Effects on Steroid 5-Alpha Reductase Gene Expression of Thai Rice Bran Extracts and Molecular Dynamics Study on SRD5A2
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Plant Material and Extraction
2.3. Determination of Bioactive Compounds
2.3.1. γ-Oryzanol and Tocopherols
2.3.2. Total Phenolic Content
2.3.3. Total Flavonoid Content
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. RNA Extraction and Semiquantitative RT-PCR Analysis
2.6.1. RNA Extraction
2.6.2. Semi-Quantitative RT-PCR
2.7. Computational Method Details
2.7.1. Protein and Ligand Preparation
2.7.2. Molecular Docking Study for 5-Alpha Reductase 2
2.7.3. Molecular Dynamics Simulation
2.7.4. Trajectory Analysis
2.8. Statistical Analysis
3. Results and Discussion
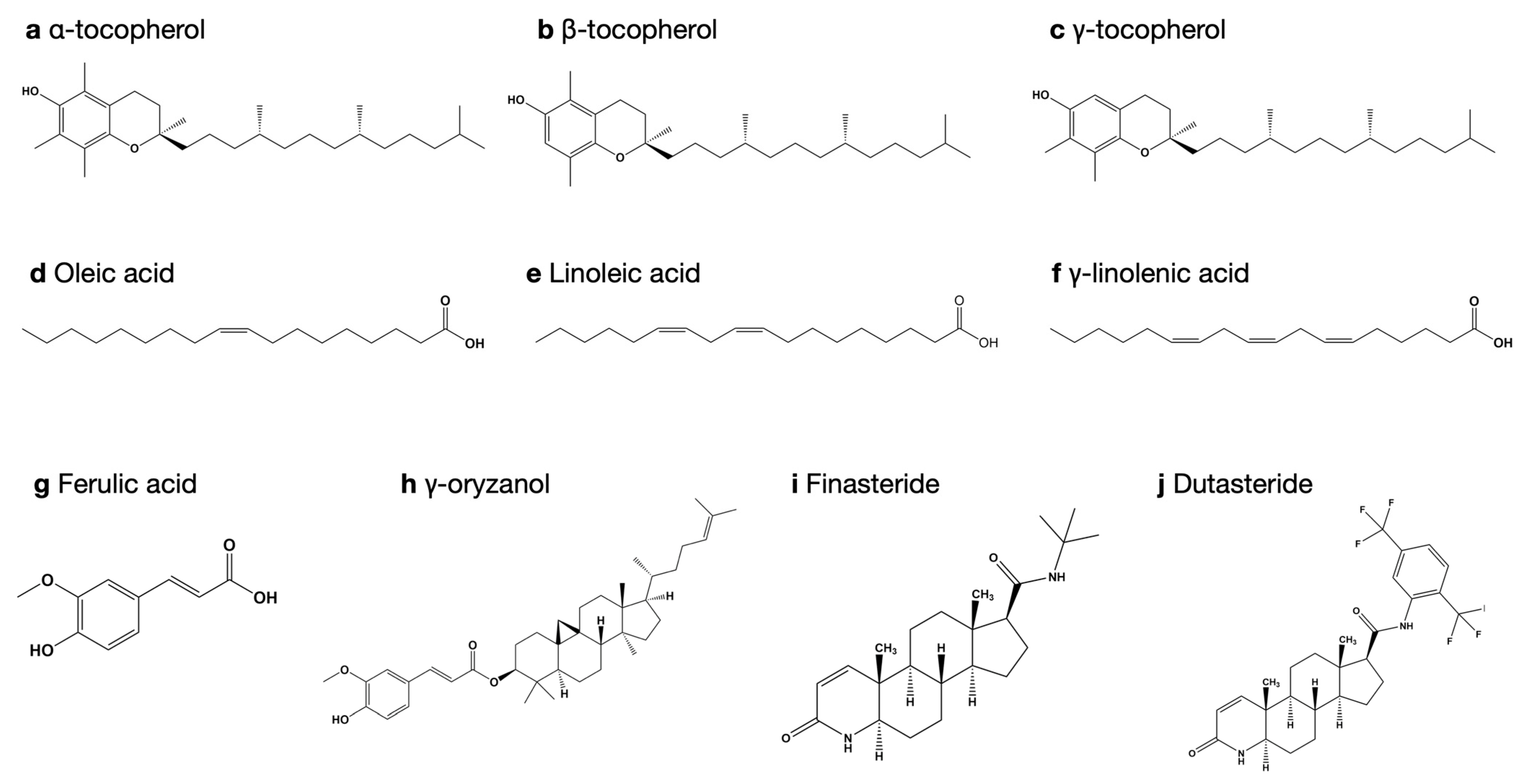
3.1. Extraction Yield and Bioactive Compounds
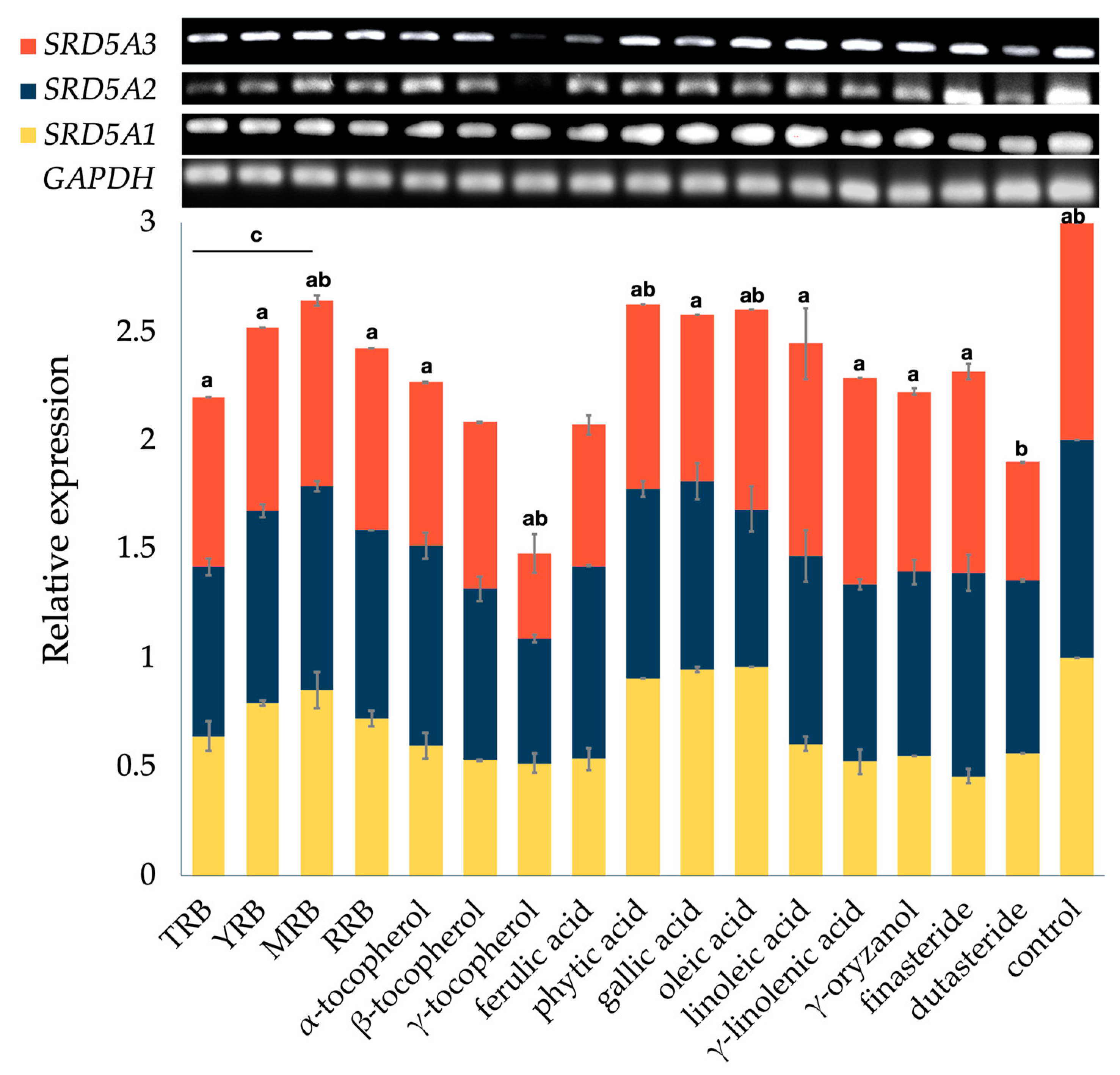
3.2. Effect on the Expression of 5-Alpha Reductase Isoenzymes
3.3. Correlation Analysis
3.3.1. Pearson’s Correlation
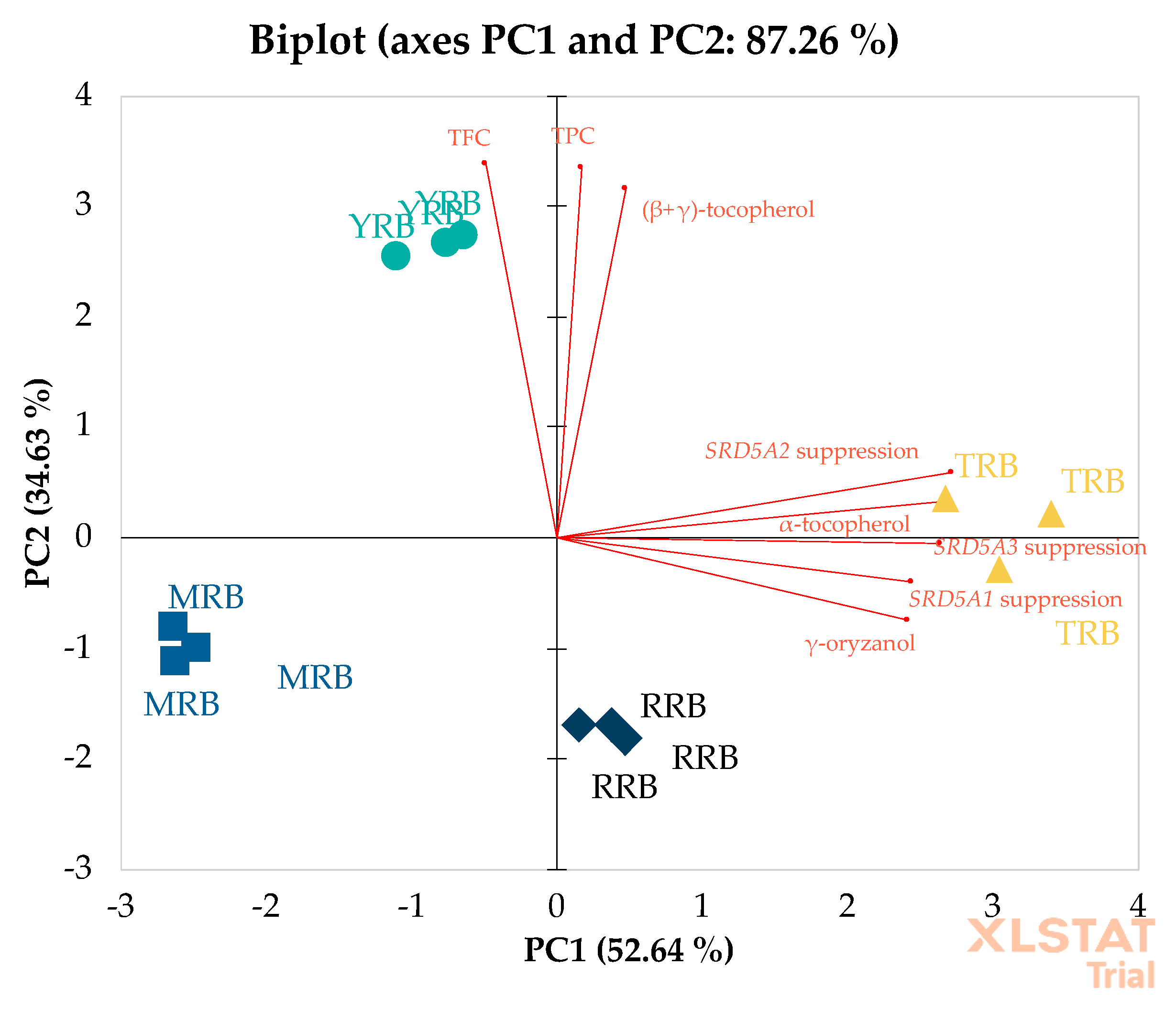
3.3.2. Correlation by Principal Component Analysis
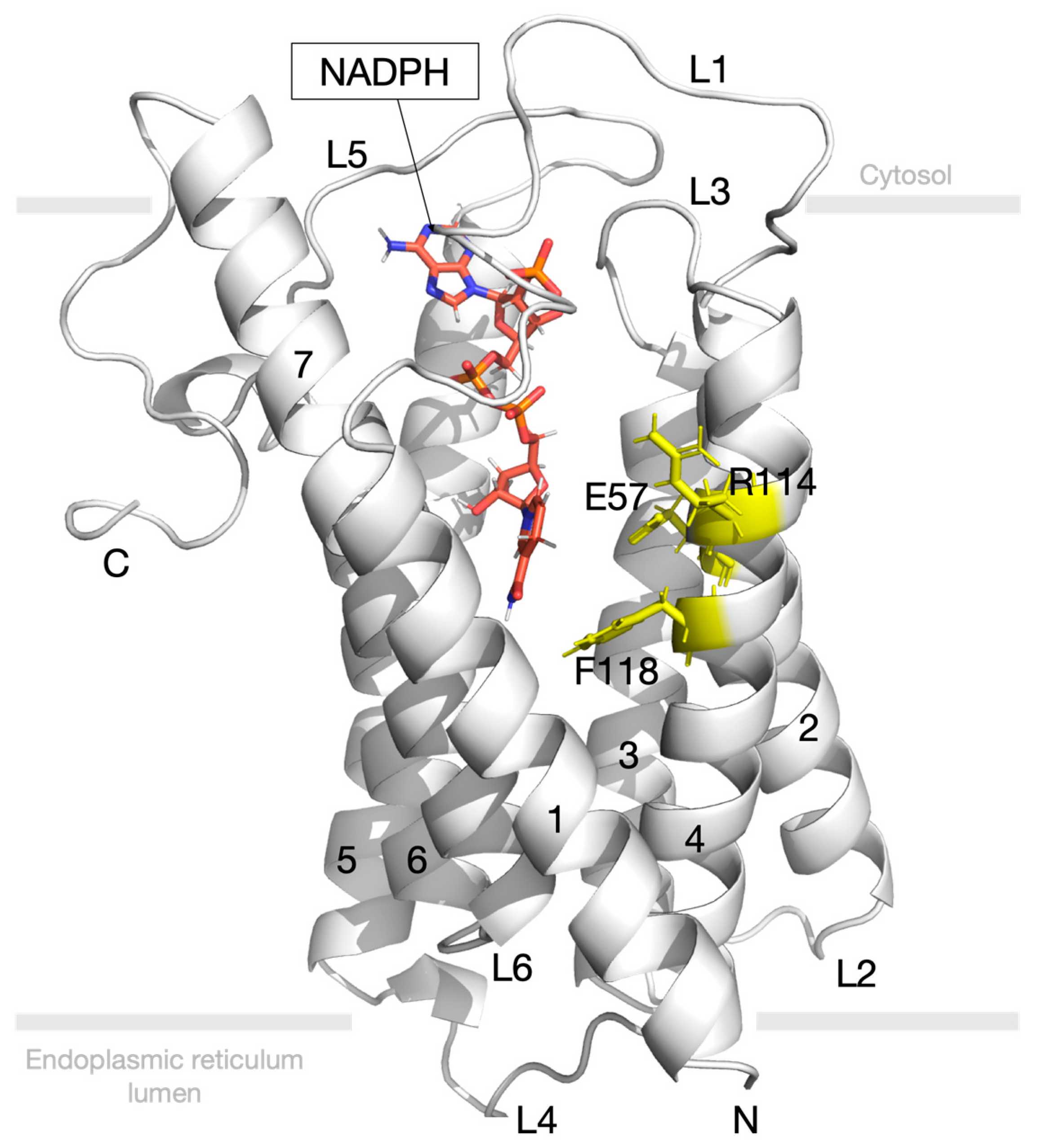
3.4. Molecular Docking Study for 5-Alpha Reductase 2
3.5. Molecular Dynamics Simulation
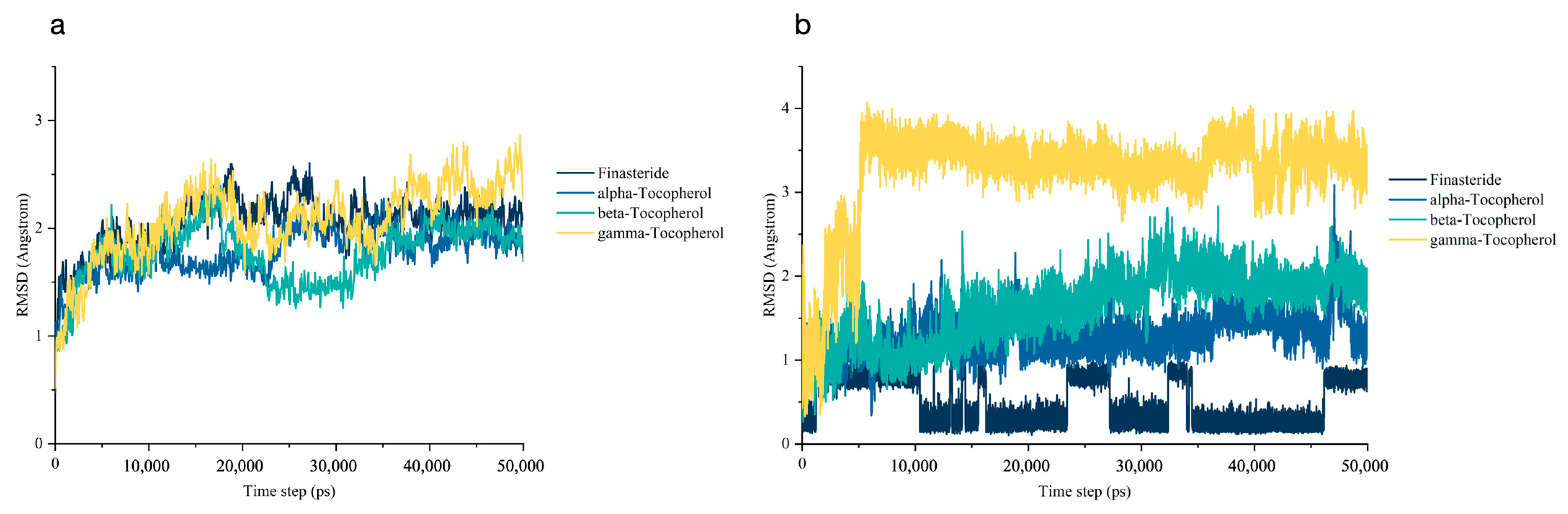
3.5.1. Stability of the Molecular Dynamics Trajectories
3.5.2. Binding Free Energy Analysis
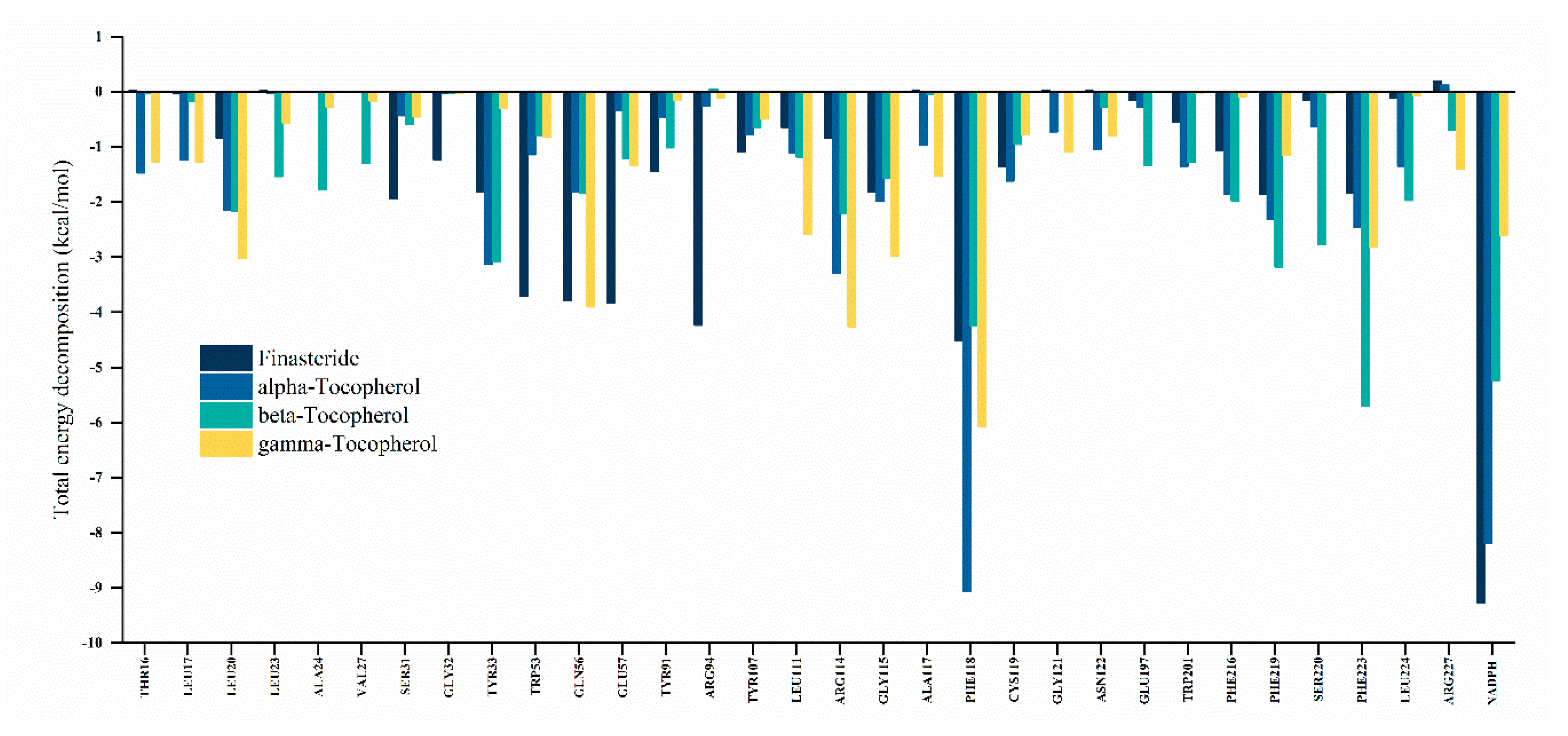
3.5.3. Decomposition of Binding Free Energy
3.6. Post-Molecular Dynamics Simulation Binding Mode Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Gupta, S.; Goyal, I.; Mahendra, A. Quality of life assessment in patients with androgenetic alopecia. Int. J. Trichol. 2019, 11, 147–152. [Google Scholar] [CrossRef]
- Gibson, D.A.; Saunders, P.T.K.; McEwan, I.J. Androgens and androgen receptor: Above and beyond. Mol. Cell. Endocrinol. 2018, 465, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, J.M.; Leirós, G.J.; Balañá, M.E. Androgens and androgen receptor action in skin and hair follicles. Mol. Cell. Endocrinol. 2018, 465, 122–133. [Google Scholar] [CrossRef]
- Azzouni, F.; Godoy, A.; Li, Y.; Mohler, J. The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. Adv. Urol. 2011, 2012, 530121. [Google Scholar] [CrossRef]
- Robitaille, J.; Langlois, V.S. Consequences of steroid-5α-reductase deficiency and inhibition in vertebrates. Gen. Comp. Endocrinol. 2020, 290, 113400. [Google Scholar] [CrossRef] [PubMed]
- Katharopoulos, E.; Sauter, K.; Pandey, A.V.; Flück, C.E. In silico and functional studies reveal novel loss-of-function variants of SRD5A2, but no variants explaining excess 5α-reductase activity. J. Steroid Biochem. Mol. Biol. 2019, 190, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Chávez, B.; Ramos, L.; García-Becerra, R.; Vilchis, F. Hamster SRD5A3 lacks steroid 5α-reductase activity in vitro. Steroids 2015, 94, 41–50. [Google Scholar] [CrossRef]
