Cell-Based Neuroprotection of Retinal Ganglion Cells in Animal Models of Optic Neuropathies
Simple Summary
Abstract
1. Introduction
2. Diverse Genetically Non-Modified Cell Types Promote Retinal Ganglion Cell Survival
2.1. Mesenchymal Stem Cells
2.1.1. Bone Marrow-Derived Mesenchymal Stem Cells
2.1.2. Umbilical Cord-Derived Mesenchymal Stem Cells
2.1.3. Adipose-Derived Mesenchymal Stem Cells
2.1.4. Dental Pulp Stem Cells
2.2. Other Cell Types
2.3. Proposed Mechanisms of Neuroprotection Conferred by Grafted Cells
3. Retinal Ganglion Cell Protection Using Genetically Modified Cells
3.1. Brain-Derived Neurotrophic Factor
3.2. Ciliary Neurotrophic Factor
3.3. Glial Cell Line-Derived Neurotrophic Factor
3.4. Other Neuroprotective Factors
4. Boosting Retinal Ganglion Cell Survival with Cell-Based Combinatorial Neuroprotective Approaches
5. Retinal Ganglion Cell Protection with Neurotrophic Factor Combinations
Cell-Based Administration of Neurotrophic Factor Combinations
6. Translating Cell-Based Neuroprotective Approaches to Clinical Applications
7. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanes, J.R.; Masland, R.H. The types of retinal ganglion cells: Current status and implications for neuronal classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef]
- Baden, T.; Berens, P.; Franke, K.; Roson, M.R.; Bethge, M.; Euler, T. The functional diversity of retinal ganglion cells in the mouse. Nature 2016, 529, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Rheaume, B.A.; Jereen, A.; Bolisetty, M.; Sajid, M.S.; Yang, Y.; Renna, K.; Sun, L.; Robson, P.; Trakhtenberg, E.F. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat. Commun. 2018, 9, 2759. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Ahmed, Z.; Logan, A. Return of function after CNS axon regeneration: Lessons from injury-responsive intrinsically photosensitive and alpha retinal ganglion cells. Prog. Retin. Eye Res. 2019, 71, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Qiao, M.; Bei, F.; Kim, I.J.; He, Z.; Sanes, J.R. Subtype-specific regeneration of retinal ganglion cells following axotomy: Effects of osteopontin and mTOR signaling. Neuron 2015, 85, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Ren, C.; Sollars, P.J.; Pickard, G.E.; So, K.F. The injury resistant ability of melanopsin-expressing intrinsically photosensitive retinal ganglion cells. Neuroscience 2015, 284, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Della Santina, L.; Ou, Y. Who’s lost first? Susceptibility of retinal ganglion cell types in experimental glaucoma. Exp. Eye Res. 2017, 158, 43–50. [Google Scholar] [CrossRef]
- VanderWall, K.B.; Lu, B.; Alfaro, J.S.; Allsop, A.R.; Carr, A.S.; Wang, S.; Meyer, J.S. Differential susceptibility of retinal ganglion cell subtypes in acute and chronic models of injury and disease. Sci. Rep. 2020, 10, 17359. [Google Scholar] [CrossRef]
- Pascale, A.; Drago, F.; Govoni, S. Protecting the retinal neurons from glaucoma: Lowering ocular pressure is not enough. Pharmacol. Res. 2012, 66, 19–32. [Google Scholar] [CrossRef]
- Caprioli, J. Neuroprotection of the optic nerve in glaucoma. Acta Ophthalmol. Scand. 1997, 75, 364–367. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hussein, M.; Bengtsson, B.; Hyman, L.; Komaroff, E.; Early Manifest Glaucoma Trial, G. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch. Ophthalmol. 2003, 121, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Levin, L.A. Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annu. Rev. Vis. Sci. 2017, 3, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Vargas, J.L.C.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Qu, J.; Wang, D.; Grosskreutz, C.L. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp. Eye Res. 2010, 91, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Gupta, S.K.; Agarwal, P.; Saxena, R.; Agrawal, S.S. Current concepts in the pathophysiology of glaucoma. Indian J. Ophthalmol. 2009, 57, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, L.; Bruttini, C.; Micheletti, E.; Konstas, A.G.P.; Michelessi, M.; Oddone, F.; Katsanos, A.; Sbardella, D.; De Angelis, G.; Riva, I. Glaucoma and neuroinflammation: An overview. Surv. Ophthalmol. 2021, 66, 693–713. [Google Scholar] [CrossRef]
- Pang, I.H.; Clark, A.F. Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef]
- Williams, P.A.; Morgan, J.E.; Votruba, M. Mouse models of dominant optic atrophy: What do they tell us about the pathophysiology of visual loss? Vis. Res. 2011, 51, 229–234. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, S.J.; Schlamp, C.L.; Nickells, R.W. Mouse models of retinal ganglion cell death and glaucoma. Exp. Eye Res. 2009, 88, 816–824. [Google Scholar] [CrossRef]
- Johnson, T.V.; Tomarev, S.I. Rodent models of glaucoma. Brain Res. Bull. 2010, 81, 349–358. [Google Scholar] [CrossRef]
- Bastakis, G.G.; Ktena, N.; Karagogeos, D.; Savvaki, M. Models and treatments for traumatic optic neuropathy and demyelinating optic neuritis. Dev. Neurobiol. 2019, 79, 819–836. [Google Scholar] [CrossRef]
- Bernstein, S.L.; Miller, N.R. Ischemic optic neuropathies and their models: Disease comparisons, model strengths and weaknesses. Jpn. J. Ophthalmol. 2015, 59, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Levkovitch-Verbin, H. Animal models of optic nerve diseases. Eye 2004, 18, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, Y.; Yao, K. Protection of retinal ganglion cells in glaucoma: Current status and future. Exp. Eye Res. 2021, 205, 108506. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lo, A.C.; Lai, J.S.; Shih, K.C. The role of electrical stimulation therapy in ophthalmic diseases. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 171–176. [Google Scholar] [CrossRef]
