Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
2.2. DPPH Free Radical-Scavenging Capacity
2.3. ABTS Acid Cation Radical-Scavenging Capacity
2.4. Antimicrobial Activity
2.4.1. Test Microorganisms
2.4.2. Agar-Well-Diffusion Method
2.4.3. Determination of Minimum Inhibitory Concentration (MIC)
2.5. Determination of Essential Oil Compounds by GC-MS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Composition of the Essential Oils
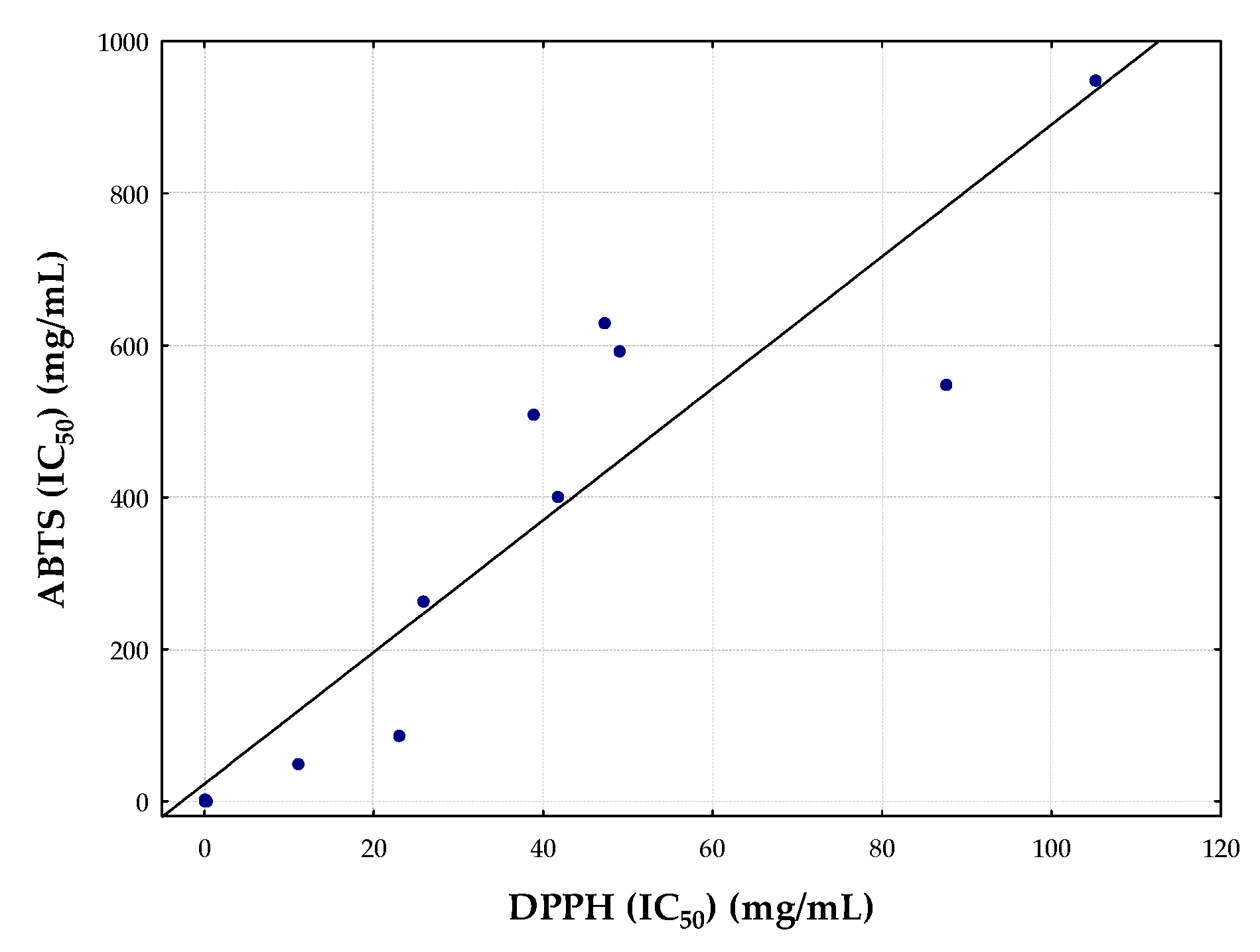
3.2. Antioxidant Activity of the Essential Oils
3.3. Antimicrobial Activity against Bacteria and C. albicans
3.4. Antifungal Activity of Essential Oils against B. cinerea
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021, 5, 133. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Do Nascimento, L.D.; de Moraes, A.A.B.; da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; de Aguiar Andrade, E.H.; de Faria, L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Vianna, T.C.; Marinho, C.O.; Marangoni Júnior, L.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Razola-Díaz, M.; Guerra-Hernández, E.J.; García-Villanova, B.; Verardo, V. Recent developments in extraction and encapsulation techniques of orange essential oil. Food Chem. 2021, 354, 129575. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free redical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Parejo, I.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH·(2,2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hayes, A.J.; Markovic, B. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem. Toxicol. 2002, 40, 535–543. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ben Lajnef, H.; Ferioli, F.; Pasini, F.; Politowicz, J.; Khaldi, A.; D’Antuono, L.F.; Caboni, M.F.; Nasri, N. Chemical composition and antioxidant activity of the volatile fraction extracted from air-dried fruits of Tunisian Eryngium maritimum L. ecotypes. J. Sci. Food Agric. 2018, 98, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; de Aquino Garcia Moura, L.; de Melo, N.R.; Sanches-Silva, A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Influence of phenological stage on chemical composition and antioxidant activity of Salvia lavandulifolia Vahl. essential oils. Ind. Crops Prod. 2014, 53, 71–77. [Google Scholar] [CrossRef]
- Zorga, J.; Kunicka-Styczynska, A.; Gruska, R.; Smigielski, K. Ultrasound-Assisted Hydrodistillation of Essential Oil from Celery Seeds (Apium graveolens L.) and Its Biological and Aroma Profile. Molecules 2020, 25, 5322. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.; Soran, M.L.; Varodi, C.; Lung, I.; Copolovici, L.; Mǎruţoiu, C. Extraction and GC determination of volatile aroma compounds from extracts of three plant species of the Apiaceae family. AIP Conf. Proc. 2013, 1565, 75–78. [Google Scholar]
- Singh, B.; Singh, J.P.; Kaur, A.; Yadav, M.P. Insights into the chemical composition and bioactivities of citrus peel essential oils. Food Res. Int. 2021, 143, 110231. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, F.; Giambanelli, E.; D’Antuono, L.F. Fennel (Foeniculum vulgare Mill. subsp. piperitum) florets, a traditional culinary spice in Italy: Evaluation of phenolics and volatiles in local populations, and comparison with the composition of other plant parts. J. Sci. Food Agric. 2017, 97, 5369–5380. [Google Scholar] [CrossRef] [PubMed]
- Heer, A.; Guleria, S.; Razdan, V.K. Chemical composition, antioxidant and antimicrobial activities and characterization of bioactive compounds from essential oil of Cinnamomum tamala grown in north-western Himalaya. J. Plant Biochem. Biotechnol. 2017, 26, 191–198. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Ahmad, F.; Yen, K.H. Antioxidant and anticholinesterase activities of essential oils of cinnamomum griffithii and C. macrocarpum. Nat. Prod. Commun. 2015, 10, 1465–1468. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.H.; Shani, B. Haseena Antioxidant potential and antimicrobial activity of Cinnamomum malabathrum (Batka). Orient. J. Chem. 2010, 26, 1449–1453. [Google Scholar]
