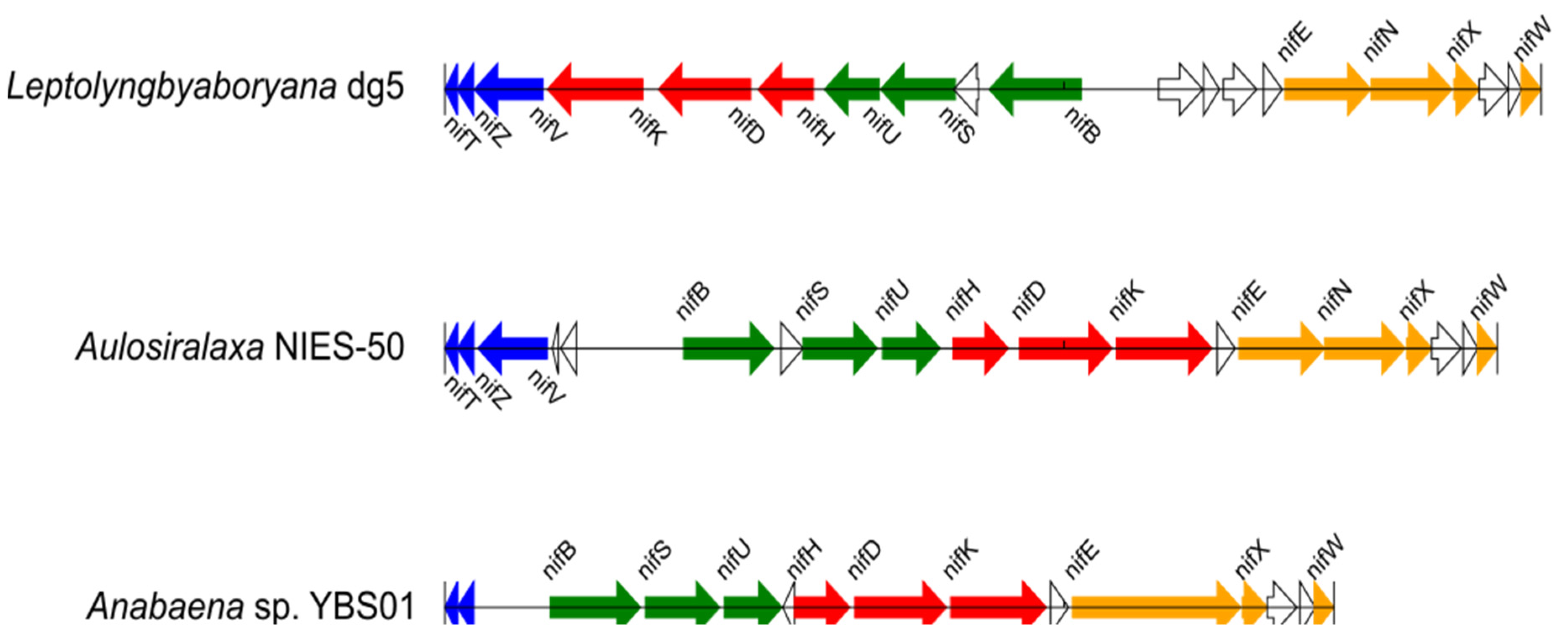
2.3. Detection of nif Gene Operon Structures
2.4. Selection of nif Genes from Multiple nif Genes in An Operon Structure
2.5. Phylogenetic Analysis of Cyanobacterial nif Proteins
2.6. Discovery and Phylogenetic Analysis of nifs in Non-Diazotrophic Cyanobacteria
3. Results and Discussion
3.1. Phylogenetic Tree of 179 Cyanobacteria Species
3.2. Operon Structures of the nif Genes
3.3. Phylogenetic Analysis of nif Proteins
3.4. Nif Genes in Non-Diazotrophic Cyanobacteria
3.5. General Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, E.S.; Hamilton, T.L.; Peters, J.W. An alternative path for the evolution of biological nitrogen fixation. Front. Microbiol. 2011, 2, 205. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The natural history of nitrogen fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef]
- Staley, J.T.; Reysenbach, A.L. Biodiversity of Microbial Life: Foundation of Earth’s Biosphere; Wiley & Sons: Hoboken, NJ, USA, 2002; p. 592. [Google Scholar]
- Xiong, J.; Fischer, W.M.; Inoue, K.; Nakahara, M.; Bauer, C.E. Molecular evidence for the early evolution of photosynthesis. Science 2000, 289, 1724–1730. [Google Scholar] [CrossRef]
- Mus, F.; Colman, D.R.; Peters, J.W.; Boyd, E.S. Geobiological feedbacks, oxygen, and the evolution of nitrogenase. Free Radic. Biol. Med. 2019, 140, 250–259. [Google Scholar] [CrossRef]
- Boyd, E.S.; Peters, J.W. New insights into the evolutionary history of biological nitrogen fixation. Front. Microbiol. 2013, 4, 201. [Google Scholar] [CrossRef]
- Turner, S.; Huang, T.C.; Chaw, S.M. Molecular phylogeny of nitrogen-fixing unicellular cyanobacteria. Bot. Bull. Acad. Sin. 2001, 42, 181–186. [Google Scholar]
- Berman-Frank, I.; Lundgren, P.; Falkowski, P. Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res. Microbiol. 2003, 154, 157–164. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Hayes, P.K.; Blank, C.E. Morphological and habitat evolution in the Cyanobacteria using a compartmentalization approach. Geobiology 2005, 3, 145–165. [Google Scholar] [CrossRef]
- Shi, T.; Falkowski, P.G. Genome evolution in cyanobacteria: The stable core and the variable shell. Proc. Natl. Acad. Sci. USA 2008, 105, 2510–2515. [Google Scholar] [CrossRef]
- Larsson, J.; Nylander, J.A.A.; Bergman, B. Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. BMC Evol. Biol. 2011, 11, 187. [Google Scholar] [CrossRef]
- Latysheva, N.; Junker, V.L.; Palmer, W.J.; Codd, G.A.; Barker, D. The evolution of nitrogen fixation in cyanobacteria. Bioinformatics 2012, 28, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Yerrapragada, S.; Siefert, J.L.; Fox, G.E. Horizontal gene transfer in cyanobacterial signature genes. Methods Mol. Biol. 2009, 532, 339–366. [Google Scholar]