- Amore, G.; Romagnoli, M.; Carbonelli, M.; Barboni, P.; Carelli, V.; La Morgia, C. Therapeutic Options in Hereditary Optic Neuropathies. Drugs 2021, 81, 57–86. [Google Scholar] [CrossRef]
- Fu, L.; Kwok, S.S.; Chan, Y.K.; Lai, J.S.M.; Pan, W.; Nie, L.; Shih, K.C. Therapeutic Strategies for Attenuation of Retinal Ganglion Cell Injury in Optic Neuropathies: Concepts in Translational Research and Therapeutic Implications. Biomed. Res. Int. 2019, 2019, 8397521. [Google Scholar] [CrossRef]
- Tribble, J.R.; Hui, F.; Joe, M.; Bell, K.; Chrysostomou, V.; Crowston, J.G.; Williams, P.A. Targeting Diet and Exercise for Neuroprotection and Neurorecovery in Glaucoma. Cells 2021, 10, 295. [Google Scholar] [CrossRef]
- Adornetto, A.; Russo, R.; Parisi, V. Neuroinflammation as a target for glaucoma therapy. Neural Regen. Res. 2019, 14, 391–394. [Google Scholar] [CrossRef]
- Rolle, T.; Ponzetto, A.; Malinverni, L. The Role of Neuroinflammation in Glaucoma: An Update on Molecular Mechanisms and New Therapeutic Options. Front. Neurol. 2020, 11, 612422. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Shih, K.C. Use of Gene Therapy in Retinal Ganglion Cell Neuroprotection: Current Concepts and Future Directions. Biomolecules 2021, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Miltner, A.M.; La Torre, A. Retinal Ganglion Cell Replacement: Current Status and Challenges Ahead. Dev. Dyn. 2019, 248, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.Q.; Liu, H.; Wang, N.; Jin, Z.B. Towards stem cell-based neuronal regeneration for glaucoma. Prog. Brain Res. 2020, 257, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.B.; Gao, M.L.; Deng, W.L.; Wu, K.C.; Sugita, S.; Mandai, M.; Takahashi, M. Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 2019, 69, 38–56. [Google Scholar] [CrossRef]
- Rabesandratana, O.; Goureau, O.; Orieux, G. Pluripotent Stem Cell-Based Approaches to Explore and Treat Optic Neuropathies. Front. Neurosci. 2018, 12, 651. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Aguzzi, E.A.; Johnson, T.V. Retinal Ganglion Cell Transplantation: Approaches for Overcoming Challenges to Functional Integration. Cells 2021, 10, 1426. [Google Scholar] [CrossRef] [PubMed]
- Boia, R.; Ruzafa, N.; Aires, I.D.; Pereiro, X.; Ambrosio, A.F.; Vecino, E.; Santiago, A.R. Neuroprotective Strategies for Retinal Ganglion Cell Degeneration: Current Status and Challenges Ahead. Int. J. Mol. Sci. 2020, 21, 2262. [Google Scholar] [CrossRef]
- Khatib, T.Z.; Martin, K.R. Neuroprotection in Glaucoma: Towards Clinical Trials and Precision Medicine. Curr. Eye Res. 2020, 45, 327–338. [Google Scholar] [CrossRef]
- Tsai, J.C. Innovative IOP-Independent Neuroprotection and Neuroregeneration Strategies in the Pipeline for Glaucoma. J. Ophthalmol. 2020, 2020, 9329310. [Google Scholar] [CrossRef]
- Claes, M.; De Groef, L.; Moons, L. Target-Derived Neurotrophic Factor Deprivation Puts Retinal Ganglion Cells on Death Row: Cold Hard Evidence and Caveats. Int. J. Mol. Sci. 2019, 20, 4314. [Google Scholar] [CrossRef] [PubMed]
- Fudalej, E.; Justyniarska, M.; Kasarello, K.; Dziedziak, J.; Szaflik, J.P.; Cudnoch-Jedrzejewska, A. Neuroprotective factors of the retina and their role in promoting survival of retinal ganglion cells: A review. Ophthalmic. Res. 2021, 64, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.R.; Ooi, J.W.; Rodger, J. Neurotrophic factors and the regeneration of adult retinal ganglion cell axons. Int. Rev. Neurobiol. 2012, 106, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Di Polo, A. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Ther. 2012, 19, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Fellabaum, C.; Arsenijevic, A.; Markovic, B.S.; Djonov, V.; Volarevic, V. Therapeutic Potential of Mesenchymal Stem Cells and Their Secretome in the Treatment of Glaucoma. Stem Cells Int. 2019, 2019, 7869130. [Google Scholar] [CrossRef] [PubMed]
- Holan, V.; Palacka, K.; Hermankova, B. Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials. Cells 2021, 10, 588. [Google Scholar] [CrossRef]
- Johnson, T.V.; Martin, K.R. Cell transplantation approaches to retinal ganglion cell neuroprotection in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Plant, G.W.; Harvey, A.R.; Leaver, S.G.; Lee, S.V. Olfactory ensheathing glia: Repairing injury to the mammalian visual system. Exp. Neurol. 2011, 229, 99–108. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. Retinal ganglion cell neuroprotection by growth factors and exosomes: Lessons from mesenchymal stem cells. Neural Regen. Res. 2018, 13, 228–229. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. Extracellular vesicle therapy for retinal diseases. Prog. Retin. Eye Res. 2020, 79, 100849. [Google Scholar] [CrossRef]
- Lucas-Ruiz, F.; Galindo-Romero, C.; Garcia-Bernal, D.; Norte-Munoz, M.; Rodriguez-Ramirez, K.T.; Salinas-Navarro, M.; Millan-Rivero, J.E.; Vidal-Sanz, M.; Agudo-Barriuso, M. Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters? Neural Regen. Res. 2019, 14, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Berry, M.; Logan, A.; Scott, R.A.; Leadbeater, W.; Scheven, B.A. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015, 14, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Concise Review: Dental Pulp Stem Cells: A Novel Cell Therapy for Retinal and Central Nervous System Repair. Stem Cells 2017, 35, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mesentier-Louro, L.A.; Zaverucha-do-Valle, C.; Rosado-de-Castro, P.H.; Silva-Junior, A.J.; Pimentel-Coelho, P.M.; Mendez-Otero, R.; Santiago, M.F. Bone Marrow-Derived Cells as a Therapeutic Approach to Optic Nerve Diseases. Stem Cells Int. 2016, 2016, 5078619. [Google Scholar] [CrossRef]