- Sriramavaratharajan, V.; Murugan, R. Chemical profile of leaf essential oil of cinnamomum walaiwarense and comparison of its antioxidant and hypoglycemic activities with the major constituent benzyl benzoate. Nat. Prod. Commun. 2018, 13, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Gogoi, R.; Sarma, N.; Loying, R.; Pandey, S.K.; Begum, T.; Lal, M. A Comparative Analysis of Bark and Leaf Essential Oil and their Chemical Composition, Antioxidant, Anti-inflammatory, Antimicrobial Activities and Genotoxicity of North East Indian Cinnamomum zeylanicum Blume. Nat. Prod. J. 2021, 11, 74–84. [Google Scholar]
- Abdelwahab, S.I.; Mariod, A.A.; Taha, M.M.E.; Zaman, F.Q.; Abdelmageed, A.H.A.; Khamis, S.; Sivasothy, Y.; Awang, K. Chemical composition and antioxidant properties of the essential oil of Cinnamomum altissimum Kosterm. (Lauraceae). Arab. J. Chem. 2017, 10, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Ross, S.A.; Salkini, M.A.; Alam, P. Simultaneous Estimation of Cinnamaldehyde and Eugenol in Essential Oils and Traditional and Ultrasound-Assisted Extracts of Different Species of Cinnamon Using a Sustainable/Green HPTLC Technique. Molecules 2021, 26, 2054. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Al-Waili, N.; Aboulghazi, A.; Abdellaoui, A.; Al-Waili, T.; Lyoussi, B. Chemical composition and antioxidant content of Thymus vulgaris honey and Origanum vulgare essential oil; their effect on carbon tetrachlorideinduced toxicity. Vet. World 2021, 14, 292–301. [Google Scholar]
- Sokmen, A.; Abdel-Baki, A.A.S.; Al-Malki, E.S.; Al-Quraishy, S.; Abdel-Haleem, H.M. Constituents of essential oil of Origanum minutiflorum and its in vitro antioxidant, scolicidal and anticancer activities. J. King Saud Univ.-Sci. 2020, 32, 2377–2382. [Google Scholar] [CrossRef]
- Boskovic, M.; Glisic, M.; Djordjevic, J.; Starcevic, M.; Glamoclija, N.; Djordjevic, V.; Baltic, M.Z. Antioxidative Activity of Thyme (Thymus vulgaris) and Oregano (Origanum vulgare) Essential Oils and Their Effect on Oxidative Stability of Minced Pork Packaged Under Vacuum and Modified Atmosphere. J. Food Sci. 2019, 84, 2467–2474. [Google Scholar] [CrossRef]
- Sokmen, A.; Gulluce, M.; Akpulat, H.A.; Daferera, D.; Tepe, B.; Polissiou, M.; Sokmen, M.; Sahin, F. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 2004, 15, 627–634. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Chraibi, M.; Farah, A.; Elamin, O.; Iraqui, H.; Fikri-Benbrahim, K. Characterization, antioxidant, antimycobacterial, antimicrobial effcts of Moroccan rosemary essential oil, and its synergistic antimicrobial potential with carvacrol. J. Adv. Pharm. Technol. Res. 2020, 11, 25–29. [Google Scholar]
- Leporini, M.; Bonesi, M.; Loizzo, M.R.; Passalacqua, N.G.; Tundis, R. The essential oil of salvia rosmarinus spenn. From Italy as a source of health-promoting compounds: Chemical profile and antioxidant and cholinesterase inhibitory activity. Plants 2020, 9, 798. [Google Scholar] [CrossRef]
- Lakehal, S.; Chaouia, C.; Benrebiha, F.Z. Antibacterial and Antioxidant Activities of Rosemary (Rosmarinus officinalis L.) Essential Oil Growing in Djelfa (Algeria). In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, Vols I and II; Kallel, A., Ksibi, M., BenDhia, H., Khelifi, N., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1253–1254. [Google Scholar]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. Ind. Crops Prod. 2017, 107, 305–311. [Google Scholar] [CrossRef]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Hadjipavlou-Litina, D.; Bilia, A.R. Liposomes loaded with Salvia triloba and Rosmarinus officinalis essential oils: In Vitro assessment of antioxidant, antiinflammatory and antibacterial activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatemi, F.; Dini, S.; Rezaei, M.B.; Dadkhah, A.; Dabbagh, R.; Naij, S. The effect of γ-irradiation on the chemical composition and antioxidant activities of peppermint essential oil and extract. J. Essent. Oil Res. 2014, 26, 97–104. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Stanojevic, J.S.; Savic, V.L.; Cvetkovic, D.J.; Kolarevic, A.; Marjanovic-Balaban, Z.; Nikolic, L.B. Peppermint and Basil Essential Oils: Chemical Composition, in vitro Antioxidant Activity and in vivo Estimation of Skin Irritation. J. Essent. Oil-Bearing Plants 2019, 22, 979–993. [Google Scholar] [CrossRef]
