Curcuma amarissima Extract Activates Growth and Survival Signal Transduction Networks to Stimulate Proliferation of Human Keratinocyte
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ethanolic Extract from the Rhizomes of Curcuma aeruginosa (CA)
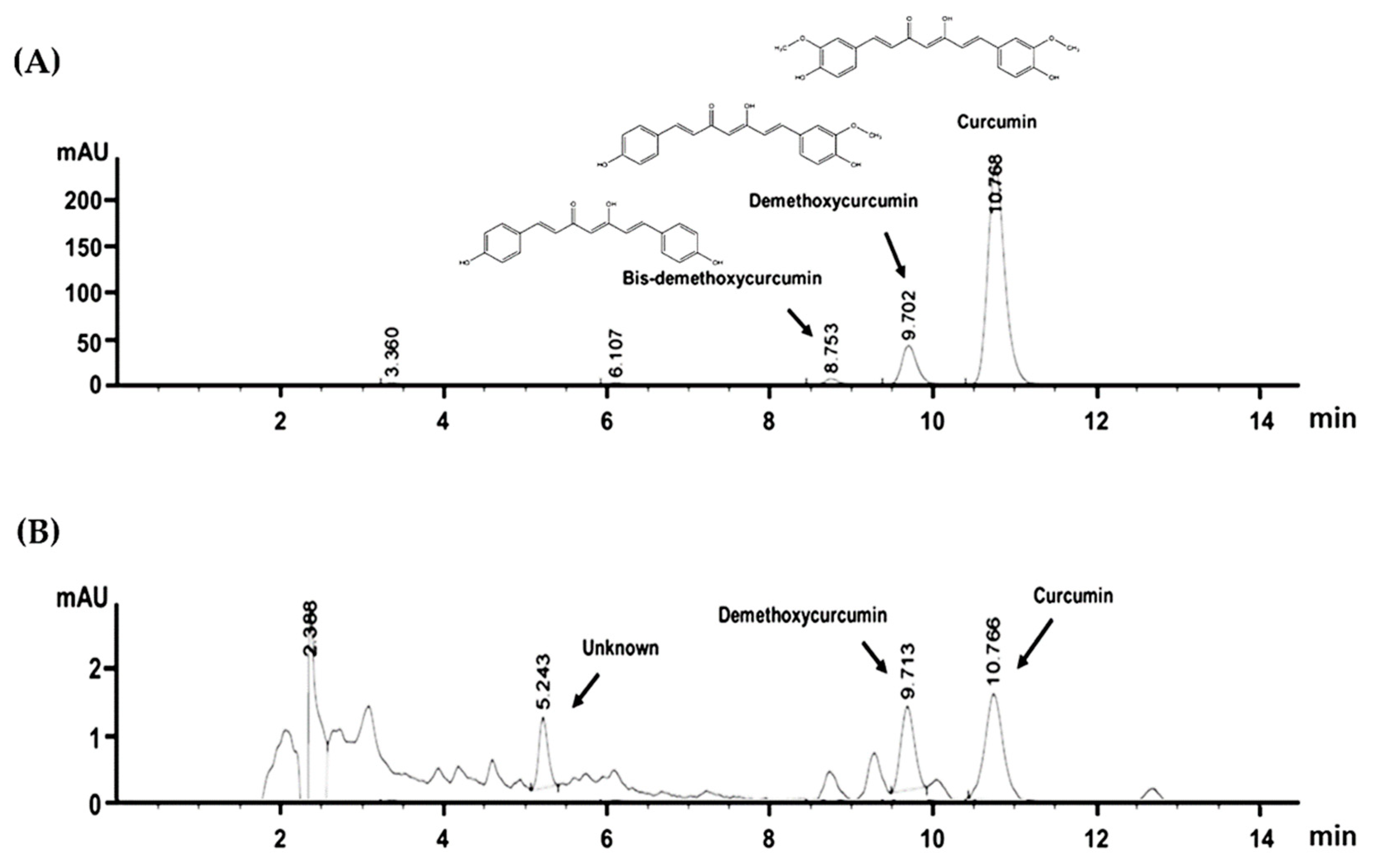
2.2. HPLC Fingerprint of CA Extract
2.3. Culture of Human Keratinocyte Cell Line, HaCaT
2.4. Cell Viability Determination of HaCaT Cells Treated with CA Extract
2.5. Determination of HaCaT Cell Monolayer Healing by Scratch Wounding Assay
2.6. Effects of CA Extract on Increasing Number of HaCaT Cells
2.7. Effects of CA Extract on Stimulating Molecular Signaling Pathways
2.8. Detection of Early Signaling Pathway Induced by CA Extract in Individual Cells by Immunofluorescence Study
2.9. Data and Statistical Analysis
3. Results
3.1. Curcuma Amarissima (CA) Extract Enhances Cell Viability of HaCaT Cells
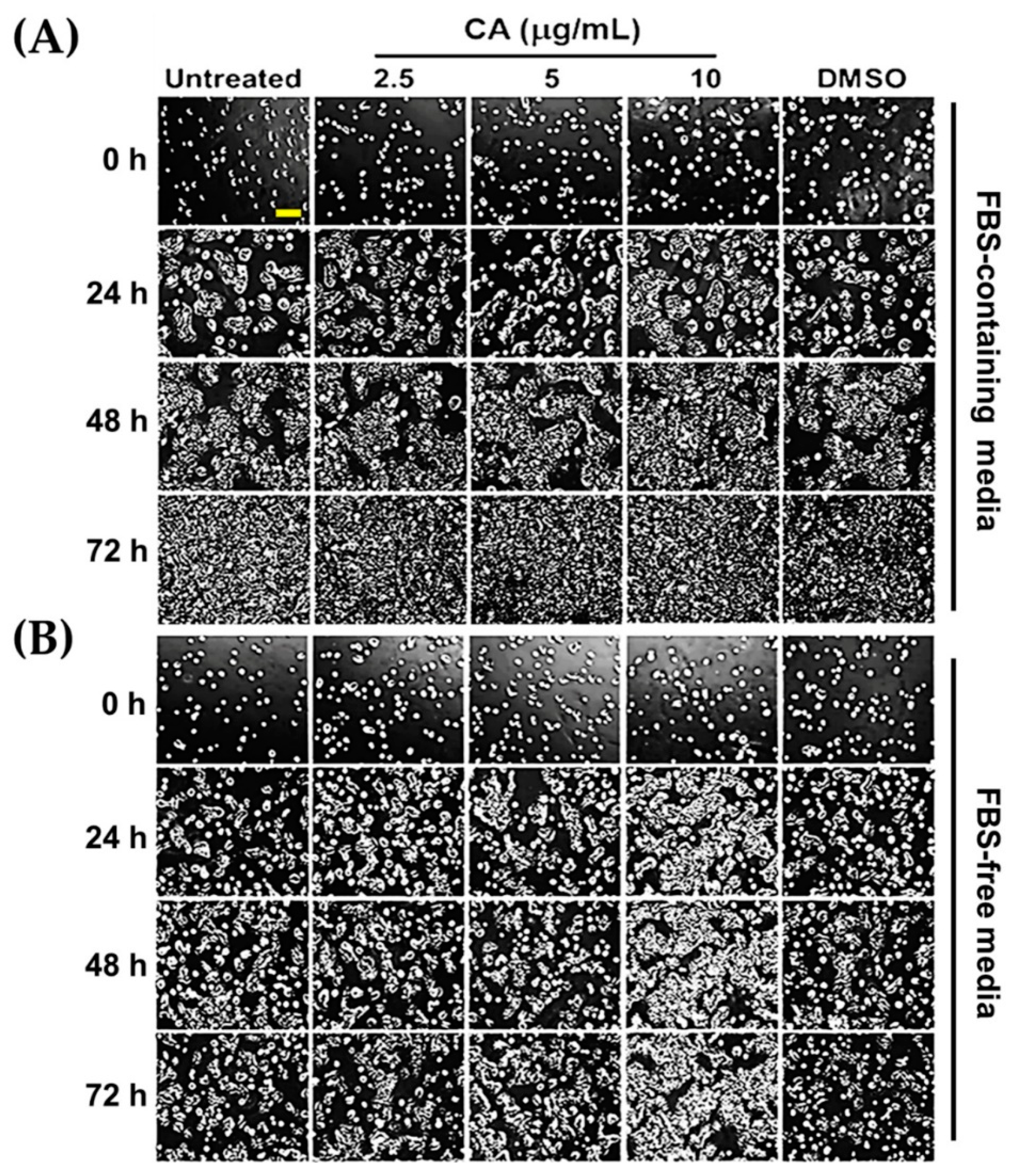
3.2. CA Extract Stimulates Colony Formation and Proliferation of HaCaT Cells
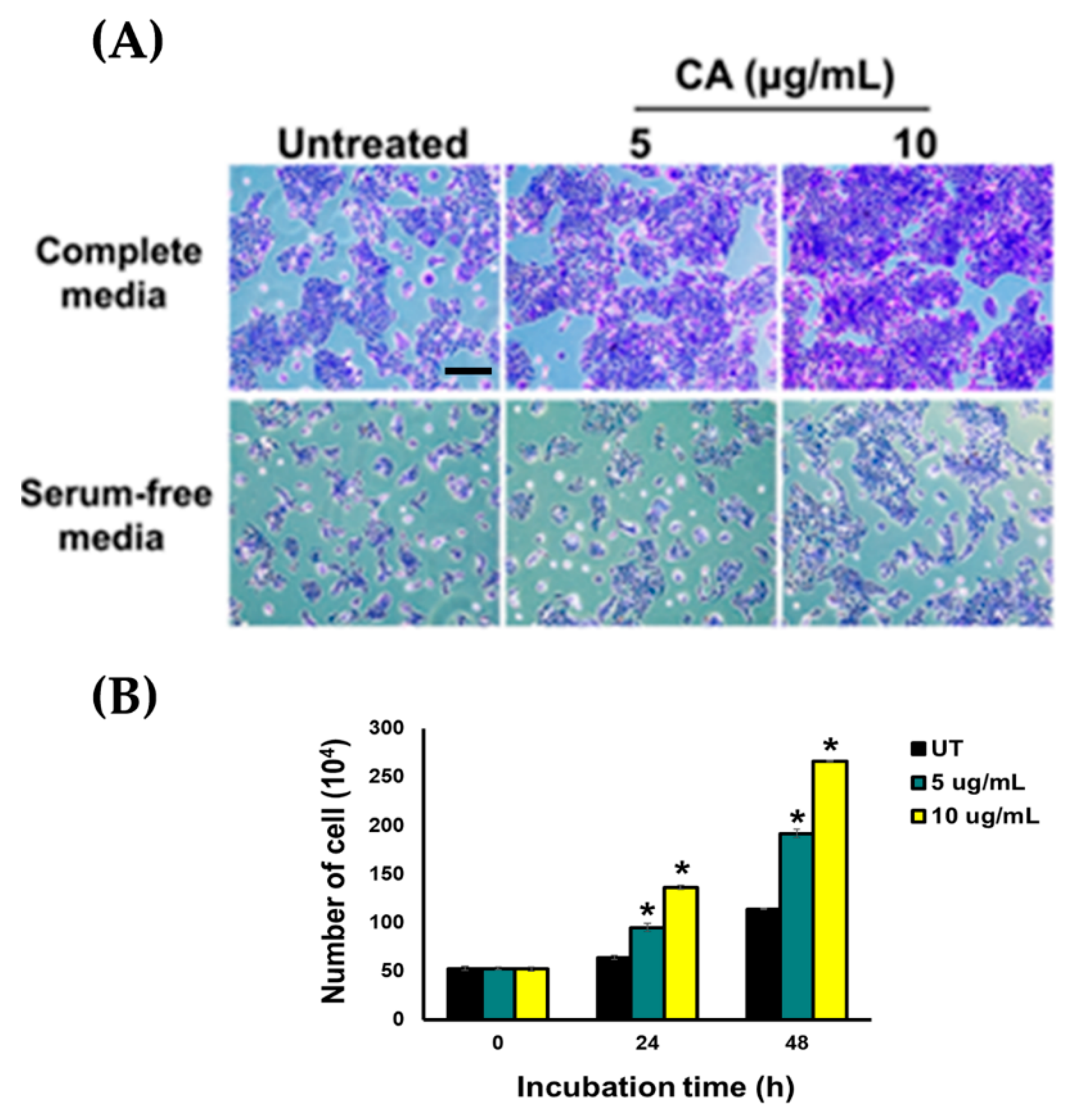
3.3. CA Extract Induces Migration of HaCaT Cell Monolayer into The Wounded Area
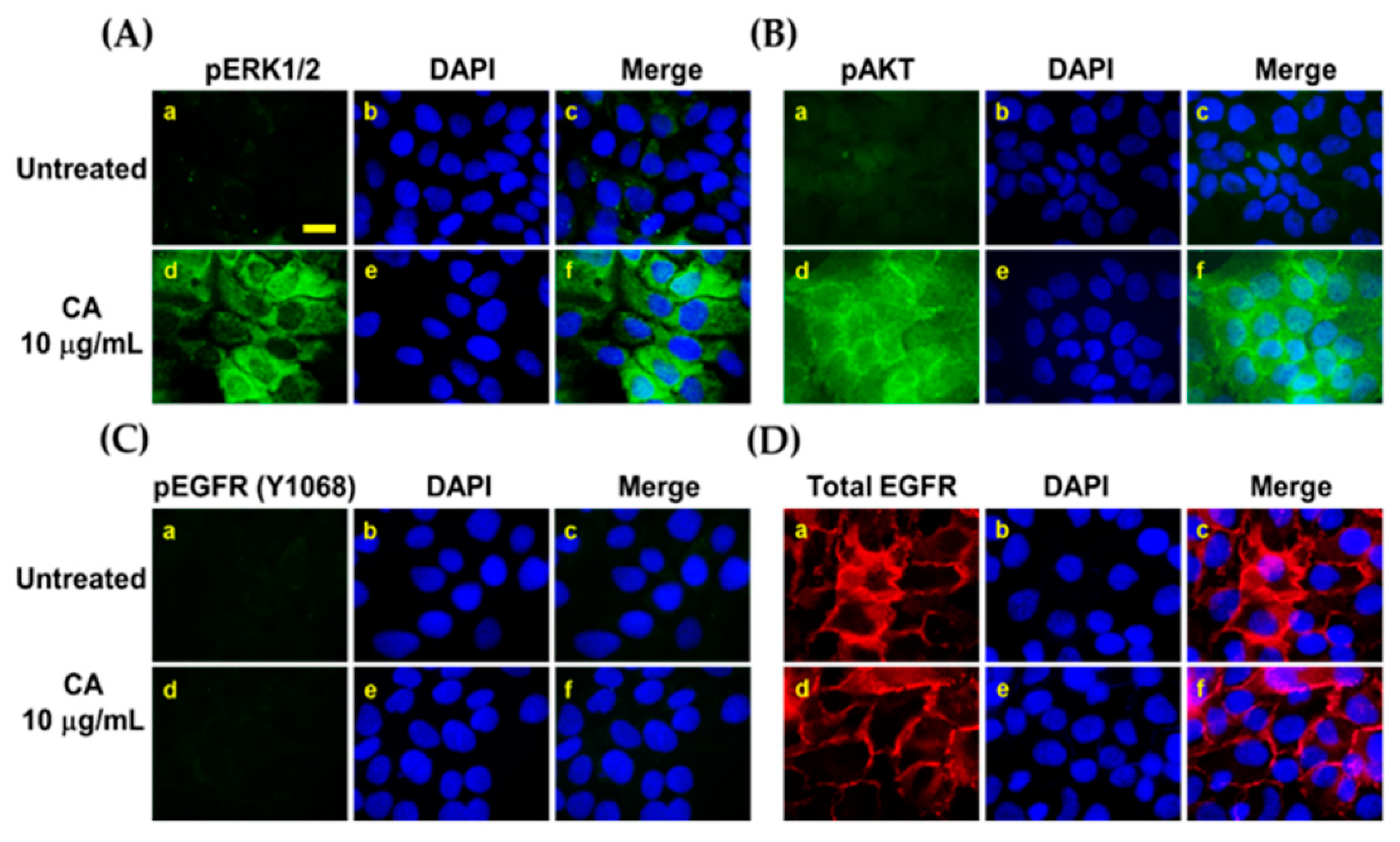
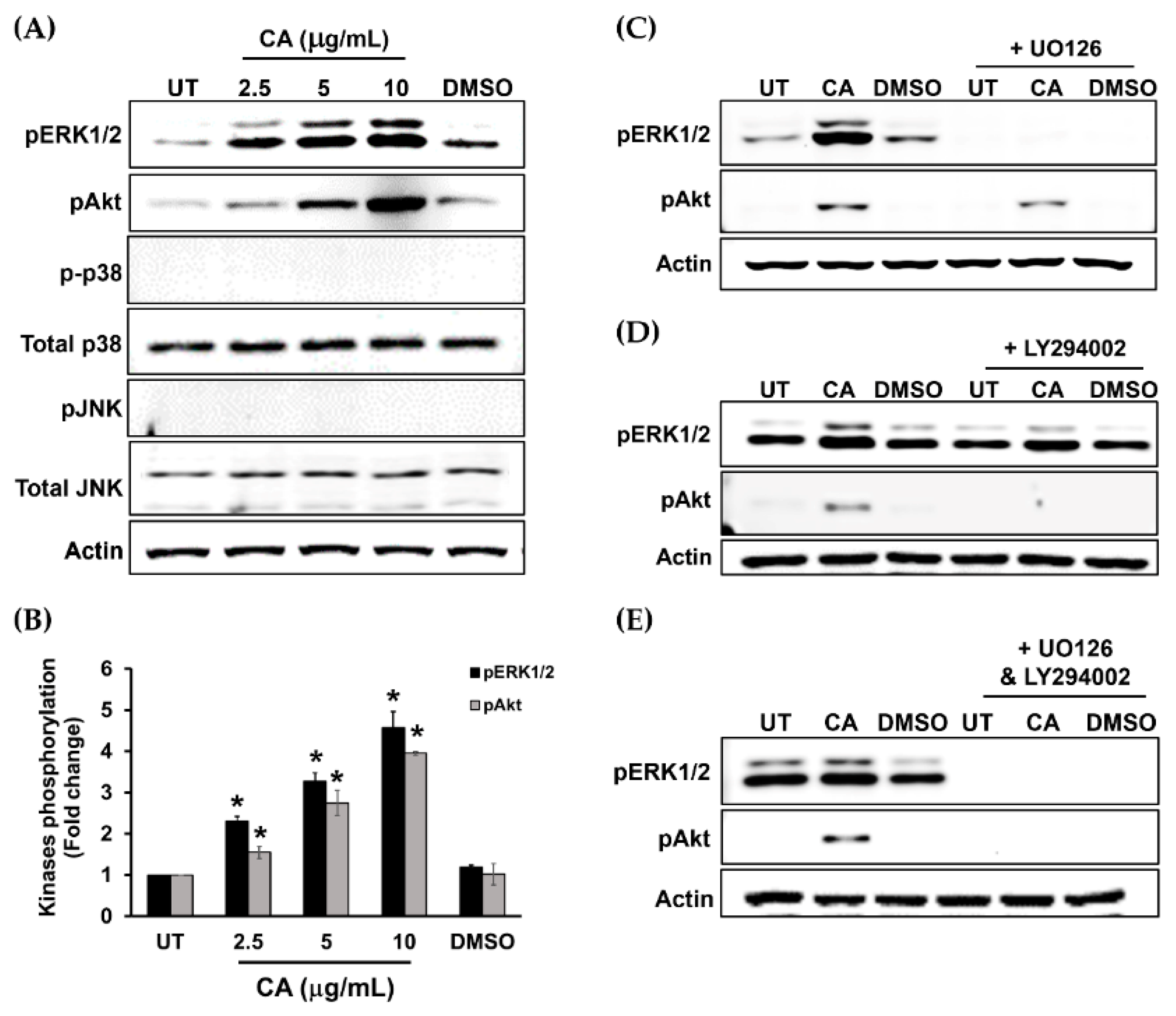
3.4. Proliferation and Survival Signal Transductions Are Induced in HaCaT Cells upon Treatment with CA Extract