- Jain, R.; Monthakantirat, O.; Tengamnuay, P.; Deeknamkul, W. Identification of a new plant extract for androgenic alopecia treatment using a non-radioactive human hair dermal papilla cell-based assay. BMC Complement. Med. Ther. 2015, 16, 18. [Google Scholar] [CrossRef]
- Inui, S.; Itami, S. Androgen actions on the human hair follicle: Perspectives. Exp. Dermatol. 2013, 22, 168–171. [Google Scholar] [CrossRef]
- Scaglione, A.; Montemiglio, L.C.; Parisi, G.; Asteriti, I.A.; Bruni, R.; Cerutti, G.; Testi, C.; Savino, C.; Mancia, F.; Lavia, P.; et al. Subcellular localization of the five members of the human steroid 5α-reductase family. Biochim. Open 2017, 4, 99–106. [Google Scholar] [CrossRef]
- Leirós, G.J.; Ceruti, J.M.; Castellanos, M.L.; Kusinsky, A.G.; Balañá, M.E. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol. Cell. Endocrinol. 2017, 439, 26–34. [Google Scholar] [CrossRef]
- Sawaya, M.E.; Price, V.H. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J. Investig. Dermatol. 1997, 109, 296–300. [Google Scholar] [CrossRef]
- Cantagrel, V.; Lefeber, D.J.; Ng, B.G.; Guan, Z.; Silhavy, J.L.; Bielas, S.L.; Lehle, L.; Hombauer, H.; Adamowicz, M.; Swiezewska, E.; et al. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell 2010, 142, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Tamura, K.; Chung, S.; Honma, S.; Okuyama, A.; Nakamura, Y.; Nakagawa, H. Novel 5α-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008, 99, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Yamana, K.; Labrie, F.; Luu The, V. Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Horm. Mol. Biol. Clin. Investig. 2010, 2, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.; Kawinski, E.; Li, Y.; Oka, D.; Alexiev, B.; Azzouni, F.; Titus, M.A.; Mohler, J.L. 5α-reductase type 3 expression in human benign and malignant tissues: A comparative analysis during prostate cancer progression. Prostate 2011, 71, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.K.; Levine, P.H.; Cleary, S.D.; Hoffman, H.J.; Graubard, B.I.; Cook, M.B. Male pattern baldness in relation to prostate cancer-specific mortality: A prospective analysis in the nhanes i epidemiologic follow-up study. Am. J. Epidemiol. 2016, 183, 210–217. [Google Scholar] [CrossRef]
- Jin, T.; Wu, T.; Luo, Z.; Duan, X.; Deng, S.; Tang, Y. Association between male pattern baldness and prostate disease: A meta-analysis. Urol. Oncol. 2018, 36, 80.e7–80.e15. [Google Scholar] [CrossRef]
