Effect of Temperature Exposition of Casting Solution on Properties of Polysulfone Hollow Fiber Membranes
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Preparation of Spinning Solution
2.3. Flat Membranes Preparation
2.4. Fabrication of Hollow Fiber Membranes
2.5. Gas Permeance Measurements
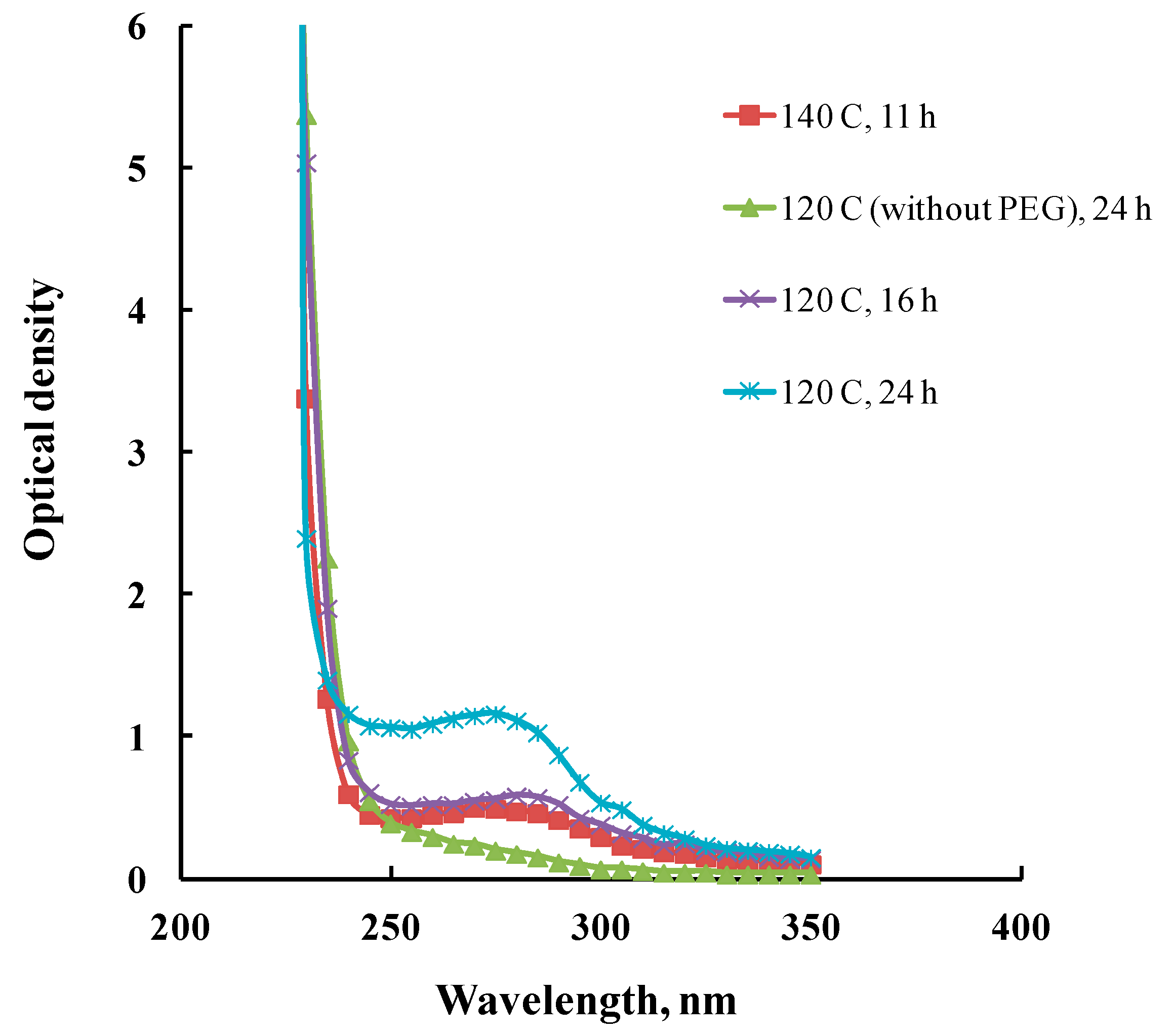
2.6. Spectrophotometric Analysis
2.7. Coagulation Value
2.8. Scanning Electron Microscopy
3. Results and Discussion
3.1. Effect of Heat Treatment on Spinning Solution Properties
3.2. Flat Membranes
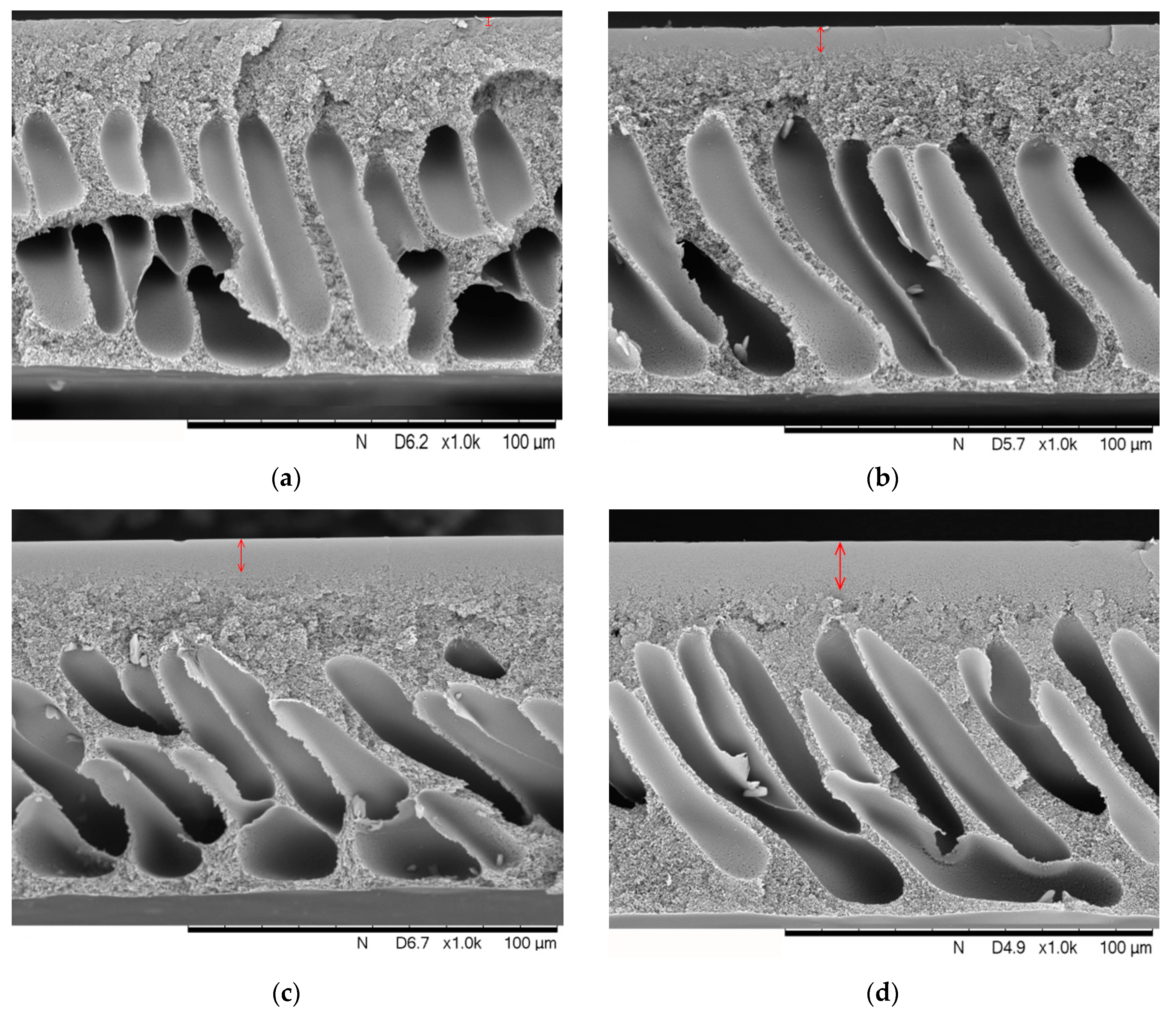
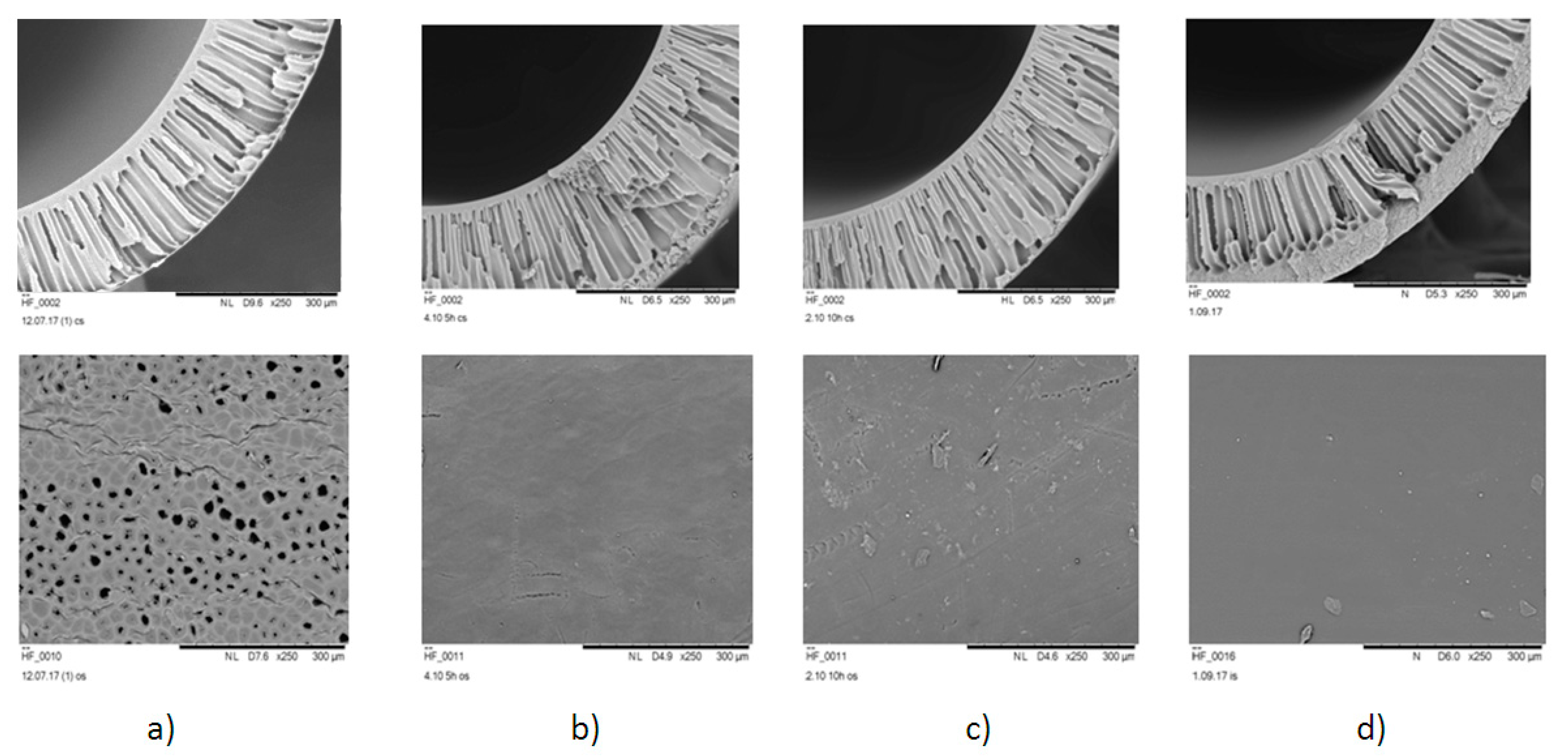
3.3. Hollow Fiber Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons Ltd: Chichester, UK, 2004; pp. 191–354. [Google Scholar]
- Mulder, J. Basic Principles of Membrane Technology; Kluwer Academic: Dordrecht, The Netherlands, 1996; pp. 51–58. [Google Scholar]
- Ran, F. Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1598–1600. [Google Scholar]
- Ida, S. Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1528–1534. [Google Scholar]
- Hołda, A.K.; Vankelecom, I.F. Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—Part A: Influence of high molecular weight additives. J. Membr. Sci. 2014, 450, 512–521. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F. Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—Part B: Influence of low molecular weight additives. J. Membr. Sci. 2014, 450, 499–511. [Google Scholar] [CrossRef]
- Ovcharova, A.; Vasilevsky, V.; Borisov, I.; Bazhenov, S.; Volkov, A.; Bildyukevich, A.; Volkov, V. Polysulfone porous hollow fiber membranes for ethylene-ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2017, 183, 162–172. [Google Scholar] [CrossRef]
- Kostyanaya, M.; Bazhenov, S.; Borisov, I.; Plisko, T.; Vasilevsky, V. Surface Modified Polysulfone Hollow Fiber Membranes for Ethane/Ethylene Separation Using Gas-Liquid Membrane Contactors with Ionic Liquid-Based Absorbent. Fibers 2019, 7, 4. [Google Scholar] [CrossRef]