3.5. Suppression of ERK1/2 and Akt Activation by Specific Inhibitors Attenuates CA Extract-Induced HaCaT Cell Monolayer Wound Healing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuwahara, M.; Tada, H.; Mashiba, K.; Yurugi, S.; Iioka, H.; Niitsuma, K.; Yasuda, Y. Mortality and recurrence rate after pressure ulcer operation for elderly long-term bedridden patients. Ann. Plast. Surg. 2005, 54, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Boyko, E.J.; Ahroni, J.H.; Smith, D.G.; Davignon, D. Increased mortality associated with diabetic foot ulcer. Diabet. Med. 1996, 13, 967–972. [Google Scholar] [CrossRef]
- Sundaram, G.M.; Quah, S.; Sampath, P. Cancer: The dark side of wound healing. FEBS J. 2018, 285, 4516–4534. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Van de Vijver, E.; Maddalena, A.; Sanal, O.; Holland, S.M.; Uzel, G.; Madkaikar, M.; de Boer, M.; van Leeuwen, K.; Koker, M.Y.; Parvaneh, N.; et al. Hematologically important mutations: Leukocyte adhesion deficiency (first update). Blood Cells Mol. Dis. 2012, 48, 53–61. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Kawasumi, A.; Sagawa, N.; Hayashi, S.; Yokoyama, H.; Tamura, K. Wound healing in mammals and amphibians: Toward limb regeneration in mammals. Curr. Top. Microbiol. Immunol. 2013, 367, 33–49. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Usui, M.L.; Mansbridge, J.N.; Carter, W.G.; Fujita, M.; Olerud, J.E. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J. Histochem. Cytochem. 