- Sánchez, P.; Serrano Falcón, C.; Torres, J.; Serrano, S.; Ortega, E. 5α-Reductase isozymes and aromatase mRNA levels in plucked hair from young women with female pattern hair loss. Arch. Dermatol. Res. 2018, 310, 77–83. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, S.; Gao, Z.; Wu, J.; Ma, J.; Cui, Y. The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: A systematic review and meta-analysis. Clin. Interv. Aging 2019, 14, 399–406. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Mechanism of action of herbs and their active constituents used in hair loss treatment. Fitoterapia 2016, 114, 18–25. [Google Scholar] [CrossRef]
- Khatun, A.; Waters, D.L.E.; Liu, L. A review of rice starch digestibility: Effect of composition and heat-moisture processing. Starke 2019, 71, 1900090. [Google Scholar] [CrossRef]
- Liu, J.; Rahman, S.; Sriboonchitta, S.; Wiboonpongse, A. Enhancing productivity and resource conservation by eliminating inefficiency of Thai rice farmers: A zero inefficiency stochastic frontier approach. Sustainability 2017, 9, 770. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Kalita, P. Rice—Not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Technol. 2020, 97, 265–285. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Bioactive compounds of rice (Oryza sativa L.): Review on paradigm and its potential benefit in human health. Trends Food Sci. Technol. 2020, 97, 355–365. [Google Scholar] [CrossRef]
- Liang, T.; Liao, S. Inhibition of steroid 5 α-reductase by specific aliphatic unsaturated fatty acids. Biochem. J. 1992, 285, 557–562. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Manosroi, J.; Abe, M.; Manosroi, W.; Manosroi, A. 5α-Reductase type 1 inhibition of Oryza sativa bran extract prepared by supercritical carbon dioxide fluid. J. Supercrit. Fluids 2011, 59, 61–71. [Google Scholar] [CrossRef]
- Camacho-Martinez, F.M. Hair loss in women. In Seminars in Cutaneous Medicine and Surgery; Elsevier: Amsterdam, The Netherlands, 2009; pp. 19–32. [Google Scholar]
- Pestana Bauer, V.R.; Zambiazi, R.C.; Mendonca, C.R.; Beneito Cambra, M.; Ramis Ramos, G. γ-Oryzanol and tocopherol contents in residues of rice bran oil refining. Food Chem. 2012, 134, 1479–1483. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Li, Y.; Chen, Z. Phenolic contents, cellular antioxidant activity and antiproliferative capacity of different varieties of oats. Food Chem. 2018, 239, 260–267. [Google Scholar] [CrossRef]
- Zeng, J.; Shang, X.; Zhang, P.; Wang, H.; Gu, Y.; Tan, J.-N. Combined use of deep eutectic solvents, macroporous resins, and preparative liquid chromatography for the isolation and purification of flavonoids and 20-hydroxyecdysone from Chenopodium quinoa willd. Biomolecules 2019, 9, 776. [Google Scholar] [CrossRef]