- El Jery, A.; Hasan, M.; Rashid, M.M.; Al Mesfer, M.K.; Danish, M.; Ben Rebah, F. Phytochemical characterization, and antioxidant and antimicrobial activities of essential oil from leaves of the common sage Salvia officinalis L. from Abha, Saudi Arabia. Asian Biomed. 2020, 14, 261–270. [Google Scholar] [CrossRef]
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Fang, S.P.; Xing, X.; Lai, P.X.; Huang, J.J. Chemical Composition and Antioxidant Activity of the Essential Oil from Salvia kiangsiensis. Chem. Nat. Compd. 2018, 54, 591–592. [Google Scholar] [CrossRef]
- Hassanen, N.H.; Eissa, A.M.F.; Hafez, S.A.M.; Mosa, E.A. Antioxidant and antimicrobial activity of celery (Apium graveolens) and coriander (Coriandrum sativum) herb and seed essential oils. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 284–296. [Google Scholar]
- Kamal, G.M.; Ashraf, M.Y.; Hussain, A.I.; Shahzadi, A.; Chughtai, M.I. Antioxidant potential of peel essential oils of three Pakistani Citrus species: Citrus reticulata, Citrus sinensis, and Citrus paradisii. Pak. J. Bot. 2013, 45, 1449–1454. [Google Scholar]
- Phi, N.T.L.; Van Hung, P.; Chi, P.T.L.; Dung, N.H. Impact of Extraction Methods on Antioxidant and Antimicrobial Activities of Citrus Essential Oils. J. Essent. Oil Bear. Plants 2015, 18, 806–817. [Google Scholar] [CrossRef]
- Chi, P.T.L.; Van Hung, P.; Le Thanh, H.; Phi, N.T.L. Valorization of Citrus Leaves: Chemical Composition, Antioxidant and Antibacterial Activities of Essential Oils. Waste Biomass Valorization 2020, 11, 4849–4857. [Google Scholar] [CrossRef]
- Kaanin-Boudraa, G.; Brahmi, F.; Wrona, M.; Nerín, C.; Hadjal, S.; Madani, K.; Boulekbache-Makhlouf, L. Citrus × paradisi essential oil as a promising agent for margarine storage stability: Composition and antioxidant capacity. J. Food Process. Preserv. 2021, 45, e15374. [Google Scholar] [CrossRef]
- Ou, M.C.; Liu, Y.H.; Sun, Y.W.; Chan, C.F. The Composition, Antioxidant and Antibacterial Activities of Cold-Pressed and Distilled Essential Oils of Citrus paradisi and Citrus grandis (L.) Osbeck. Evid.-Based Complement. Altern. Med. 2015, 2015, 804091. [Google Scholar] [CrossRef] [Green Version]
- Ben Miri, Y.; Arino, A.; Djenane, D. Study of Antifungal, Anti-aflatoxigenic, Antioxidant Activity and Phytotoxicity of Algerian Citrus limon var. Eureka and Citrus sinensis var. Valencia Essential oils. J. Essent. Oil-Bearing Plants 2018, 21, 345–361. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.J.; Gao, Z.P.; Xia, J.L.; Ritenour, M.A.; Li, G.Y.; Shan, Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. Lwt 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Zara, S.; Sanna, M.L.; Addis, R.; Maldini, M.; Foddai, M.; Rourke, J.P.; Chessa, M.; Pintore, G. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT-Food Sci. Technol. 2016, 69, 579–585. [Google Scholar] [CrossRef]
- Kalleli, F.; Bettaieb Rebey, I.; Wannes, W.A.; Boughalleb, F.; Hammami, M.; Saidani Tounsi, M.; M’hamdi, M. Chemical composition and antioxidant potential of essential oil and methanol extract from Tunisian and French fennel (Foeniculum vulgare Mill.) seeds. J. Food Biochem. 2019, 43, e12935. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Shi, M.; Liu, C.; Kang, W. Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.)seeds from Egypt and China. Food Sci. Hum. Wellness 2019, 8, 67–72. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the antifungal activity and mode of action of thymus vulgaris, citrus limonum, pelargonium graveolens, cinnamomum cassia, ocimum basilicum, and eugenia caryophyllus essential oils. Molecules 2018, 23, 1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; De Carvalho, M.G.; Catunda, F.E.A. Antibacterial and Antibiofilm Activities of Cinnamomum Sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Shen, S.; Xu, J.; Lin, S.; Yuan, Y.; Jones, G.S. Synergistic interactions of cinnamaldehyde in combination with carvacrol against food-borne bacteria. Food Control 2013, 34, 619–623. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J. Agric. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016, 16, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- McCabe-Sellers, B.J.; Beattie, S.E. Food safety: Emerging trends in foodborne illness surveillance and prevention. J. Am. Diet. Assoc. 2004, 104, 1708–1717. [Google Scholar] [CrossRef]
- Sergelidis, D.; Angelidis, A.S. Methicillin-resistant Staphylococcus aureus: A controversial food-borne pathogen. Lett. Appl. Microbiol. 2017, 64, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, N.; Alves, S.; Gonçalves, A.; Amaral, J.S.; Poeta, P. Antimicrobial activity of essential oils from mediterranean aromatic plants against several foodborne and spoilage bacteria. Food Sci. Technol. Int. 2013, 19, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K.; et al. Carvacrol Induces Candida albicans Apoptosis Associated With Ca2+/Calcineurin Pathway. Front. Cell. Infect. Microbiol. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-Vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Tosi, G.; Massi, P.; Pistelli, L.; Mancianti, F. In Vitro antimicrobial activity of essential oils against salmonella enterica serotypes enteritidis and typhimurium strains isolated from poultry. Molecules 2019, 24, 900. [Google Scholar] [CrossRef] [Green Version]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brnawi, W.I.; Hettiarachchy, N.S.; Horax, R.; Kumar-Phillips, G.; Ricke, S. Antimicrobial activity of leaf and bark cinnamon essential oils against Listeria monocytogenes and Salmonella typhimurium in broth system and on celery. J. Food Process. Preserv. 2019, 43, e13888. [Google Scholar] [CrossRef]
- Blaszyk, M.; Holley, R.A. Interaction of monolaurin, eugenol and sodium citrate on growth of common meat spoilage and pathogenic organisms. Int. J. Food Microbiol. 1998, 39, 175–183. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, L.F.; Xu, J.G.; Hu, Q.P. Comparison study on antioxidant, DNA damage protective and antibacterial activities of eugenol and isoeugenol against several foodborne pathogens. Food Nutr. Res. 2017, 61, 1353356. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef] [PubMed]
- El-Mogy, M.M.; Alsanius, B.W. Cassia oil for controlling plant and human pathogens on fresh strawberries. Food Control 2012, 28, 157–162. [Google Scholar] [CrossRef]
- Soylu, E.M.; Kurt, Ş.; Soylu, S. In Vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef]
- De Clerck, C.; Dal Maso, S.; Parisi, O.; Dresen, F.; Zhiri, A.; Haissam Jijakli, M. Screening of Antifungal and Antibacterial Activity of 90 Commercial Essential Oils against 10 Pathogens of Agronomical Importance. Foods 2020, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- Palfi, M.; Konjevoda, P.; Vrandečić, K.; Ćosić, J. Antifungal activity of essential oils on mycelial growth of Fusarium oxysporum and Bortytis cinerea. Emir. J. Food Agric. 2019, 31, 544–554. [Google Scholar] [CrossRef]