- Pearson, C.; Martin, K. Stem cell approaches to glaucoma: From aqueous outflow modulation to retinal neuroprotection. Prog. Brain Res. 2015, 220, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Williams, A.; Waisbourd, M.; Iacovitti, L.; Katz, L.J. Stem cell therapy for glaucoma: Science or snake oil? Surv. Ophthalmol. 2015, 60, 93–105. [Google Scholar] [CrossRef]
- Patel, D.M.; Shah, J.; Srivastava, A.S. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013, 2013, 496218. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Alvarez-Sanchez, S.; Gonzalez-Zamora, J.; Carretero-Barrio, I.; Pastor, J.C.; Fernandez-Bueno, I.; Srivastava, G.K. Mesenchymal stem cell therapy in retinal and optic nerve diseases: An update of clinical trials. World J. Stem Cells 2016, 8, 376–383. [Google Scholar] [CrossRef]
- Xu, W.; Xu, G.X. Mesenchymal stem cells for retinal diseases. Int. J. Ophthalmol. 2011, 4, 413–421. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Johnson, T.V.; Bull, N.D.; Hunt, D.P.; Marina, N.; Tomarev, S.I.; Martin, K.R. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2051–2059. [Google Scholar] [CrossRef]
- Hu, Y.; Tan, H.B.; Wang, X.M.; Rong, H.; Cui, H.P.; Cui, H. Bone marrow mesenchymal stem cells protect against retinal ganglion cell loss in aged rats with glaucoma. Clin. Interv. Aging 2013, 8, 1467–1470. [Google Scholar] [CrossRef]
- Yu, S.; Tanabe, T.; Dezawa, M.; Ishikawa, H.; Yoshimura, N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem. Biophys. Res. Commun. 2006, 344, 1071–1079. [Google Scholar] [CrossRef]
- Roubeix, C.; Godefroy, D.; Mias, C.; Sapienza, A.; Riancho, L.; Degardin, J.; Fradot, V.; Ivkovic, I.; Picaud, S.; Sennlaub, F.; et al. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res. Ther. 2015, 6, 177. [Google Scholar] [CrossRef]
- Mead, B.; Hill, L.J.; Blanch, R.J.; Ward, K.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18, 487–496. [Google Scholar] [CrossRef]
- Emre, E.; Yuksel, N.; Duruksu, G.; Pirhan, D.; Subasi, C.; Erman, G.; Karaoz, E. Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow-derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 2015, 17, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Levkovitch-Verbin, H.; Sadan, O.; Vander, S.; Rosner, M.; Barhum, Y.; Melamed, E.; Offen, D.; Melamed, S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6394–6400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, Z.L.; Li, N.; Wei, X.; Tang, L.; Wang, T.H.; Chen, X.M. Neuroprotective effects of BDNF and GDNF in intravitreally transplanted mesenchymal stem cells after optic nerve crush in mice. Int. J. Ophthalmol. 2017, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mesentier-Louro, L.A.; Zaverucha-do-Valle, C.; da Silva-Junior, A.J.; Nascimento-Dos-Santos, G.; Gubert, F.; de Figueiredo, A.B.; Torres, A.L.; Paredes, B.D.; Teixeira, C.; Tovar-Moll, F.; et al. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS ONE 2014, 9, e110722. [Google Scholar] [CrossRef]
- Tan, H.; Kang, X.; Lu, S.; Liu, L. The therapeutic effects of bone marrow mesenchymal stem cells after optic nerve damage in the adult rat. Clin. Interv. Aging 2015, 10, 487–490. [Google Scholar] [CrossRef][Green Version]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7544–7556. [Google Scholar] [CrossRef] [PubMed]
- Millan-Rivero, J.E.; Nadal-Nicolas, F.M.; Garcia-Bernal, D.; Sobrado-Calvo, P.; Blanquer, M.; Moraleda, J.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Human Wharton’s jelly mesenchymal stem cells protect axotomized rat retinal ganglion cells via secretion of anti-inflammatory and neurotrophic factors. Sci. Rep. 2018, 8, 16299. [Google Scholar] [CrossRef]
- da Silva-Junior, A.J.; Mesentier-Louro, L.A.; Nascimento-Dos-Santos, G.; Teixeira-Pinheiro, L.C.; Vasques, J.F.; Chimeli-Ormonde, L.; Bodart-Santos, V.; de Carvalho, L.R.P.; Santiago, M.F.; Mendez-Otero, R. Human mesenchymal stem cell therapy promotes retinal ganglion cell survival and target reconnection after optic nerve crush in adult rats. Stem Cell Res. Ther. 2021, 12, 69. [Google Scholar] [CrossRef]
- Ji, S.; Lin, S.; Chen, J.; Huang, X.; Wei, C.C.; Li, Z.; Tang, S. Neuroprotection of Transplanting Human Umbilical Cord Mesenchymal Stem Cells in a Microbead Induced Ocular Hypertension Rat Model. Curr. Eye Res. 2018, 43, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiang, Z.; Cai, J. The anti-apoptotic and neuro-protective effects of human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) on acute optic nerve injury is transient. Brain Res. 2013, 1532, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef]
- Xie, L.; Mao, M.; Zhou, L.; Zhang, L.; Jiang, B. Signal Factors Secreted by 2D and Spheroid Mesenchymal Stem Cells and by Cocultures of Mesenchymal Stem Cells Derived Microvesicles and Retinal Photoreceptor Neurons. Stem Cells Int. 2017, 2017, 2730472. [Google Scholar] [CrossRef]
- Huang, W.; Wang, C.; Xie, L.; Wang, X.; Zhang, L.; Chen, C.; Jiang, B. Traditional two-dimensional mesenchymal stem cells (MSCs) are better than spheroid MSCs on promoting retinal ganglion cells survival and axon regeneration. Exp. Eye Res. 2019, 185, 107699. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, S.; Wang, L. Therapeutic effect of adiposederived stem cell transplantation on optic nerve injury in rats. Mol. Med. Rep. 2018, 17, 2529–2534. [Google Scholar] [CrossRef]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: Comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS ONE 2014, 9, e109305. [Google Scholar] [CrossRef]