- Papazisis, K.; Geromichalos, G.; Dimitriadis, K.; Kortsaris, A. Optimization of the sulforhodamine B colorimetric assay. J. Immunol. Methods 1997, 208, 151–158. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, L.; Supekar, S.; Shen, T.; Liu, H.; Ye, F.; Huang, J.; Fan, H.; Wei, Z.; Zhang, C. Structure of human steroid 5α-reductase 2 with the anti-androgen drug finasteride. Nat. Commun. 2020, 11, 5430. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Nadvorny, D.; Soares Sobrinho, J.L.; de La Roca Soares, M.F.; Ribeiro, A.J.; Veiga, F.; Seabra, G.M. Molecular dynamics simulations reveal the influence of dextran sulfate in nanoparticle formation with calcium alginate to encapsulate insulin. J. Biomol. Struct. Dyn. 2018, 36, 1255–1260. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Beck, D.A.; Daggett, V. Methods for molecular dynamics simulations of protein folding/unfolding in solution. Methods 2004, 34, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.W.; Brooks, B.R.; Szabo, A. An analysis of the accuracy of Langevin and molecular dynamics algorithms. Mol. Phys. 1988, 65, 1409–1419. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- DeLano, W.L. PyMOL; Texas A&M University: College Station, TX, USA, 2002. [Google Scholar]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R., III; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA. py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]
- Cruz, J.N.; Costa, J.F.; Khayat, A.S.; Kuca, K.; Barros, C.A.; Neto, A. Molecular dynamics simulation and binding free energy studies of novel leads belonging to the benzofuran class inhibitors of Mycobacterium tuberculosis Polyketide Synthase 13. J. Biomol. Struct. Dyn. 2019, 37, 1616–1627. [Google Scholar] [CrossRef]
- Onufriev, A.; Bashford, D.; Case, D.A. Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins 2004, 55, 383–394. [Google Scholar] [CrossRef]
- Weiser, J.; Shenkin, P.S.; Still, W.C. Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J. Comput. Chem. 1999, 20, 217–230. [Google Scholar] [CrossRef]
- Bhat, F.M.; Riar, C.S.; Seesuriyachan, P.; Sommano, S.R.; Chaiyaso, T.; Promuthai, C. Status of bioactive compounds from bran of pigmented traditional rice varieties and their scope in production of medicinal food with nutraceutical importance. Agronomy 2020, 10, 1817. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Ling, B.; Lyng, J.G.; Wang, S. Effects of hot air-assisted radio frequency heating on enzyme inactivation, lipid stability and product quality of rice bran. LWT 2018, 91, 453–459. [Google Scholar] [CrossRef]
- Perretti, G.; Miniati, E.; Montanari, L.; Fantozzi, P. Improving the value of rice by-products by SFE. J. Supercrit. Fluids 2003, 26, 63–71. [Google Scholar] [CrossRef]
- Tsuzuki, W.; Komba, S.; Kotakec Nara, E. Diversity in γ-oryzanol profiles of Japanese black-purple rice varieties. J. Food Sci. Technol. 2019, 56, 2778–2786. [Google Scholar] [CrossRef]
- Surin, S.; Surayot, U.; Seesuriyachan, P.; You, S.; Phimolsiripol, Y. Antioxidant and immunomodulatory activities of sulphated polysaccharides from purple glutinous rice bran (Oryza sativa L.). Int. J. Food Sci. Technol. 2018, 53, 994–1004. [Google Scholar] [CrossRef]