- Marino, T.; Blasi, E.; Tornaghi, S.; Di Nicolò, E.; Figoli, A. Polyethersulfone membranes prepared with Rhodiasolv® Polarclean as water soluble green solvent. J. Membr. Sci. 2018, 549, 192–204. [Google Scholar] [CrossRef]
- Dong, X.; Al-Jumaily, A.; Escobar, I. Investigation of the use of a bio-derived solvent for non-solvent-induced phase separation (NIPS) fabrication of polysulfone membranes. Membranes 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Jung, J.T.; Kim, J.F.; Kim, S.; Drioli, E.; Lee, Y.M. A novel green solvent alternative for polymeric membrane preparation via nonsolvent-induced phase separation (NIPS). J. Membr. Sci. 2019, 574, 44–54. [Google Scholar] [CrossRef]
- Marino, T.; Galiano, F.; Simone, S.; Figoli, A. DMSO EVOL™ as novel non-toxic solvent for polyethersulfone membrane preparation. Environ. Sci. Pollut. Res. 2018, 26, 14774–14785. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Galiano, F.; Molino, A.; Figoli, A. New frontiers in sustainable membrane preparation: Cyrene™ as green bioderived solvent. J. Membr. Sci. 2019, 580, 224–234. [Google Scholar] [CrossRef]
- Kim, I.C.; Lee, K.H. Effect of various additives on pore size of polysulfone membrane by phase-inversion process. J. Appl. Polym. Sci. 2003, 89, 2562–2566. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Effect of molecular weight of PEG on membrane morphology and transport properties. J. Membr. Sci. 2008, 309, 209–221. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, K.H. Effect of PEG additive on membrane formation by phase inversion. J. Membr. Sci. 1998, 138, 153–163. [Google Scholar] [CrossRef]
- Zheng, Q.Z.; Wang, P.; Yang, Y.N. Rheological and thermodynamic variation in polysulfone solution by PEG introduction and its effect on kinetics of membrane formation via phase-inversion process. J. Membr. Sci. 2006, 279, 230–237. [Google Scholar] [CrossRef]