- Su, Z.; He, C. Olfactory ensheathing cells: Biology in neural development and regeneration. Prog. Neurobiol. 2010, 92, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.H.; Lu, C.Y.; Wei, L.; Fan, J.R.; Yu, Y.L.; Shyu, W.C. The potential therapeutic applications of olfactory ensheathing cells in regenerative medicine. Cell Transplant. 2014, 23, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Khaw, P.T.; Yin, Z.Q.; Li, D.; Raisman, G.; Li, Y. Olfactory Ensheathing Cells Rescue Optic Nerve Fibers in a Rat Glaucoma Model. Transl. Vis. Sci. Technol. 2012, 1, 3. [Google Scholar] [CrossRef]
- Wu, M.M.; Fan, D.G.; Tadmori, I.; Yang, H.; Furman, M.; Jiao, X.Y.; Young, W.; Sun, D.; You, S.W. Death of axotomized retinal ganglion cells delayed after intraoptic nerve transplantation of olfactory ensheathing cells in adult rats. Cell Transplant. 2010, 19, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sauve, Y.; Li, D.; Lund, R.D.; Raisman, G. Transplanted olfactory ensheathing cells promote regeneration of cut adult rat optic nerve axons. J. Neurosci. 2003, 23, 7783–7788. [Google Scholar] [CrossRef] [PubMed]
- Bull, N.D.; Irvine, K.A.; Franklin, R.J.; Martin, K.R. Transplanted oligodendrocyte precursor cells reduce neurodegeneration in a model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4244–4253. [Google Scholar] [CrossRef]
- Bray, G.M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Aguayo, A.J. The use of peripheral nerve grafts to enhance neuronal survival, promote growth and permit terminal reconnections in the central nervous system of adult rats. J. Exp. Biol. 1987, 132, 5–19. [Google Scholar] [CrossRef]
- Dezawa, M.; Adachi-Usami, E. Role of Schwann cells in retinal ganglion cell axon regeneration. Prog. Retin. Eye Res. 2000, 19, 171–204. [Google Scholar] [CrossRef]
- Harvey, A.R.; Hu, Y.; Leaver, S.G.; Mellough, C.B.; Park, K.; Verhaagen, J.; Plant, G.W.; Cui, Q. Gene therapy and transplantation in CNS repair: The visual system. Prog. Retin. Eye Res. 2006, 25, 449–489. [Google Scholar] [CrossRef]
- Johnson, T.V.; DeKorver, N.W.; Levasseur, V.A.; Osborne, A.; Tassoni, A.; Lorber, B.; Heller, J.P.; Villasmil, R.; Bull, N.D.; Martin, K.R.; et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2014, 137, 503–519. [Google Scholar] [CrossRef]
- Wang, T.; Cong, R.; Yang, H.; Wu, M.M.; Luo, N.; Kuang, F.; You, S.W. Neutralization of BDNF attenuates the in vitro protective effects of olfactory ensheathing cell-conditioned medium on scratch-insulted retinal ganglion cells. Cell. Mol. Neurobiol. 2011, 31, 357–364. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, N.; Xu, W.; Xu, G. Mesenchymal stem cells attenuate hydrogen peroxide-induced oxidative stress and enhance neuroprotective effects in retinal ganglion cells. Vitro Cell. Dev. Biol. Anim. 2017, 53, 328–335. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, J.X. Gender difference in the neuroprotective effect of rat bone marrow mesenchymal cells against hypoxia-induced apoptosis of retinal ganglion cells. Neural Regen. Res. 2016, 11, 846–853. [Google Scholar] [CrossRef]
- Yu, K.; Ge, J.; Summers, J.B.; Li, F.; Liu, X.; Ma, P.; Kaminski, J.; Zhuang, J. TSP-1 secreted by bone marrow stromal cells contributes to retinal ganglion cell neurite outgrowth and survival. PLoS ONE 2008, 3, e2470. [Google Scholar] [CrossRef] [PubMed]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Di Lauro, S.; Garcia-Gutierrez, M.T.; Srivastava, G.K.; Pastor, J.C.; Fernandez-Bueno, I. Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp. Eye Res. 2019, 185, 107671. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.R.; Yuan, J.Q. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 503–514. [Google Scholar] [CrossRef]
- Johnson, T.V.; Bull, N.D.; Martin, K.R. Identification of barriers to retinal engraftment of transplanted stem cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 960–970. [Google Scholar] [CrossRef]
- Dreixler, J.C.; Poston, J.N.; Balyasnikova, I.; Shaikh, A.R.; Tupper, K.Y.; Conway, S.; Boddapati, V.; Marcet, M.M.; Lesniak, M.S.; Roth, S. Delayed administration of bone marrow mesenchymal stem cell conditioned medium significantly improves outcome after retinal ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3785–3796. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Dreixler, J.C.; Mathew, B.; Balyasnikova, I.; Mann, J.R.; Boddapati, V.; Xue, L.; Lesniak, M.S. Hypoxic-Preconditioned Bone Marrow Stem Cell Medium Significantly Improves Outcome After Retinal Ischemia in Rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3522–3532. [Google Scholar] [CrossRef] [PubMed]
- Zwart, I.; Hill, A.J.; Al-Allaf, F.; Shah, M.; Girdlestone, J.; Sanusi, A.B.; Mehmet, H.; Navarrete, R.; Navarrete, C.; Jen, L.S. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp. Neurol. 2009, 216, 439–448. [Google Scholar] [CrossRef]
- Lipson, A.C.; Widenfalk, J.; Lindqvist, E.; Ebendal, T.; Olson, L. Neurotrophic properties of olfactory ensheathing glia. Exp. Neurol. 2003, 180, 167–171. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Y.; Tang, L.; Li, Y.; Fan, F.; Jiang, B. Protective effects of human umbilical cord blood stem cell intravitreal transplantation against optic nerve injury in rats. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1021–1028. [Google Scholar] [CrossRef]
- Wilkins, A.; Majed, H.; Layfield, R.; Compston, A.; Chandran, S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: A novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J. Neurosci. 2003, 23, 4967–4974. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.; Chandran, S.; Compston, A. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia 2001, 36, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Froger, N.; Matonti, F.; Roubeix, C.; Forster, V.; Ivkovic, I.; Brunel, N.; Baudouin, C.; Sahel, J.A.; Picaud, S. VEGF is an autocrine/paracrine neuroprotective factor for injured retinal ganglion neurons. Sci. Rep. 2020, 10, 12409. [Google Scholar] [CrossRef]