2008, 56, 687–696. [Google Scholar] [CrossRef]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired wound healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Jinnin, M.; Muchemwa, F.C.; Fukushima, S.; Kogushi-Nishi, H.; Moriya, C.; Igata, T.; Fujisawa, A.; Johno, T.; Ihn, H. Basic fibroblast growth factor stimulates the proliferation of human dermal fibroblasts via the ERK1/2 and JNK pathways. Br. J. Dermatol. 2010, 162, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y.; Ebisuya, M.; Honjoh, S.; Nishida, E. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr. Biol. 2004, 14, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Shono, T.; Kanetake, H.; Kanda, S. The role of mitogen-activated protein kinase activation within focal adhesions in chemotaxis toward FGF-2 by murine brain capillary endothelial cells. Exp. Cell Res. 2001, 264, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Anand-Apte, B.; Zetter, B.R.; Viswanathan, A.; Qiu, R.G.; Chen, J.; Ruggieri, R.; Symons, M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J. Biol. Chem. 1997, 272, 30688–30692. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Pallero, M.A.; Gupta, K.; Chang, P.; Ware, M.F.; Witke, W.; Kwiatkowski, D.J.; Lauffenburger, D.A.; Murphy-Ullrich, J.E.; Wells, A. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility-associated PLCgamma signaling pathway. J. Cell Sci. 1998, 111 Pt 5, 615–624. [Google Scholar] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef]
- Zeng, F.; Harris, R.C. Epidermal growth factor, from gene organization to bedside. Semin. Cell Dev. Biol. 2014, 28, 2–11. [Google Scholar] [CrossRef]