- Gruľová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; de Martino, L.; Sedlák, V.; Gogaľová, Z.; Poráčová, J.; Camele, I.; de Feo, V. Thymol Chemotype Origanum vulgare L. Essential oil as a potential selective bio-based herbicide on monocot plant species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Ahmadu, T.; Ahmad, K.; Ismail, S.I.; Rashed, O.; Asib, N.; Omar, D. Antifungal efficacy of moringa oleifera leaf and seed extracts against botrytis cinerea causing gray mold disease of tomato (Solanum lycopersicum L.). Braz. J. Biol. 2021, 81, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Leroux, P. Chemical Control of Botrytis and its Resistance to Chemical Fungicides. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 195–222. [Google Scholar]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C.; Bach, J.; Gredt, M.; Chapeland, F. Mechanisms of resistance to fungicides in field strains of B. cinerea. Pest Manag. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef]
- Leroux, P.; Gredt, M.; Leroch, M.; Walker, A.S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microbiol. 2010, 76, 6615–6630. [Google Scholar] [CrossRef] [Green Version]
- Bardas, G.A.; Veloukas, T.; Koutita, O.; Karaoglanidis, G.S. Multiple resistance of Botrytis cinerea from kiwifruit to SDHIs, QoIs and fungicides of other chemical groups. Pest Manag. Sci. 2010, 66, 967–973. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Akthar, M.S.; Degaga, B.; Azam, T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. Issues Biol. Sci. Pharm. Res. 2014, 2, 001–007. [Google Scholar]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]

| Common Name | Scientific Name | Part Used |
|---|---|---|
| Aromatic herbs and spices | ||
| True cinnamon | Cinnamomum zeylanicum J. Presl | Leaf |
| Cinnamon | Cinnamomum cassia J. Presl | Bark |
| Oregano | Origanum vulgare L. | Leaf |
| Thyme | Thymus vulgaris L. | Flower/leaf |
| Rosemary | Rosmarinus officinalis (L.) Schleid. | Leaf |
| Peppermint | Mentha piperita L. | Leaf |
| Sage | Salvia lavandulifolia Vahl | Leaf |
| Fruits and vegetables | ||
| Celery | Apium graveolens L. | Seed |
| Fennel | Foeniculum vulgare Mill. var. dulce | Not reported |
| Mandarin | Citrus reticulata Blanco | Peel |
| Sweet orange | Citrus sinensis Osbeck | Peel |
| Lemon | Citrus limon L. | Peel |
| Grapefruit | Citrus paradisi Macfad. | Peel |
| Compound | Cinnamomum cassia | Cinnamomum zeylanicum | Mentha piperita | Origanum vulgare | Rosmarinus officinalis | Salvia lavandulifolia | Thymus vulgaris | |
|---|---|---|---|---|---|---|---|---|
| 1 | Tricyclene | n.d. | n.d. | n.d. | n.d. | 0.2 ± 0.02 | 0.2 ± 0.01 | 0.08 ± 0.00 |
| 2 | Thujene | n.d. | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.3 ± 0.01 | 0.2 ± 0.00 | 0.2 ± 0.01 | 0.6 ± 0.03 |
| 3 | α-pinene | 0.05 ± 0.00 | 0.2 ± 0.03 | 0.7 ± 0.07 | 1.0 ± 0.10 | 11.9 ± 0.90 | 5.5 ± 0.30 | 0.7 ± 0.06 |
| 4 | Camphene | n.d. | 0.08 ± 0.00 | 0.06 ± 0.00 | 0.2 ± 0.00 | 5.0 ± 0.30 | 7.7 ± 0.40 | 0.7 ± 0.01 |
| 5 | Sabinene | n.d. | n.d. | 0.2 ± 0.00 | n.d. | n.d. | 1.1 ± 0.30 | 0.004 ± 0.00 |
| 6 | β-pinene | 0.03 ± 0.00 | 0.07 ± 0.00 | 0.9 ± 0.02 | 0.07 ± 0.01 | 2.5 ± 0.30 | 5.1 ± 0.20 | 0.1 ± 0.01 |
| 7 | β-myrcene | n.d. | 0.008 ± 0.00 | n.d. | 0.5 ± 0.30 | 30.7 ± 0.40 | 2.0 ± 0.08 | 0.7 ± 0.03 |
| 8 | α-phellandrene | n.d. | 0.2 ± 0.01 | 0.01 ± 0.00 | 0.07 ± 0.00 | 0.7 ± 0.02 | 0.04 ± 0.00 | 0.1 ± 0.00 |
| 9 | α-terpinene | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.1 ± 0.00 | 0.5 ± 0.02 | 0.6 ± 0.02 | 0.3 ± 0.01 | 1.2 ± 0.10 |
| 10 | p-cymene | 0.05 ± 0.00 | 0.1 ± 0.01 | 0.3 ± 0.03 | 5.3 ± 0.20 | 1.6 ± 0.10 | 0.3 ± 0.02 | 16.5 ± 0.30 |
| 11 | D-limonene | 1.5 ± 0.20 | 0.09 ± 0.00 | 2.1 ± 0.20 | 0.2 ± 0.01 | 2.9 ± 0.30 | 4.7 ± 0.40 | 0.3 ± 0.02 |
| 12 | 1,8-cineole (Eucalyptol) | n.d. | n.d. | 4.9 ± 0.30 | 0.07 ± 0.00 | 14.8 ± 0.50 | 38.6 ± 0.80 | n.d. |
| 13 | cis-ocimene | n.d. | n.d. | n.d. | n.d. | 0.1 ± 0.00 | 0.06 ± 0.00 | n.d. |
| 14 | γ-terpinene | n.d. | n.d. | 0.1 ± 0.03 | 2.4 ± 0.20 | 0.7 ± 0.02 | 0.4 ± 0.01 | 7.1 ± 0.30 |
| 15 | Terpinolene | n.d. | 0.02 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.3 ± 0.03 | 0.2 ± 0.00 | 0.05 ± 0.00 |
| 16 | Linalool | 1.8 ± 0.10 | 0.4 ± 0.01 | n.d. | 0.7 ± 0.04 | 0.5 ± 0.06 | 0.7 ± 0.00 | 2.5 ± 0.08 |
| 17 | Pinone | n.d. | n.d. | 0.004 ± 0.00 | n.d. | n.d. | 0.3 ± 0.01 | n.d. |