- Bampton, E.T.; Ma, C.H.; Tolkovsky, A.M.; Taylor, J.S. Osteonectin is a Schwann cell-secreted factor that promotes retinal ganglion cell survival and process outgrowth. Eur. J. Neurosci. 2005, 21, 2611–2623. [Google Scholar] [CrossRef]
- Osborne, A.; Sanderson, J.; Martin, K.R. Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells. Stem Cells 2018, 36, 65–78. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, C.; Huang, L.; Chen, J.; Xu, N. Protective effects of intravitreal administration of mesenchymal stem cell-derived exosomes in an experimental model of optic nerve injury. Exp. Cell Res. 2021, 407, 112792. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Pan, D.; Chang, X.; Xu, M.; Zhang, M.; Zhang, S.; Wang, Y.; Luo, X.; Xu, J.; Yang, X.; Sun, X. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J. Chem. Neuroanat. 2019, 96, 134–139. [Google Scholar] [CrossRef]
- Seyedrazizadeh, S.Z.; Poosti, S.; Nazari, A.; Alikhani, M.; Shekari, F.; Pakdel, F.; Shahpasand, K.; Satarian, L.; Baharvand, H. Extracellular vesicles derived from human ES-MSCs protect retinal ganglion cells and preserve retinal function in a rodent model of optic nerve injury. Stem Cell Res. Ther. 2020, 11, 203. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Guo, M.; Dong, X.; Liao, M.; Du, M.; Wang, X.; Yin, H.; Yan, H. Exosome-Mediated Delivery of the Neuroprotective Peptide PACAP38 Promotes Retinal Ganglion Cell Survival and Axon Regeneration in Rats With Traumatic Optic Neuropathy. Front. Cell Dev. Biol. 2021, 9, 659783. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Ahmed, Z.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5473–5480. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Amaral, J.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in Rodent Models of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 702–714. [Google Scholar] [CrossRef]
- Lani-Louzada, R.; Dias, M.S.; Linden, R.; de Toledo Ribas, V.; Petrs-Silva, H. Gene Therapy Strategies for Glaucomatous Neurodegeneration. Curr. Gene Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; Bull, N.D.; Martin, K.R. Neurotrophic factor delivery as a protective treatment for glaucoma. Exp. Eye Res. 2011, 93, 196–203. [Google Scholar] [CrossRef]
- Castillo, B., Jr.; del Cerro, M.; Breakefield, X.O.; Frim, D.M.; Barnstable, C.J.; Dean, D.O.; Bohn, M.C. Retinal ganglion cell survival is promoted by genetically modified astrocytes designed to secrete brain-derived neurotrophic factor (BDNF). Brain Res. 1994, 647, 30–36. [Google Scholar] [CrossRef]
- Harper, M.M.; Adamson, L.; Blits, B.; Bunge, M.B.; Grozdanic, S.D.; Sakaguchi, D.S. Brain-derived neurotrophic factor released from engineered mesenchymal stem cells attenuates glutamate- and hydrogen peroxide-mediated death of staurosporine-differentiated RGC-5 cells. Exp. Eye Res. 2009, 89, 538–548. [Google Scholar] [CrossRef]
- Harper, M.M.; Grozdanic, S.D.; Blits, B.; Kuehn, M.H.; Zamzow, D.; Buss, J.E.; Kardon, R.H.; Sakaguchi, D.S. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4506–4515. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zeng, M.; Ruan, Y.; Wu, H.; Chen, J.; Fan, Z.; Zhen, H. Protection of retinal ganglion cells against glaucomatous neuropathy by neurotrophin-producing, genetically modified neural progenitor cells in a rat model. Chin. Med. J. 2002, 115, 1394–1400. [Google Scholar] [PubMed]
- Hu, Y.; Leaver, S.G.; Plant, G.W.; Hendriks, W.T.J.; Niclou, S.P.; Verhaagen, J.; Harvey, A.R.; Cui, Q. Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Mol. Ther. 2005, 11, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Flachsbarth, K.; Kruszewski, K.; Jung, G.; Jankowiak, W.; Riecken, K.; Wagenfeld, L.; Richard, G.; Fehse, B.; Bartsch, U. Neural stem cell-based intraocular administration of ciliary neurotrophic factor attenuates the loss of axotomized ganglion cells in adult mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7029–7039. [Google Scholar] [CrossRef] [PubMed]
- Flachsbarth, K.; Jankowiak, W.; Kruszewski, K.; Helbing, S.; Bartsch, S.; Bartsch, U. Pronounced synergistic neuroprotective effect of GDNF and CNTF on axotomized retinal ganglion cells in the adult mouse. Exp. Eye Res. 2018, 176, 258–265. [Google Scholar] [CrossRef]
- Dulz, S.; Bassal, M.; Flachsbarth, K.; Riecken, K.; Fehse, B.; Schlichting, S.; Bartsch, S.; Bartsch, U. Intravitreal Co-Administration of GDNF and CNTF Confers Synergistic and Long-Lasting Protection against Injury-Induced Cell Death of Retinal Ganglion Cells in Mice. Cells 2020, 9, 2082. [Google Scholar] [CrossRef]
- Ma, J.; Guo, C.; Guo, C.; Sun, Y.; Liao, T.; Beattie, U.; Lopez, F.J.; Chen, D.F.; Lashkari, K. Transplantation of Human Neural Progenitor Cells Expressing IGF-1 Enhances Retinal Ganglion Cell Survival. PLoS ONE 2015, 10, e0125695. [Google Scholar] [CrossRef]
- Xie, J.X.; Feng, Y.; Yuan, J.M.; You, Z.D.; Lin, H.Y.; Lu, C.L.; Xu, J.J. Positive effects of bFGF modified rat amniotic epithelial cells transplantation on transected rat optic nerve. PLoS ONE 2015, 10, e0119119. [Google Scholar] [CrossRef]
- Zhang, W.M.; Zhang, Z.R.; Zhang, Y.G.; Gao, Y.S. Neural Stem Cell-based Intraocular Administration of Pigment Epithelium-derived Factor Promotes Retinal Ganglion Cell Survival and Axon Regeneration after Optic Nerve Crush Injury in Rat: An Experimental Study. Iran. J. Med Sci. 2016, 41, 382–390. [Google Scholar]