- Anderson, K.S.; Petersson, S.; Wong, J.; Shubbar, E.; Lokko, N.N.; Carlstrom, M.; Enerback, C. Elevation of serum epidermal growth factor and interleukin 1 receptor antagonist in active Psoriasis vulgaris. Br. J. Dermatol. 2010, 163, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.K.; Choudhury, P.K.; Srivastava, R.; Sharma, M. Antimicrobial, anti-inflammatory and wound healing activity of polyherbal formulation. Biomed. Pharmacother. 2019, 111, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bartolo, P.J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Maver, U.; Stana Kleinschek, K.; Smrke, D.M.; Kreft, S. A review of herbal medicines in wound healing. Int. J. Dermatol. 2015, 54, 740–751. [Google Scholar] [CrossRef]
- Liu, C.L.; Tam, J.C.; Sanders, A.J.; Ko, C.H.; Fung, K.P.; Leung, P.C.; Harding, K.G.; Jiang, W.G.; Lau, C.B. Molecular angiogenic events of a two-herb wound healing formula involving MAPK and Akt signaling pathways in human vascular endothelial cells. Wound Repair Regen. 2013, 21, 579–587. [Google Scholar] [CrossRef]
- Bueno, F.G.; Moreira, E.A.; Morais, G.R.; Pacheco, I.A.; Baesso, M.L.; Leite-Mello, E.V.; Mello, J.C. Enhanced Cutaneous Wound Healing In Vivo by Standardized Crude Extract of Poincianella pluviosa. PLoS ONE 2016, 11, e0149223. [Google Scholar] [CrossRef]