- Boom, R.M.; Van den Boomgaard, T.; Smolders, C.A. Mass transfer and thermodynamics during immersion precipitation for a two-polymer system: Evaluation with the system PES-PVP-NMP-water. J. Membr. Sci. 1994, 90, 231–249. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Preparation, characterization and performance studies of polysulfone membranes using PVP as an additive. J. Membr. Sci. 2008, 315, 36–47. [Google Scholar] [CrossRef]
- Miyano, T.; Matsuura, T.; Sourirajan, S. Effect of polyvinylpyrrolidone additive on the pore size and the pore size distribution of polyethersulfone (Victrex) membranes. Chem. Eng. Commun. 1993, 119, 23–39. [Google Scholar] [CrossRef]
- Tsai, H.A.; Li, L.D.; Lee, K.R.; Wang, Y.C.; Li, C.L.; Huang, J.J.; Lai, Y. Effect of surfactant addition on the morphology and pervaporation performance of asymmetric polysulfone membranes. J. Membr. Sci. 2000, 176, 97–103. [Google Scholar] [CrossRef]
- Fung-Ching, L.; Da-Ming, W.; Cheng-Lee, L.; Juin-Yih, L. Effect of surfactants on the structure of PMMA membranes. J. Membr. Sci. 1997, 123, 281–291. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zadhoush, A. Investigation of the relation between viscoelastic properties of polysulfone solutions, phase inversion process and membrane morphology: The effect of solvent power. J. Membr. Sci. 2017, 532, 47–57. [Google Scholar] [CrossRef]
- Reynolds, D.W.; Galvani, M.; Hicks, S.R.; Joshi, B.J.; Kennedy-Gabb, S.A.; Kleinman, M.H.; Parmar, P.Z. The use of N-methylpyrrolidone as a cosolvent and oxidant in pharmaceutical stress testing. J. Pharm. Sci. 2012, 101, 761–776. [Google Scholar] [CrossRef]
- Glastrup, J. Degradation of polyethylene glycol. A study of the reaction mechanism in a model molecule: Tetraethylene glycol. Polym. Degrad. Stabil. 1996, 52, 217–222. [Google Scholar] [CrossRef]