- Bohm, M.R.; Pfrommer, S.; Chiwitt, C.; Bruckner, M.; Melkonyan, H.; Thanos, S. Crystallin-beta-b2-overexpressing NPCs support the survival of injured retinal ganglion cells and photoreceptors in rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8265–8279. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Bibel, M.; Barde, Y.A. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000, 14, 2919–2937. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Di Polo, A.; Aigner, L.J.; Dunn, R.J.; Bray, G.M.; Aguayo, A.J. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc. Natl. Acad. Sci. USA 1998, 95, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, S.; Klocker, N.; Gravel, C.; Bahr, M. Short communication: Protection of axotomized retinal ganglion cells by adenovirally delivered BDNF in vivo. Eur. J. Neurosci. 1998, 10, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Mansour-Robaey, S.; Clarke, D.B.; Wang, Y.C.; Bray, G.M.; Aguayo, A.J. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc. Natl. Acad. Sci. USA 1994, 91, 1632–1636. [Google Scholar] [CrossRef]
- Mey, J.; Thanos, S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993, 602, 304–317. [Google Scholar] [CrossRef]
- Martin, K.R.; Quigley, H.A.; Zack, D.J.; Levkovitch-Verbin, H.; Kielczewski, J.; Valenta, D.; Baumrind, L.; Pease, M.E.; Klein, R.L.; Hauswirth, W.W. Gene therapy with brain-derived neurotrophic factor as a protection: Retinal ganglion cells in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4357–4365. [Google Scholar] [CrossRef]
- Cheng, L.; Sapieha, P.; Kittlerova, P.; Hauswirth, W.W.; Di Polo, A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J. Neurosci. 2002, 22, 3977–3986. [Google Scholar] [CrossRef]
- Bauer, S.; Kerr, B.J.; Patterson, P.H. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat. Rev. Neurosci. 2007, 8, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, M.; Pollett, M.A.; Harvey, A.R. Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J. Neurotrauma 2011, 28, 2475–2483. [Google Scholar] [CrossRef]
- Ji, J.Z.; Elyaman, W.; Yip, H.K.; Lee, V.W.; Yick, L.W.; Hugon, J.; So, K.F. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: The possible involvement of STAT3 pathway. Eur. J. Neurosci. 2004, 19, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.; Rau, C.R.; Storch, M.K.; Sattler, M.B.; Demmer, I.; Weissert, R.; Taheri, N.; Kuhnert, A.V.; Bahr, M.; Diem, R. Ciliary neurotrophic factor protects retinal ganglion cells from secondary cell death during acute autoimmune optic neuritis in rats. Brain Pathol. 2004, 14, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Parrilla-Reverter, G.; Agudo, M.; Sobrado-Calvo, P.; Salinas-Navarro, M.; Villegas-Perez, M.P.; Vidal-Sanz, M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: A quantitative in vivo study. Exp. Eye Res. 2009, 89, 32–41. [Google Scholar] [CrossRef]
- van Adel, B.A.; Kostic, C.; Deglon, N.; Ball, A.K.; Arsenijevic, Y. Delivery of ciliary neurotrophic factor via lentiviral-mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum. Gene Ther. 2003, 14, 103–115. [Google Scholar] [CrossRef]
- Leaver, S.G.; Cui, Q.; Plant, G.W.; Arulpragasam, A.; Hisheh, S.; Verhaagen, J.; Harvey, A.R. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006, 13, 1328–1341. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Hauk, T.G.; Leibinger, M.; Marienfeld, R.; Fischer, D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol. Cell. Neurosci. 2009, 41, 233–246. [Google Scholar] [CrossRef]
- Pernet, V.; Joly, S.; Dalkara, D.; Jordi, N.; Schwarz, O.; Christ, F.; Schaffer, D.V.; Flannery, J.G.; Schwab, M.E. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol. Dis. 2013, 51, 202–213. [Google Scholar] [CrossRef]
- Cui, Q.; Pollett, M.A.; Symons, N.A.; Plant, G.W.; Harvey, A.R. A new approach to CNS repair using chimeric peripheral nerve grafts. J. Neurotrauma 2003, 20, 17–31. [Google Scholar] [CrossRef]
- Jung, G.; Sun, J.; Petrowitz, B.; Riecken, K.; Kruszewski, K.; Jankowiak, W.; Kunst, F.; Skevas, C.; Richard, G.; Fehse, B.; et al. Genetically modified neural stem cells for a local and sustained delivery of neuroprotective factors to the dystrophic mouse retina. Stem Cells Transl. Med. 2013, 2, 1001–1010. [Google Scholar] [CrossRef]
- Jankowiak, W.; Kruszewski, K.; Flachsbarth, K.; Skevas, C.; Richard, G.; Ruther, K.; Braulke, T.; Bartsch, U. Sustained Neural Stem Cell-Based Intraocular Delivery of CNTF Attenuates Photoreceptor Loss in the nclf Mouse Model of Neuronal Ceroid Lipofuscinosis. PLoS ONE 2015, 10, e0127204. [Google Scholar] [CrossRef]
- Conti, L.; Pollard, S.M.; Gorba, T.; Reitano, E.; Toselli, M.; Biella, G.; Sun, Y.; Sanzone, S.; Ying, Q.L.; Cattaneo, E.; et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005, 3, e283. [Google Scholar] [CrossRef]
- Pollard, S.M.; Conti, L.; Sun, Y.; Goffredo, D.; Smith, A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb. Cortex 2006, 16 (Suppl. 1), i112–i120. [Google Scholar] [CrossRef]
- Ibanez, C.F.; Andressoo, J.O. Biology of GDNF and its receptors—Relevance for disorders of the central nervous system. Neurobiol. Dis. 2017, 97, 80–89. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef]
- Ishikawa, H.; Takano, M.; Matsumoto, N.; Sawada, H.; Ide, C.; Mimura, O.; Dezawa, M. Effect of GDNF gene transfer into axotomized retinal ganglion cells using in vivo electroporation with a contact lens-type electrode. Gene Ther. 2005, 12, 289–298. [Google Scholar] [CrossRef]
- Jiang, C.; Moore, M.J.; Zhang, X.; Klassen, H.; Langer, R.; Young, M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol. Vis. 2007, 13, 1783–1792. [Google Scholar]
- Klocker, N.; Braunling, F.; Isenmann, S.; Bahr, M. In vivo neurotrophic effects of GDNF on axotomized retinal ganglion cells. Neuroreport 1997, 8, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, P.D.; Ball, A.K. Effects of GDNF on retinal ganglion cell survival following axotomy. Vis. Res. 1998, 38, 1505–1515. [Google Scholar] [CrossRef]