- Ruttanapattanakul, J.; Wikan, N.; Okonogi, S.; Na Takuathung, M.; Buacheen, P.; Pitchakarn, P.; Potikanond, S.; Nimlamool, W. Boesenbergia rotunda extract accelerates human keratinocyte proliferation through activating ERK1/2 and PI3K/Akt kinases. Biomed. Pharmacother. 2021, 133, 111002. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Kheeree, N.; Sangvanich, P.; Puthong, S.; Karnchanatat, A. Antifungal and antiproliferative activities of lectin from the rhizomes of Curcuma amarissima Roscoe. Appl. Biochem. Biotechnol. 2010, 162, 912–925. [Google Scholar] [CrossRef]
- Muthachan, T.; Tewtrakul, S. Anti-inflammatory and wound healing effects of gel containing Kaempferia marginata extract. J. Ethnopharmacol. 2019, 240, 111964. [Google Scholar] [CrossRef]
- Srirod, S.; Tewtrakul, S. Anti-inflammatory and wound healing effects of cream containing Curcuma mangga extract. J. Ethnopharmacol. 2019, 238, 111828. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Mohamed, T.A.; Essa, A.F.; Abd-ElGawad, A.M.; Alqahtani, A.S.; Shahat, A.A.; Yoneyama, T.; Farrag, A.R.H.; Noji, M.; El-Seedi, H.R.; et al. Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review. Nutrients 2019, 11, 2396. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Akarchariya, N.; Sirilun, S.; Julsrigival, J.; Chansakaowa, S. Chemical profiling and antimicrobial activity of essential oil from Curcuma aeruginosa Roxb., Curcuma glans K. Larsen & J. Mood and Curcuma cf. xanthorrhiza Roxb. collected in Thailand. Asian Pac. J. Trop. Biomed. 