- Muntana, N.; Prasong, S. Study on total phenolic contents and their antioxidant activities of Thai white, red and black rice bran extracts. Pak. J. Biol. Sci. 2010, 13, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The genetic basis and nutritional benefits of pigmented rice grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef]
- Surin, S.; You, S.; Seesuriyachan, P.; Muangrat, R.; Wangtueai, S.; Jambrak, A.R.; Phongthai, S.; Jantanasakulwong, K.; Chaiyaso, T.; Phimolsiripol, Y. Optimization of ultrasonic-assisted extraction of polysaccharides from purple glutinous rice bran (Oryza sativa L.) and their antioxidant activities. Sci. Rep. 2020, 10, 10410. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Gu, L.; McClung, A.M.; Bergman, C.J.; Chen, M.H. Free and bound total phenolic concentrations, antioxidant capacities, and profiles of proanthocyanidins and anthocyanins in whole grain rice (Oryza sativa L.) of different bran colours. Food Chem. 2012, 133, 715–722. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Harun, M.S.; Wong, T.W.; Fong, C.W. Advancing skin delivery of α-tocopherol and γ-tocotrienol for dermatitis treatment via nanotechnology and microwave technology. Int. J. Pharm. 2021, 593, 120099. [Google Scholar] [CrossRef]
- Nachbar, F.; Korting, H. The role of vitamin E in normal and damaged skin. J. Mol. Med. 1995, 73, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.; Sasaki, N. Effect of topical application of vitamin E on the hair growth of rabbits. J. Vitaminol. 1965, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nohno, T.; Yoshida, H.; Yokoya, H. Trans-4-hydroxy-3-methoxycinnamic acid (ferulic acid) inhibits the effect of androgens on the rat prostate. Experientia 1979, 35, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Abu El Saad, A.S.; Mahmoud, H.M. Phytic acid exposure alters aflatoxinb1-induced reproductive and oxidative toxicity in albino rats (Rattus norvegicus). Evid. Based Complement. Alternat. Med. 2009, 6, 107398. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Abe, M.; Manosroi, W.; Manosroi, J. Transfollicular enhancement of gel containing cationic niosomes loaded with unsaturated fatty acids in rice (Oryza sativa) bran semi-purified fraction. Eur. J. Pharm. Biopharm. 2012, 81, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Ruksiriwanich, W.; Abe, M.; Sakai, H.; Aburai, K.; Manosroi, W.; Manosroi, J. Physico-chemical properties of cationic niosomes loaded with fraction of rice (Oryza sativa) bran extract. J. Nanosci. Nanotechnol. 2012, 12, 7339–7345. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.T.; Zheng, Q.C.; Wu, Y.J.; Zhang, J.L.; Liang, C.Y.; Chen, L.; Xue, Q.; Zhang, H.X. Molecular dynamics (MD) simulations and binding free energy calculation studies between inhibitors and type II dehydroquinase (DHQ2). Mol. Simul. 2013, 39, 137–144. [Google Scholar] [CrossRef]