- Ray, W.J., Jr.; Puvathingal, J.M. A simple procedure for removing contaminating aldehydes and peroxides from aqueous solutions of polyethylene glycols and of nonionic detergents that are based on the polyoxyethylene linkage. Anal. Biochem. 1985, 146, 307–312. [Google Scholar] [CrossRef]
- Han, S.; Kim, C.; Kwon, D. Thermal/oxidative degradation and stabilization of polyethylene glycol. Polymer 1997, 38, 317–323. [Google Scholar] [CrossRef]
- Ismail, A.F.; Mansourizadeh, A. A comparative study on the structure and performance of porous polyvinylidene fluoride and polysulfone hollow fiber membranes for CO2 absorption. J. Membr. Sci. 2010, 365, 319–328. [Google Scholar] [CrossRef]
- Idris, A.; Zain, N.M.; Noordin, M.Y. Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives. Desalination 2007, 207, 324–339. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.Y.; Zhu, B.K.; Zhang, F.; Zhu, L.P. Preparation of hydrophilic and fouling resistant poly (vinylidene fluoride) hollow fiber membranes. J. Membr. Sci. 2009, 345, 331–339. [Google Scholar] [CrossRef]
- Loh, C.H.; Wang, R.; Shi, L.; Fane, A.G. Fabrication of high performance polyethersulfone UF hollow fiber membranes using amphiphilic Pluronic block copolymers as pore-forming additives. J. Membr. Sci. 2011, 380, 114–123. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F.; Aroon, M.A. Sustainable Membrane Technology for Energy, Water, and Environment; Ismail, A.F., Matsuura, T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 191–201. [Google Scholar]
- Ohya, H.; Shiki, S.; Kawakami, H. Fabrication study of polysulfone hollow-fiber microfiltration membranes: Optimal dope viscosity for nucleation and growth. J. Membr. Sci. 2009, 326, 293–302. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Characteristics, performance and stability of polyethersulfone ultrafiltration membranes prepared by phase separation method using different macromolecular additives. J. Membr. Sci. 2009, 327, 125–135. [Google Scholar] [CrossRef]