| 18 | Camphor | n.d. | n.d. | 0.09 ± 0.00 | 0.07 ± 0.00 | 20.7 ± 0.30 | 23.6 ± 0.50 | 0.6 ± 0.02 |
| 19 | Menthone | n.d. | n.d. | 29.8 ± 0.50 | n.d. | n.d. | n.d. | n.d. |
| 20 | Menthofuran | 0.002 ± 0.00 | n.d. | 1.9 ± 0.30 | n.d. | 0.004 ± 0.00 | n.d. | n.d. |
| 21 | Isomenthone | 0.002 ± 0.00 | 0.002 ± 0.00 | 4.2 ± 0.20 | n.d. | 0.01 ± 0.00 | n.d. | n.d. |
| 22 | Isomenthol | n.d. | n.d. | 3.6 ± 0.10 | n.d. | n.d. | n.d. | n.d. |
| 23 | Borneol | n.d. | 0.004 ± 0.00 | n.d. | 0.3 ± 0.04 | 0.9 ± 0.10 | 2.0 ± 0.20 | 1.3 ± 0.10 |
| 24 | Menthol | n.d. | n.d. | 39.7 ± 0.30 | n.d. | n.d. | n.d. | n.d. |
| 25 | Terpinen-4-ol | n.d. | 0.02 ± 0.00 | n.d. | 0.3 ± 0.00 | 0.4 ± 0.01 | 0.4 ± 0.02 | 1.0 ± 0.1 |
| 26 | α-terpineol | n.d. | 0.04 ± 0.00 | 0.2 ± 0.00 | n.d. | 1.0 ± 0.06 | 0.4 ± 0.01 | 0.1 ± 0.01 |
| 27 | Estragole | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 |
| 28 | Verbenone | 0.006 ± 0.00 | n.d. | n.d. | n.d. | 0.3 ± 0.00 | n.d. | 0.01 ± 0.00 |
| 29 | Methyl thymyl ether | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 ± 0.02 |
| 30 | Pulegone | n.d. | n.d. | 0.8 ± 0.10 | n.d. | n.d. | n.d. | n.d. |
| 31 | Linalyl anthranilate | n.d. | n.d. | n.d. | n.d. | n.d. | 2.2 ± 0.30 | n.d. |
| 32 | Piperitone | n.d. | n.d. | 0.5 ± 0.03 | n.d. | n.d. | n.d. | n.d. |
| 33 | Neomenthol acetate | 0.003 ± 0.00 | n.d. | 0.3 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| 34 | Cinnamaldehyde | 91.9 ± 1.70 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 35 | Bornyl acetate | n.d. | n.d. | n.d. | n.d. | 0.8 ± 0.02 | 0.8 ± 0.01 | n.d. |
| 36 | Myrtenyl acetate | n.d. | n.d. | n.d. | n.d. | n.d. | 1.1 ± 0.02 | n.d. |
| 37 | Menthyl acetate | n.d. | n.d. | 6.1 ± 0.70 | n.d. | n.d. | n.d. | n.d. |
| 38 | β-isosafrole | n.d. | 0.1 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 39 | Thymol | n.d. | n.d. | n.d. | 1.6 ± 0.20 | n.d. | n.d. | 61.2 ± 1.10 |
| 40 | Carvacrol | n.d. | n.d. | n.d. | 84.5 ± 1.60 | n.d. | n.d. | 2.6 ± 0.5 |
| 41 | Terpinyl acetate | n.d. | n.d. | n.d. | n.d. | n.d. | 0.6 ± 0.02 | 0.06 ± 0.00 |
| 42 | Eugenol | 4.2 ± 0.30 | 95.2 ± 1.40 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 43 | β-caryophyllene | 0.5 ± 0.02 | 1.6 ± 0.20 | 2.7 ± 0.10 | 1.6 ± 0.10 | 2.2 ± 0.20 | 0.7 ± 0.10 | 1.9 ± 0.20 |
| 44 | α-caryophyllene | 0.03 ± 0.00 | 0.3 ± 0.04 | 0.2 ± 0.00 | 0.1 ± 0.00 | 0.7 ± 0.08 | 0.4 ± 0.03 | 0.05 ± 0.00 |
| 45 | β-eudesmene | n.d. | n.d. | 0.08 ± 0.00 | n.d. | n.d. | n.d. | 0.07 ± 0.00 |
| 46 | Eremophilane | n.d. | n.d. | 0.03 ± 0.00 | n.d. | n.d. | 0.2 ± 0.01 | 0.01 ± 0.00 |
| 47 | Bisabolene | n.d. | n.d. | n.d. | 0.12 | n.d. | n.d. | 0.07 ± 0.00 |
| 48 | Isoeugenol | n.d. | 0.4 ± 0.10 | 0.2 ± 0.00 | n.d. | 0.3 ± 0.03 | 0.1 ± 0.00 | 0.07 ± 0.00 |
| 49 | 2-(2-propenyl)-furan | n.d. | 0.007 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 50 | Benzyl benzoate | n.d. | 1.0 ± 0.30 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Monoterpenes | 3.4 | 1.3 | 39.9 | 11.4 | 58.2 | 28.9 | 30.8 | |
| Oxygenated monoterpenes | n.d. | n.d. | 5.0 | 0.1 | 35.5 | 62.2 | 0.6 | |
| Alcohols | n.d. | 0.1 | 43.6 | 0.5 | 2.3 | 2.8 | 2.4 | |
| Ethers | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 | |
| Esthers | n.d. | 1.0 | 6.4 | n.d. | 0.8 | 2.5 | 0.1 | |
| Sesquiterpene hydrocarbons | 0.5 | 1.8 | 3.1 | 1.8 | 2.9 | 1.3 | 2.1 | |
| Aldehydes | 91.9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Phenols | 4.2 | 95.7 | 0.2 | 86.1 | 0.3 | 0.1 | 63.9 | |
| Others | n.d. | 0.2 | 1.9 | n.d. | n.d. | 2.2 | n.d. |
| Compound | Apium graveolens | Citrus limon | Citrus paradisi | Citrus reticulata | Citrus sinensis | Foeniculum vulgare | |
|---|---|---|---|---|---|---|---|
| 1 | Tricyclene | n.d. | n.d. | n.d. | 0.003 ± 0.00 | n.d. | 0.004 ± 0.00 |
| 2 | Thujene | 0.009 ± 0.00 | 0.1 ± 0.02 | n.d. | 0.01 ± 0.00 | n.d. | n.d. |
| 3 | α-pinene | 0.3 ± 0.04 | 0.8 ± 0.05 | 0.1 ± 0.00 | 0.2 ± 0.02 | 0.2 ± 0.03 | 1.2 ± 0.10 |
| 4 | Camphene | n.d. | 0.03 ± 0.00 | n.d. | n.d. | n.d. | 0.01 ± 0.00 |