- Takayasu, S.; Adachi, K. The conversion of testosterone to 17β-hydroxy-5α-androstan-3-one (dihydrotestosterone) by human hair follicles. J. Clin. Endocrinol. Metab. 1972, 34, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef]
- Smith, C.M.; Ballard, S.A.; Worman, N.; Buettner, R.; Masters, J.R. 5 alpha-reductase expression by prostate cancer cell lines and benign prostatic hyperplasia in vitro. J. Clin. Endocrinol. Metab. 1996, 81, 1361–1366. [Google Scholar]
- Lourith, N.; Kanlayavattanakul, M.; Chaikul, P. Para rubber seed oil: The safe and efficient bio-material for hair loss treatment. J. Cosmet. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fertig, R.; Shapiro, J.; Bergfeld, W.; Tosti, A. Investigation of the plausibility of 5-alpha-reductase inhibitor syndrome. Skin Appendage Disord. 2016, 2, 120–129. [Google Scholar] [CrossRef]
- Ganzer, C.A.; Jacobs, A.R.; Iqbal, F. Persistent sexual, emotional, and cognitive impairment post-finasteride: A survey of men reporting symptoms. Am. J. Men’s Health 2015, 9, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Coskuner, E.R.; Ozkan, B.; Culha, M.G. Sexual problems of men with androgenic alopecia treated with 5-alpha reductase inhibitors. Sex. Med. Rev. 2019, 7, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Chaikul, P.; Chankhampan, C.; Ruksiriwanich, W.; Manosroi, W.; Manosroi, J. 5α-reductase inhibition and melanogenesis induction of the selected Thai plant extracts. Chiang Mai J. Sci. 2018, 45, 220–236. [Google Scholar]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair follicle dermal papilla cells at a glance. J. Cell Sci. 2011, 124, 1179–1182. [Google Scholar] [CrossRef]
- Imperato Mcginley, J.; Peterson, R.E.; Gautier, T.; Cooper, G.; Danner, R.; Arthur, A.; Morris, P.L.; Sweeney, W.J.; Shackleton, C. Hormonal evaluation of a large kindred with complete androgen insensitivity: Evidence for secondary 5α-reductase deficiency. J. Clin. Endocrinol. Metab. 1982, 54, 931–941. [Google Scholar] [CrossRef]
- Sharifi, N.; Auchus, R.J. Steroid biosynthesis and prostate cancer. Steroids 2012, 77, 719–726. [Google Scholar] [CrossRef]
- Han, Y.; Zhuang, Q.; Sun, B.; Lv, W.; Wang, S.; Xiao, Q.; Pang, B.; Zhou, Y.; Wang, F.; Chi, P.; et al. Crystal structure of steroid reductase SRD5A reveals conserved steroid reduction mechanism. Nat. Commun. 2021, 1, 449. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Iamsumang, W.; Leerunyakul, K. Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: A review of the current literature. J. Dermatol. Treat. 2020, 31, 1–6. [Google Scholar] [CrossRef]