- Schmeer, C.; Straten, G.; Kugler, S.; Gravel, C.; Bahr, M.; Isenmann, S. Dose-dependent rescue of axotomized rat retinal ganglion cells by adenovirus-mediated expression of glial cell-line derived neurotrophic factor in vivo. Eur. J. Neurosci. 2002, 15, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Straten, G.; Schmeer, C.; Kretz, A.; Gerhardt, E.; Kugler, S.; Schulz, J.B.; Gravel, C.; Bahr, M.; Isenmann, S. Potential synergistic protection of retinal ganglion cells from axotomy-induced apoptosis by adenoviral administration of glial cell line-derived neurotrophic factor and X-chromosome-linked inhibitor of apoptosis. Neurobiol. Dis. 2002, 11, 123–133. [Google Scholar] [CrossRef]
- Ward, M.S.; Khoobehi, A.; Lavik, E.B.; Langer, R.; Young, M.J. Neuroprotection of retinal ganglion cells in DBA/2J mice with GDNF-loaded biodegradable microspheres. J. Pharm. Sci. 2007, 96, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Wang, J.; Matheson, C.R.; Urich, J.L. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: Comparison to and combination with brain-derived neurotrophic factor (BDNF). J. Neurobiol. 1999, 38, 382–390. [Google Scholar] [CrossRef]
- Zhang, Q.; Lai, D. Application of human amniotic epithelial cells in regenerative medicine: A systematic review. Stem Cell Res. Ther. 2020, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jiang, Y.; Zhang, J.S.; Li, N. Repairing effect of Schwann cells combined with mesenchymal stem cells on optic nerve injury in rats. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 277–288. [Google Scholar] [CrossRef]
- Nascimento-Dos-Santos, G.; Teixeira-Pinheiro, L.C.; da Silva-Junior, A.J.; Carvalho, L.R.P.; Mesentier-Louro, L.A.; Hauswirth, W.W.; Mendez-Otero, R.; Santiago, M.F.; Petrs-Silva, H. Effects of a combinatorial treatment with gene and cell therapy on retinal ganglion cell survival and axonal outgrowth after optic nerve injury. Gene Ther. 2020, 27, 27–39. [Google Scholar] [CrossRef]
- Pang, I.H.; Zeng, H.; Fleenor, D.L.; Clark, A.F. Pigment epithelium-derived factor protects retinal ganglion cells. BMC Neurosci. 2007, 8, 11. [Google Scholar] [CrossRef]
- Vigneswara, V.; Berry, M.; Logan, A.; Ahmed, Z. Pigment epithelium-derived factor is retinal ganglion cell neuroprotective and axogenic after optic nerve crush injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2624–2633. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Z.; Liu, L.; Sun, H. Combined effect of olfactory ensheathing cell (OEC) transplantation and glial cell line-derived neurotrophic factor (GDNF) intravitreal injection on optic nerve injury in rats. Mol. Vis. 2010, 16, 2903–2910. [Google Scholar]
- Yin, D.P.; Chen, Q.Y.; Liu, L. Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation). Neural Regen. Res. 2016, 11, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Yin, Z.Q.; Wang, Y. Synergistic effect of olfactory ensheathing cells and alpha-crystallin on restoration of adult rat optic nerve injury. Neurosci. Lett. 2017, 638, 167–174. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, X.B.; Xiong, S.Q. Neuro-protection of retinal stem cells transplantation combined with copolymer-1 immunization in a rat model of glaucoma. Mol. Cell. Neurosci. 2013, 54, 1–8. [Google Scholar] [CrossRef]
- Logan, A.; Ahmed, Z.; Baird, A.; Gonzalez, A.M.; Berry, M. Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain 2006, 129, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Franke, A.; Kaplan, M.R.; Pfrieger, F.W.; Barres, B.A. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 1995, 15, 805–819. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Ball, A.K. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: Combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience 2002, 110, 555–567. [Google Scholar] [CrossRef]
- Del Rio, P.; Irmler, M.; Arango-Gonzalez, B.; Favor, J.; Bobe, C.; Bartsch, U.; Vecino, E.; Beckers, J.; Hauck, S.M.; Ueffing, M. GDNF-induced osteopontin from Muller glial cells promotes photoreceptor survival in the Pde6brd1 mouse model of retinal degeneration. Glia 2011, 59, 821–832. [Google Scholar] [CrossRef]
- Harada, C.; Harada, T.; Quah, H.M.; Maekawa, F.; Yoshida, K.; Ohno, S.; Wada, K.; Parada, L.F.; Tanaka, K. Potential role of glial cell line-derived neurotrophic factor receptors in Muller glial cells during light-induced retinal degeneration. Neuroscience 2003, 122, 229–235. [Google Scholar] [CrossRef]
- Hauck, S.M.; Kinkl, N.; Deeg, C.A.; Swiatek-de Lange, M.; Schoffmann, S.; Ueffing, M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol. Cell. Biol. 2006, 26, 2746–2757. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Bahr, M. The upregulation of GLAST-1 is an indirect antiapoptotic mechanism of GDNF and neurturin in the adult CNS. Cell Death Differ. 2008, 15, 471–483. [Google Scholar] [CrossRef]
- van Adel, B.A.; Arnold, J.M.; Phipps, J.; Doering, L.C.; Ball, A.K. Ciliary neurotrophic factor protects retinal ganglion cells from axotomy-induced apoptosis via modulation of retinal glia in vivo. J. Neurobiol. 2005, 63, 215–234. [Google Scholar] [CrossRef]
- Cen, L.P.; Ng, T.K. Stem cell therapy for retinal ganglion cell degeneration. Neural Regen. Res. 2018, 13, 1352–1353. [Google Scholar] [CrossRef] [PubMed]