2017, 7, 881–885. [Google Scholar] [CrossRef]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Rubinfeld, H.; Seger, R. The ERK cascade as a prototype of MAPK signaling pathways. Methods Mol. Biol. 2004, 250, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Rubinfeld, H.; Seger, R. The ERK cascade: A prototype of MAPK signaling. Mol. Biotechnol. 2005, 31, 151–174. [Google Scholar] [CrossRef]
- Shi, J.; Zeng, X.; Zhou, M.; Chen, Q. Activation of ERK-FAK signaling pathway and enhancement of cell migration involved in the early interaction between oral keratinocytes and Candida albicans. Mycopathologia 2009, 167, 1–7. [Google Scholar] [CrossRef]
- Wahedi, H.M.; Park, Y.U.; Moon, E.Y.; Kim, S.Y. Juglone ameliorates skin wound healing by promoting skin cell migration through Rac1/Cdc42/PAK pathway. Wound Repair Regen. 2016, 24, 786–794. [Google Scholar] [CrossRef]
- Ye, M.; Hu, D.; Tu, L.; Zhou, X.; Lu, F.; Wen, B.; Wu, W.; Lin, Y.; Zhou, Z.; Qu, J. Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 497–504. [Google Scholar] [CrossRef]
- Du, J.; Sun, C.; Hu, Z.; Yang, Y.; Zhu, Y.; Zheng, D.; Gu, L.; Lu, X. Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. PLoS ONE 2010, 5, e15940. [Google Scholar] [CrossRef]
- Xiong, W.; Cheng, B.H.; Jia, S.B.; Tang, L.S. Involvement of the PI3K/Akt signaling pathway in platelet-derived growth factor-induced migration of human lens epithelial cells. Curr. Eye Res. 2010, 35, 389–401. [Google Scholar] [CrossRef]