| 5 | Sabinene | 0.06 ± 0.00 | 0.8 ± 0.10 | 0.2 ± 0.01 | 0.2 ± 0.00 | 0.2 ± 0.00 | 0.03 ± 0.00 |
| 6 | β -pinene | 2.4 ± 0.20 | 6.0 ± 0.30 | 0.08 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.02 ± 0.00 |
| 7 | β-myrcene | 0.4 ± 0.02 | 0.4 ± 0.10 | 0.3 ± 0.03 | 0.4 ± 0.02 | 0.4 ± 0.01 | 0.01 ± 0.00 |
| 8 | α-terpinene | n.d. | 0.08 ± 0.00 | n.d. | n.d. | n.d. | 0.003 ± 0.00 |
| 9 | Cymene | 0.06 ± 0.00 | 0.1 ± 0.02 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| 10 | D-limonene | 71.4 ± 1.50 | 87.0 ± 1.20 | 99.0 ± 1.60 | 99.0 ± 1.10 | 99.2 ± 1.50 | 1.2 ± 0.40 |
| 11 | 1,8-cineole (Eucalyptol) | n.d. | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.00 |
| 12 | γ-terpinene | n.d. | 3.8 ± 0.07 | 0.04 ± 0.00 | 0.06 ± 0.00 | n.d. | 0.01 ± 0.00 |
| 13 | Terpinolene | n.d. | 0.1 ± 0.02 | n.d. | n.d. | n.d. | 0.03 ± 0.00 |
| 14 | Fenchone | 0.003 ± 0.00 | n.d. | n.d. | 0.002 ± 0.00 | n.d. | 0.3 ± 0.01 |
| 15 | Linalool | n.d. | n.d. | n.d. | n.d. | n.d. | 0.001 ± 0.00 |
| 16 | Pinone | 0.01 ± 0.00 | 0.001 ± 0.00 | n.d. | 0.002 ± 0.00 | n.d. | 0.0005 ± 0.00 |
| 17 | Camphor | n.d. | n.d. | n.d. | n.d. | 0.005 ± 0.00 | 0.003 ± 0.00 |
| 18 | 5-undecen-3-yne, (E)- | 1.2 ± 0.30 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 19 | Menthofuran | n.d. | n.d. | 0.001 ± 0.00 | 0.002 ± 0.00 | 0.002 ± 0.00 | 0.001 ± 0.00 |
| 20 | Isomenthone | 0.002 ± 0.00 | 0.008 ± 0.00 | 0.001 ± 0.00 | n.d. | n.d. | n.d. |
| 21 | Isomenthol | n.d. | n.d. | 0.01 ± 0.00 | n.d. | n.d. | 0.001 ± 0.00 |
| 22 | Borneol | n.d. | 0.004 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| 23 | Menthol | 0.01 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 24 | Terpinen-4-ol | n.d. | 0.04 ± 0.00 | n.d. | n.d. | 0.005 ± 0.00 | 0.001 ± 0.00 |
| 25 | α-terpineol | n.d. | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.01 ± 0.00 | n.d. | 0.002 ± 0.00 |
| 26 | Estragole | 0.01 ± 0.00 | n.d. | n.d. | n.d. | n.d. | 0.3 ± 0.02 |
| 27 | Verbenone | n.d. | n.d. | n.d. | n.d. | 0.003 ± 0.00 | 0.002 ± 0.00 |
| 28 | Anethol | n.d. | n.d. | n.d. | n.d. | n.d. | 96.8 ± 1.80 |
| 29 | β-caryophyllene | 0.4 ± 0.02 | 0.1 ± 0.00 | 0.1 ± 0.01 | n.d. | n.d. | n.d. |
| 30 | Bergamottin | n.d. | 0.2 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| 31 | α-caryophyllene | 0.04 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 32 | β-eudesmene | 9.8 ± 0.20 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 33 | Eremophilane | 1.2 ± 0.04 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 34 | Bisabolene | n.d. | 0.2 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| 35 | Isoeugenol | 0.05 ± 0.00 | n.d. | 0.03 ± 0.00 | n.d. | n.d. | n.d. |
| 36 | 1-(2,4-Dimethylphenyl)propan-1-one | 2.5 ± 0.30 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 37 | Allyl phenoxyacetate | 8.2 ± 0.40 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 38 | 2-(2-propenyl)-furan | 1.9 ± 0.20 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Monoterpenes | 74.6 | 99.4 | 99.8 | 100 | 100 | 2.6 | |
| Oxygenated monoterpenes | n.d. | n.d. | n.d. | n.d. | n.d. | 0.4 | |
| Alcohols | n.d. | 0.1 | 0.1 | n.d. | n.d. | n.d. | |
| Phenylpropanoids | n.d. | n.d. | n.d. | n.d. | n.d. | 97.0 | |
| Esthers | 8.2 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Sesquiterpene hydrocarbons | 11.5 | 0.4 | 0.1 | n.d. | n.d. | n.d. | |
| Phenols | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Others | 5.6 | 0.2 | n.d. | n.d. | n.d. | n.d. |
| Essential Oils | IC50 DPPH (mg/mL) |
|---|---|
| Aromatic plants EOs | |
| Cinnamomum cassia | 0.05 |
| Cinnamomum zeylanicum | 0.01 |
| Mentha piperita | 22.98 |
| Origanum vulgare | 0.29 |
| Rosmarinus officinalis | 11.19 |
| Salvia lavandulifolia | 41.82 |
| Thymus vulgaris | 0.31 |
| Fruit and vegetables EOs | |
| Apium graveolens | 25.89 |
| Citrus limon | 49.06 |
| Citrus paradisi | 87.67 |
| Citrus reticulata | 38.79 |
| Citrus sinensis | 47.30 |
| Foeniculum vulgare | 105.32 |