- Esteves-Ferreira, A.A.; Cavalcanti, J.H.F.; Vaz, M.G.M.V.; Alvarenga, L.V.; Nunes-Nesi, A.; Araújo, W.L. Cyanobacterial nitrogenases: Phylogenetic diversity, regulation and functional predictions. Genet. Mol. Biol. 2017, 40, 261–275. [Google Scholar] [CrossRef]
- Wu, M.; Scott, A.J. Phylogenomic analysis of bacterial and archaeal sequences with AMPHORA2. Bioinformatics 2012, 28, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef]
- Thiel, T.; Lyons, E.M.; Erker, J.C. Characterization of genes for a second Mo-dependent nitrogenase in the cyanobacterium Anabaena variabilis. J. Bacteriol. 1997, 179, 5222–5225. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, R.; Kamiya, N.; Fujita, Y. Transcriptional regulators ChlR and CnfR are essential for diazotrophic growth in nonheterocystous cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 6762–6767. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, B.E.; Antonelli, A.; Bagheri, H.C. The origin of multicellularity in cyanobacteria. BMC Evol. Biol. 2011, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Pratte, B.S. Regulation of three nitrogenase gene clusters in the Cyanobacterium Anabaena variabilis ATCC 29413. Life (Basel) 2014, 4, 944–967. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria Free. Microbiology 1979, 111, 1–61. [Google Scholar]
- Bolhuis, H.; Severin, I.; Confurius-Guns, V.; Wollenzien, U.I.; Stal, L.J. Horizontal transfer of the nitrogen fixation gene cluster in the cyanobacterium Microcoleus chthonoplastes. ISME J. 2010, 4, 121–130. [Google Scholar] [CrossRef] [PubMed]
| Species | Nif |
|---|---|
| Moorea producens PAL-8-15-08-1 | nifV |
| Moorea producens JHB | nifV |
| Cyanobacterium stanieri PCC 7202 | nifS |
| Cyanobacterium aponinum PCC 10605 | nifS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, T.; Horiike, T. The Evolution of Molybdenum Dependent Nitrogenase in Cyanobacteria. Biology 2021, 10, 329. https://doi.org/10.3390/biology10040329
Watanabe T, Horiike T. The Evolution of Molybdenum Dependent Nitrogenase in Cyanobacteria. Biology. 2021; 10(4):329. https://doi.org/10.3390/biology10040329
Chicago/Turabian StyleWatanabe, Tomoaki, and Tokumasa Horiike. 2021. "The Evolution of Molybdenum Dependent Nitrogenase in Cyanobacteria" Biology 10, no. 4: 329. https://doi.org/10.3390/biology10040329
APA StyleWatanabe, T., & Horiike, T. (2021). The Evolution of Molybdenum Dependent Nitrogenase in Cyanobacteria. Biology, 10(4), 329. https://doi.org/10.3390/biology10040329