- Raynaud, J.P.; Cousse, H.; Martin, P.M. Inhibition of type 1 and type 2 5alpha-reductase activity by free fatty acids, active ingredients of Permixon. J. Steroid Biochem. Mol. Biol. 2002, 82, 233–239. [Google Scholar] [CrossRef]
- Park, W.-S.; Lee, C.-H.; Lee, B.-G.; Chang, I.-S. The extract of Thujae occidentalis semen inhibited 5α-reductase and androchronogenetic alopecia of B6CBAF1/j hybrid mouse. J. Dermatol. Sci. 2003, 31, 91–98. [Google Scholar] [CrossRef]
- Kumar, T.; Chaiyasut, C.; Suttajit, M. Screening of steroid 5-reductase inhibitory activity and total phenolic content of Thai plants. J. Med. Plant Res. 2011, 5, 1265–1271. [Google Scholar]
- Ganeshpurkar, A.; Singh, R.; Gore, P.G.; Kumar, D.; Gutti, G.; Kumar, A.; Singh, S.K. Structure-based screening and molecular dynamics simulation studies for the identification of potential acetylcholinesterase inhibitors. Mol. Simul. 2020, 46, 169–185. [Google Scholar] [CrossRef]
- Sk, M.F.; Roy, R.; Jonniya, N.A.; Poddar, S.; Kar, P. Elucidating biophysical basis of binding of inhibitors to SARS-CoV-2 main protease by using molecular dynamics simulations and free energy calculations. J. Biomol. Struct. Dyn. 2020, 38, 1–13. [Google Scholar] [CrossRef]
- Shamsara, J. Homology modeling of 5-alpha-reductase 2 using available experimental data. Interdiscip. Sci. 2019, 11, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Makridakis, N.; Akalu, A.; Reichardt, J.K. Identification and characterization of somatic steroid 5 α-reductase (SRD5A2) mutations in human prostate cancer tissue. Oncogene 2004, 23, 7399–7405. [Google Scholar] [CrossRef]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef] [PubMed]
- Yui, Y.; Miyazaki, S.; Ma, Y.; Ohira, M.; Fiehn, O.; Ikegami, T.; McCalley, D.V.; Tanaka, N. Distinction of synthetic dl-α-tocopherol from natural vitamin E (d-α-tocopherol) by reversed-phase liquid chromatography enhanced selectivity of a polymeric C18 stationary phase at low temperature and/or at high pressure. J. Chromatogr. A 2016, 1450, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; De Winter, H. Molecular dynamics simulations of membrane proteins: An overview. J. Chem. Inf. Model. 2018, 58, 2193–2202. [Google Scholar] [CrossRef] [PubMed]

| Primer | NCBI Reference Sequence | Forward Primer | Reverse Primer |
|---|---|---|---|
| SRD5A1 | 001047.4 | AGCCATTGTGCAGTGTATGC | AGCCTCCCCTTGGTATTTTG |
| SRD5A2 | 000348.4 | TGAATACCCTGATGGGTGG | CAAGCCACCTTGTGGAATC |
| SRD5A3 | 024592.5 | TCCTTCTTTGCCCAAACATC | CTGATGCTCTCCCTTTACGC |
| GAPDH | 001289745.3 | GGAAGGTGAAGGTCGGAGTC | CTCAGCCTTGACGGTGCCATG |
| Sample | α-Tocopherol | (β+γ)-Tocopherol | γ-Oryzanol | TPC | TFC |
|---|---|---|---|---|---|
| mg/kg Extract | mg/kg Extract | mg/kg Extract | mg GAE/100 g | mg EGCGE/100 g | |