- Khatib, T.Z.; Martin, K.R. Protecting retinal ganglion cells. Eye 2017, 31, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Sieving, P.A.; Caruso, R.C.; Tao, W.; Coleman, H.R.; Thompson, D.J.; Fullmer, K.R.; Bush, R.A. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. USA 2006, 103, 3896–3901. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Jaffe, G.J.; Johnson, C.A.; Farsiu, S.; Lad, E.M.; Guymer, R.; Rosenfeld, P.; Hubschman, J.P.; Constable, I.; et al. Effect of Ciliary Neurotrophic Factor on Retinal Neurodegeneration in Patients with Macular Telangiectasia Type 2: A Randomized Clinical Trial. Ophthalmology 2019, 126, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.G.; Weleber, R.G.; Duncan, J.L.; Jaffe, G.J.; Tao, W.; Ciliary Neurotrophic Factor Retinitis Pigmentosa Study Group. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am. J. Ophthalmol. 2013, 156, 283–292.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Tao, W.; Williams, G.A. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef] [PubMed]
- Kauper, K.; McGovern, C.; Sherman, S.; Heatherton, P.; Rapoza, R.; Stabila, P.; Dean, B.; Lee, A.; Borges, S.; Bouchard, B.; et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7484–7491. [Google Scholar] [CrossRef]
- Talcott, K.E.; Ratnam, K.; Sundquist, S.M.; Lucero, A.S.; Lujan, B.J.; Tao, W.; Porco, T.C.; Roorda, A.; Duncan, J.L. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2219–2226. [Google Scholar] [CrossRef]
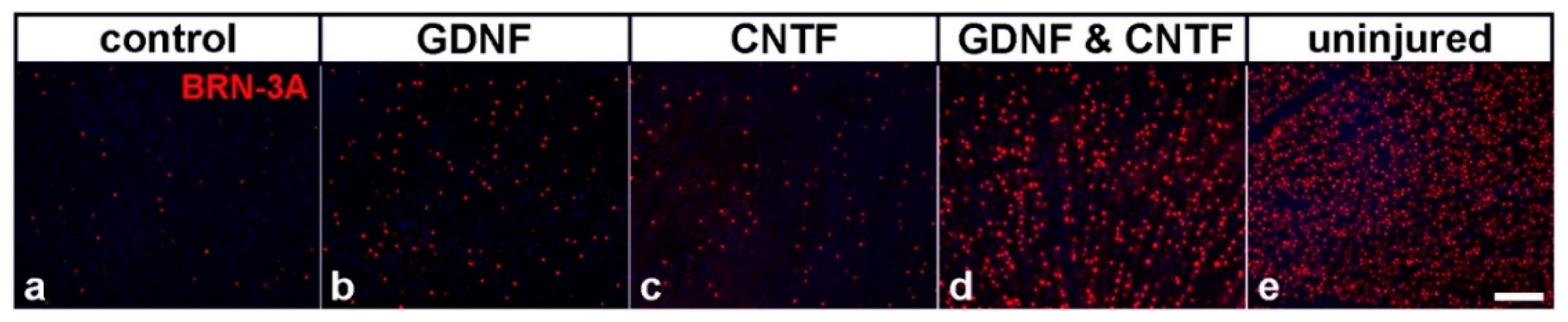
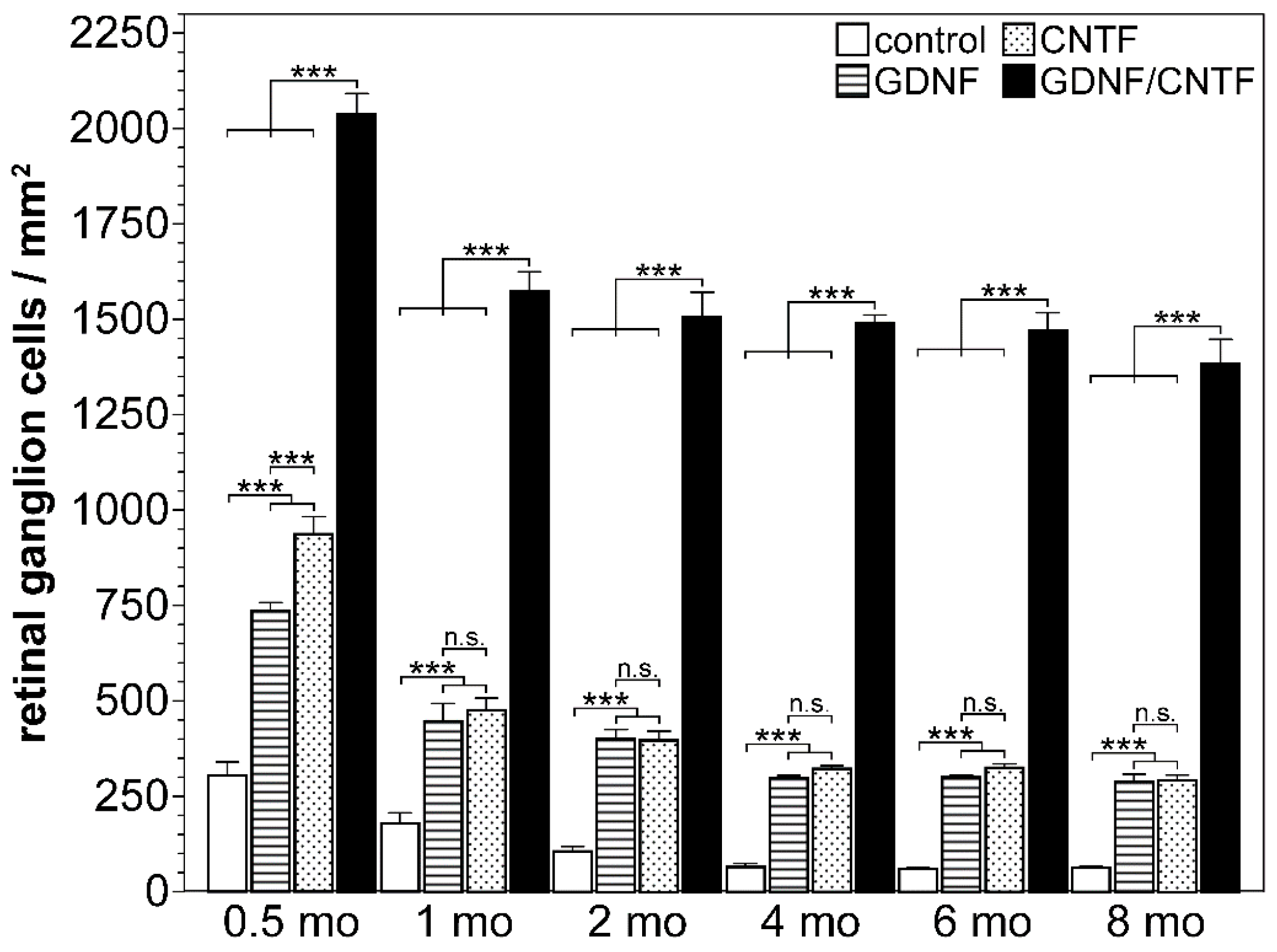
| Model | Cell Type | Neuroprotective Factor | Modification of Cells; Route of Cell Injection | Key Findings | Reference |
|---|---|---|---|---|---|
| RGC culture | ASs | BDNF |
| significantly enhanced survival of RGCs compared to conditioned medium from control ASs | [119] |
| RGC-5 culture | MSCs | BDNF |
| significantly enhanced survival of glutamate- or H2O2-treated RGC-5 cells compared to control MSCs | [120] |
| rat; OHT | MSCs | BDNF |
| significantly enhanced survival of RGCs and improved retinal function, as assessed by ERG recordings and pupillary light reflex responses ~40 days after treatment, compared to control MSCs | [121] |
| rat; ONC | NPCs | BDNF |
| significantly enhanced survival of RGCs 30 days after cell transplantation compared to control NPCs | [122] |
| rat; ONT | SCs | CNTF |
| significantly enhanced RGC survival and axon regeneration 4 weeks after grafting compared to nerve sheaths repopulated with control SCs | [123] |
| mouse; ONC | NSCs | CNTF |
|
| [124,125,126] |
| mouse; ONC | NSCs | GDNF |
| significantly enhanced RGC survival 8 months after cell transplantation compared to control NSCs | [125,126] |
| mouse; OHT | NPCs | IGF-1 |
| complete protection against IOP-induced RGC loss 30 days after cell transplantation compared to control NPCs | [127] |
| rat; ONT | AECs | FGF-2 |
| significantly enhanced RGC survival 4 weeks after cell transplantation compared to control AECs | [128] |
| rat; ONC | NSCs | PEDF |
|
| [129] |
| rat; ONC | NPCs | CRYBB2 |
| significantly enhanced RGC survival 4 weeks after cell transplantation compared to vehicle injections | [130] |
| Model | Cell Type (s) | Treatment | Key Findings | Reference |
|---|---|---|---|---|
| rat; ONC | MSCs, SCs | intravitreal co-transplantations of MSCs and SCs shortly after nerve injury | significantly enhanced RGC survival 2 weeks post-lesion after co-transplantations of both cell types compared to separate transplantations of each cell type | [165] |
| rat; ONC | MSCs | intravitreal transplantations of MSCs at the time of nerve injury combined with intravitreal injections of a PEDF-encoding AAV vector 4 weeks prior to nerve lesion |
| [166] |
| rat; incomplete ONC | OECs | transplantations of OECs into nerve injury site combined with intravitreal injections of recombinant GDNF at the time of nerve lesion |
| [169] |
| rat; ONT | OECs | transplantations of OECs at the site of nerve injury combined with intravitreal injections of recombinant CNTF at the time of nerve lesion |
| [170] |
| rat; ONC | OECs | transplantations of OECs and olfactory nerve FBs at the site of nerve injury combined with intravitreal injections of recombinant α-crystallin at the time of nerve lesion |
| [171] |
| rat; OHT | RSCs | intravitreal transplantations of RSCs 7 days after induction of an elevated IOP and vaccination with copolymer-1 | significantly enhanced RGC survival 3 weeks after elevated IOP induction after the combined treatment compared to each treatment alone | [172] |
| rat; ONT | FBs | intravitreal co-transplantations of FGF-2, NT-3 or BDNF-overexpressing FBs at the time of nerve lesion | significantly more surviving RGCs with axons extending at least 2 mm into the distal nerve stump 20 days after the combined treatment compared to cell-based treatments with FGF-2 and NT-3, FGF-2 and BDNF, or each individual factor | [173] |
| mouse; ONC | NSCs | intravitreal co-transplantations of CNTF- and GDNF-overexpressing clonal NSC lines 1 day after nerve lesion |
| [125,126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Grodzki, L.M.; Bartsch, S.; Bartsch, U. Cell-Based Neuroprotection of Retinal Ganglion Cells in Animal Models of Optic Neuropathies. Biology 2021, 10, 1181. https://doi.org/10.3390/biology10111181
Hu Y, Grodzki LM, Bartsch S, Bartsch U. Cell-Based Neuroprotection of Retinal Ganglion Cells in Animal Models of Optic Neuropathies. Biology. 2021; 10(11):1181. https://doi.org/10.3390/biology10111181
Chicago/Turabian StyleHu, Yue, Lynn Michelle Grodzki, Susanne Bartsch, and Udo Bartsch. 2021. "Cell-Based Neuroprotection of Retinal Ganglion Cells in Animal Models of Optic Neuropathies" Biology 10, no. 11: 1181. https://doi.org/10.3390/biology10111181
APA StyleHu, Y., Grodzki, L. M., Bartsch, S., & Bartsch, U. (2021). Cell-Based Neuroprotection of Retinal Ganglion Cells in Animal Models of Optic Neuropathies. Biology, 10(11), 1181. https://doi.org/10.3390/biology10111181