| Ascorbic acid | 0.003 |
| Essential Oils | S. aureus | MRSA | E. coli | S. Typhimurium | L. monocytogenes | C. albicans |
|---|---|---|---|---|---|---|
| Thymus vulgaris | 33.0 ± 1.0 | 33.0 ± 2.0 | 38.0 ± 3.6 | 41.0 ± 1.0 | 35.3 ± 5.0 | 58.0 ± 2.6 |
| Origanum vulgare | 28.7 ± 5.5 | 30.7 ± 3.8 | 33.3 ± 4.2 | 35.7 ± 1.2 | 31.7 ± 2.9 | 50.7 ± 1.2 |
| Rosmarinus officinalis | 10.7 ± 1.2 | 10.0 ± 0.0 | 11.0 ± 1.7 | 12.7 ± 1.2 | N | 18.7 ± 2.3 |
| Apium graveolens | 12.0 ± 2.6 | 11.3 ± 0.6 | N | 13.0 ± 1.0 | 11.7 ± 1.5 | 20.7 ± 1.2 |
| Salvia lavandulifolia | 10.3 ± 0.6 | 10.3 ± 0.6 | 10.7 ± 0.6 | 13.3 ± 0.6 | N | 25.0 ± 3.0 |
| Cinnamomum zeylanicum | 18.3 ± 2.3 | 18.3 ± 1.2 | 21.3 ± 1.2 | 21.7 ± 1.2 | 14.3 ± 0.6 | 34.7 ± 0.6 |
| Cinnamomum cassia | 36.0 ± 3.5 | 34.7 ± 2.3 | 29.0 ± 4.6 | 28.0 ± 1.7 | 28.0 ± 2.0 | 55.7 ± 3.5 |
| Citrus sinensis | N | N | N | N | N | N |
| Citrus reticulata | 10.3 ± 0.6 | 10.0 ± 0.0 | N | 14.0 ± 1.0 | 10.3 ± 0.6 | 22.0 ± 1.7 |
| Citrus limon | N | N | N | N | N | N |
| Citrus paradise | N | N | N | N | N | N |
| Foeniculum vulgare | N | N | N | 12.0 ± 2.0 | N | 12.7 ± 2.5 |
| Mentha piperita | 11.0 ± 0.0 | 11.0 ± 1.0 | 13.3 ± 1.5 | 22.3 ± 2.5 | N | 40.3 ± 4.0 |
| AA | 15.3 ± 0.6 | 17.3 ± 0.6 | 32.3 ± 4.0 | 30.7 ± 1.2 | 19.0 ± 1.0 | 25.7 ± 1.2 |
| Essential Oils | S. aureus | MRSA | E. coli | S. Typhimurium | L. monocytogenes | C. albicans |
|---|---|---|---|---|---|---|
| Thymus vulgaris | 2.25 | 1.125 | 1.125 | 1.125 | 1.125 | 0.56 |
| Origanum vulgare | 1.125 | 1.125 | 1.125 | 0.56 | 1.125 | 0.56 |
| Rosmarinus officinalis | 36 | 36 | 18 | 9 | 18 | 4.5 |
| Apium graveolens | 4.5 | 4.5 | 36 | 9 | 4.5 | 1.125 |
| Salvia lavandulifolia | 9 | 9 | 9 | 4.5 | 4.5 | 2.25 |
| Cinnamomum zeylanicum | 2.25 | 1.125 | 1.125 | 1.125 | 2.25 | 0.28 |
| Cinnamomum cassia | 0.28 | 0.28 | 0.28 | 0.28 | 0.14 | <0.14 |
| Citrus sinensis | 72 | 72 | 36 | 36 | 36 | 18 |
| Citrus reticulata | 36 | 36 | 36 | 36 | 18 | 4.5 |
| Citrus limon | 72 | 72 | 72 | 36 | 36 | 18 |
| Citrus paradise | 72 | 72 | 72 | 36 | 72 | 9 |
| Foeniculum vulgare | 36 | 72 | 36 | 4.5 | 36 | 2.25 |
| Mentha piperita | 4.5 | 2.25 | 2.25 | 1.125 | 4.5 | 1.125 |
| Essential Oils | Mycelium Inhibition (%) | MIC (mg/mL) |
|---|---|---|
| Thymus vulgaris | 98.2 | 1.125 |
| Origanum vulgare | 98.5 | 0.56 |
| Rosmarinus officinalis | 79.0 | 9 |
| Apium graveolens | 48.8 | 9 |
| Salvia lavandulifolia | 79.8 | 2.25 |
| Cinnamomum zeylanicum | 70.4 | 1.125 |
| Cinnamomum cassia | 81.5 | 0.14 |
| Citrus sinensis | 3.4 | 9 |
| Citrus reticulata | 3.6 | 9 |
| Citrus limon | 9.7 | 4.5 |
| Citrus paradise | 18.4 | 9 |
| Foeniculum vulgare | 93.1 | 1.125 |
| Mentha piperita | 93.8 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De-Montijo-Prieto, S.; Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; Guerra-Hernandez, E.J.; Jiménez-Valera, M.; Garcia-Villanova, B.; Ruiz-Bravo, A.; Verardo, V. Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities. Biology 2021, 10, 1091. https://doi.org/10.3390/biology10111091
De-Montijo-Prieto S, Razola-Díaz MdC, Gómez-Caravaca AM, Guerra-Hernandez EJ, Jiménez-Valera M, Garcia-Villanova B, Ruiz-Bravo A, Verardo V. Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities. Biology. 2021; 10(11):1091. https://doi.org/10.3390/biology10111091
Chicago/Turabian StyleDe-Montijo-Prieto, Soumi, María del Carmen Razola-Díaz, Ana María Gómez-Caravaca, Eduardo Jesús Guerra-Hernandez, María Jiménez-Valera, Belén Garcia-Villanova, Alfonso Ruiz-Bravo, and Vito Verardo. 2021. "Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities" Biology 10, no. 11: 1091. https://doi.org/10.3390/biology10111091
APA StyleDe-Montijo-Prieto, S., Razola-Díaz, M. d. C., Gómez-Caravaca, A. M., Guerra-Hernandez, E. J., Jiménez-Valera, M., Garcia-Villanova, B., Ruiz-Bravo, A., & Verardo, V. (2021). Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities. Biology, 10(11), 1091. https://doi.org/10.3390/biology10111091