- Wei, L.H.; Kuo, M.L.; Chen, C.A.; Chou, C.H.; Cheng, W.F.; Chang, M.C.; Su, J.L.; Hsieh, C.Y. The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI 3-K/Akt pathway. Oncogene 2001, 20, 5799–5809. [Google Scholar] [CrossRef]
- Jourdan, M.; Veyrune, J.L.; De Vos, J.; Redal, N.; Couderc, G.; Klein, B. A major role for Mcl-1 antiapoptotic protein in the IL-6-induced survival of human myeloma cells. Oncogene 2003, 22, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.Y.; Rios, A.C.; Pal, B.; Soetanto, R.; Lun, A.T.; Liu, K.; Beck, T.; Best, S.A.; Vaillant, F.; Bouillet, P.; et al. EGF-mediated induction of Mcl-1 at the switch to lactation is essential for alveolar cell survival. Nat. Cell Biol. 2015, 17, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Booy, E.P.; Henson, E.S.; Gibson, S.B. Epidermal growth factor regulates Mcl-1 expression through the MAPK-Elk-1 signalling pathway contributing to cell survival in breast cancer. Oncogene 2011, 30, 2367–2378. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef]
- Carbone, C.; Caddeo, C.; Grimaudo, M.A.; Manno, D.E.; Serra, A.; Musumeci, T. Ferulic Acid-NLC with Lavandula Essential Oil: A Possible Strategy for Wound-Healing? Nanomaterials 2020, 10, 898. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 115. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Brandner, J.M.; Zacheja, S.; Houdek, P.; Moll, I.; Lobmann, R. Expression of matrix metalloproteinases, cytokines, and connexins in diabetic and nondiabetic human keratinocytes before and after transplantation into an ex vivo wound-healing model. Diabetes Care 2008, 31, 114–120. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimlamool, W.; Potikanond, S.; Ruttanapattanakul, J.; Wikan, N.; Okonogi, S.; Jantrapirom, S.; Pitchakarn, P.; Karinchai, J. Curcuma amarissima Extract Activates Growth and Survival Signal Transduction Networks to Stimulate Proliferation of Human Keratinocyte. Biology 2021, 10, 289. https://doi.org/10.3390/biology10040289
Nimlamool W, Potikanond S, Ruttanapattanakul J, Wikan N, Okonogi S, Jantrapirom S, Pitchakarn P, Karinchai J. Curcuma amarissima Extract Activates Growth and Survival Signal Transduction Networks to Stimulate Proliferation of Human Keratinocyte. Biology. 2021; 10(4):289. https://doi.org/10.3390/biology10040289
Chicago/Turabian StyleNimlamool, Wutigri, Saranyapin Potikanond, Jirapak Ruttanapattanakul, Nitwara Wikan, Siriporn Okonogi, Salinee Jantrapirom, Pornsiri Pitchakarn, and Jirarat Karinchai. 2021. "Curcuma amarissima Extract Activates Growth and Survival Signal Transduction Networks to Stimulate Proliferation of Human Keratinocyte" Biology 10, no. 4: 289. https://doi.org/10.3390/biology10040289
APA StyleNimlamool, W., Potikanond, S., Ruttanapattanakul, J., Wikan, N., Okonogi, S., Jantrapirom, S., Pitchakarn, P., & Karinchai, J. (2021). Curcuma amarissima Extract Activates Growth and Survival Signal Transduction Networks to Stimulate Proliferation of Human Keratinocyte. Biology, 10(4), 289. https://doi.org/10.3390/biology10040289