| TRB | 20.76 ± 0.13 | 23.32 ± 0.01 | 8600.45 ± 0.13 | 180.44 ± 6.42 | 569.01 ± 90.42 |
| YRB | 12.52 ± 0.01 | 50.98 ± 0.02 | 3773.17 ± 0.01 | 254.97 ± 5.20 | 880.16 ± 22.86 |
| MRB | 7.61 ± 0.01 | 3.51 ± 0.01 | ND | 125.67 ± 0.49 | 545.30 ± 48.33 |
| RRB | 11.95 ± 0.04 | 16.97 ± 0.01 | 9174.01 ± 0.09 | 56.47 ± 2.82 | 441.49 ± 12.39 |
| Compounds | AutoDock Binding Free Energy, ∆G (kcal/mol) |
|---|---|
| Native finasteride | −10.13 |
| β-Tocopherol | −9.83 |
| γ-Tocopherol | −9.53 |
| α-Tocopherol | −9.47 |
| Dutasteride | −8.75 |
| γ-Linolenic acid | −7.03 |
| Linoleic acid | −6.62 |
| Oleic acid | −6.49 |
| Ferulic acid | −5.22 |
| γ-Oryzanol | −4.26 |
| Phytic acid | −2.02 |
| Compounds | Binding Free Energy (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|
| VDW | EEL | EGB | ESURF | ∆Ggas | ∆Gsolv | ∆GTotal | |
| Finasteride | −53.93 ± 3.23 | −18.81 ± 3.41 | 32.36 ± 3.03 | −6.15 ± 0.24 | −72.74 ± 4.33 | 26.20 ± 2.95 | −46.54 ± 3.12 |
| α-Tocopherol | −64.97 ± 3.21 | −2.26 ± 1.68 | 20.10 ± 1.80 | −7.42 ± 0.39 | −67.23 ± 3.68 | 12.68 ± 1.77 | −54.55 ± 3.67 |
| β-Tocopherol | −60.01 ± 4.30 | −8.13 ± 2.90 | 25.88 ± 2.35 | −8.15 ± 0.55 | −68.14 ± 5.02 | 17.73 ± 2.10 | −50.40 ± 4.33 |
| γ-Tocopherol | −51.24 ± 4.41 | −5.71 ± 4.69 | 23.02 ± 3.76 | −6.77 ± 0.47 | −56.96 ± 5.54 | 16.25 ± 3.65 | −40.71 ± 3.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khantham, C.; Yooin, W.; Sringarm, K.; Sommano, S.R.; Jiranusornkul, S.; Carmona, F.D.; Nimlamool, W.; Jantrawut, P.; Rachtanapun, P.; Ruksiriwanich, W. Effects on Steroid 5-Alpha Reductase Gene Expression of Thai Rice Bran Extracts and Molecular Dynamics Study on SRD5A2. Biology 2021, 10, 319. https://doi.org/10.3390/biology10040319
Khantham C, Yooin W, Sringarm K, Sommano SR, Jiranusornkul S, Carmona FD, Nimlamool W, Jantrawut P, Rachtanapun P, Ruksiriwanich W. Effects on Steroid 5-Alpha Reductase Gene Expression of Thai Rice Bran Extracts and Molecular Dynamics Study on SRD5A2. Biology. 2021; 10(4):319. https://doi.org/10.3390/biology10040319
Chicago/Turabian StyleKhantham, Chiranan, Wipawadee Yooin, Korawan Sringarm, Sarana Rose Sommano, Supat Jiranusornkul, Francisco David Carmona, Wutigri Nimlamool, Pensak Jantrawut, Pornchai Rachtanapun, and Warintorn Ruksiriwanich. 2021. "Effects on Steroid 5-Alpha Reductase Gene Expression of Thai Rice Bran Extracts and Molecular Dynamics Study on SRD5A2" Biology 10, no. 4: 319. https://doi.org/10.3390/biology10040319
APA StyleKhantham, C., Yooin, W., Sringarm, K., Sommano, S. R., Jiranusornkul, S., Carmona, F. D., Nimlamool, W., Jantrawut, P., Rachtanapun, P., & Ruksiriwanich, W. (2021). Effects on Steroid 5-Alpha Reductase Gene Expression of Thai Rice Bran Extracts and Molecular Dynamics Study on SRD5A2. Biology, 10(4), 319. https://doi.org/10.3390/biology10040319