- Wongchitphimon, S.; Wang, R.; Jiraratananon, R.; Shi, L.; Loh, C.H. Effect of polyethylene glycol (PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2011, 369, 329–338. [Google Scholar] [CrossRef]
- Zhao, L.B.; Xu, Z.L.; Liu, M.; Wei, Y.M. Preparation and characterization of PSf hollow fiber membrane from PSf-HBPE-PEG400-NMP dope solution. J. Membr. Sci. 2014, 454, 184–192. [Google Scholar] [CrossRef]
- Xu, Z.L.; Qusay, F.A. Effect of polyethylene glycol molecular weights and concentrations on polyethersulfone hollow fiber ultrafiltration membranes. J. Appl. Polym. Sci. 2004, 91, 3398–3407. [Google Scholar] [CrossRef]
- Chen, H.Z.; Xiao, Y.C.; Chung, T.S. Multi-layer composite hollow fiber membranes derived from poly (ethylene glycol) (PEG) containing hybrid materials for CO2/N2 separation. J. Membr. Sci. 2011, 381, 211–220. [Google Scholar] [CrossRef]
- Panda, S.R.; De, S. Preparation, characterization and performance of ZnCl2 incorporated polysulfone (PSF)/polyethylene glycol (PEG) blend low pressure nanofiltration membranes. Desalination 2014, 347, 52–65. [Google Scholar] [CrossRef]
- Moriya, A.; Maruyama, T.; Ohmukai, Y.; Sotani, T.; Matsuyama, H. Preparation of poly (lactic acid) hollow fiber membranes via phase separation methods. J. Membr. Sci. 2009, 342, 307–312. [Google Scholar] [CrossRef]
- Prince, J.A.; Bhuvana, S.; Boodhoo, K.V.K.; Anbharasi, V.; Singh, G. Synthesis and characterization of PEG-Ag immobilized PES hollow fiber ultrafiltration membranes with long lasting antifouling properties. J. Membr. Sci. 2014, 454, 538–548. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Plisko, T.V.; Liubimova, A.S.; Volkov, V.V.; Usosky, V.V. Hydrophilization of polysulfone hollow fiber membranes via addition of polyvinylpyrrolidone to the bore fluid. J. Membr. Sci. 2007, 524, 537–549. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Karslyan, Y.A.; Ovcharova, A.A.; Volkov, V.V. Development of high flux ultrafiltration polyphenylsulfone membranes applying the systems with upper and lower critical solution temperatures: Effect of polyethylene glycol molecular weight and coagulation bath temperature. J. Membr. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Efimov, M.N.; Vasilev, A.A.; Ivanov, V.I.; Bogdanova, Y.G.; Dolzhikova, V.D.; Karpacheva, G.P.; Bondarenko, G.N.; Volkov, A.V. Effect of IR Radiation on the Properties of Polyacrylonitrile and Membranes on Its Basis. Polym. Sci. 2017, 59, 880–890. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Usosky, V.V. Prevention of the capillary contraction of polysulfone based hollow fiber membranes. Pet. Chem. 2014, 54, 652–658. [Google Scholar] [CrossRef]
- Li, W.; Suelves, I.; Lazaro, M.J.; Zhang, S.F.; Morgan, T.J.; Herod, A.A.; Kandiyoti, R. Solvent degradation during coal liquefaction in a flowing-solvent reactor. Fuel 2004, 83, 157–179. [Google Scholar] [CrossRef]
- Berrueco, C.; Alvarez, P.; Venditti, S.; Morgan, T.J.; Herod, A.A.; Millan, M.; Kandiyoti, R. Sample contamination with NMP-oxidation products and byproduct-free NMP removal from sample solutions. Energy Fuels 2009, 23, 3008–3015. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Plisko, T.V.; Isaichykova, Y.A.; Ovcharova, A.A. Preparation of high-flux ultrafiltration polyphenylsulfone membranes. Pet. Chem. 2018, 58, 747–759. [Google Scholar] [CrossRef]
- Ismail, A.F.; Lai, P.Y. Effects of phase inversion and rheological factors on formation of defect-free and ultrathin-skinned asymmetric polysulfone membranes for gas separation. Sep. Purif. Technol. 2003, 33, 127–143. [Google Scholar] [CrossRef]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef]
- Dahe, G.J.; Teotia, R.S.; Bellare, J.R. Correlation between spinning temperature, membrane morphology, and performance of Psf/PVP/NMP/Water hollow fiber membrane forming system. J. Appl. Polym. Sci. 2012, 124, 134–146. [Google Scholar] [CrossRef]
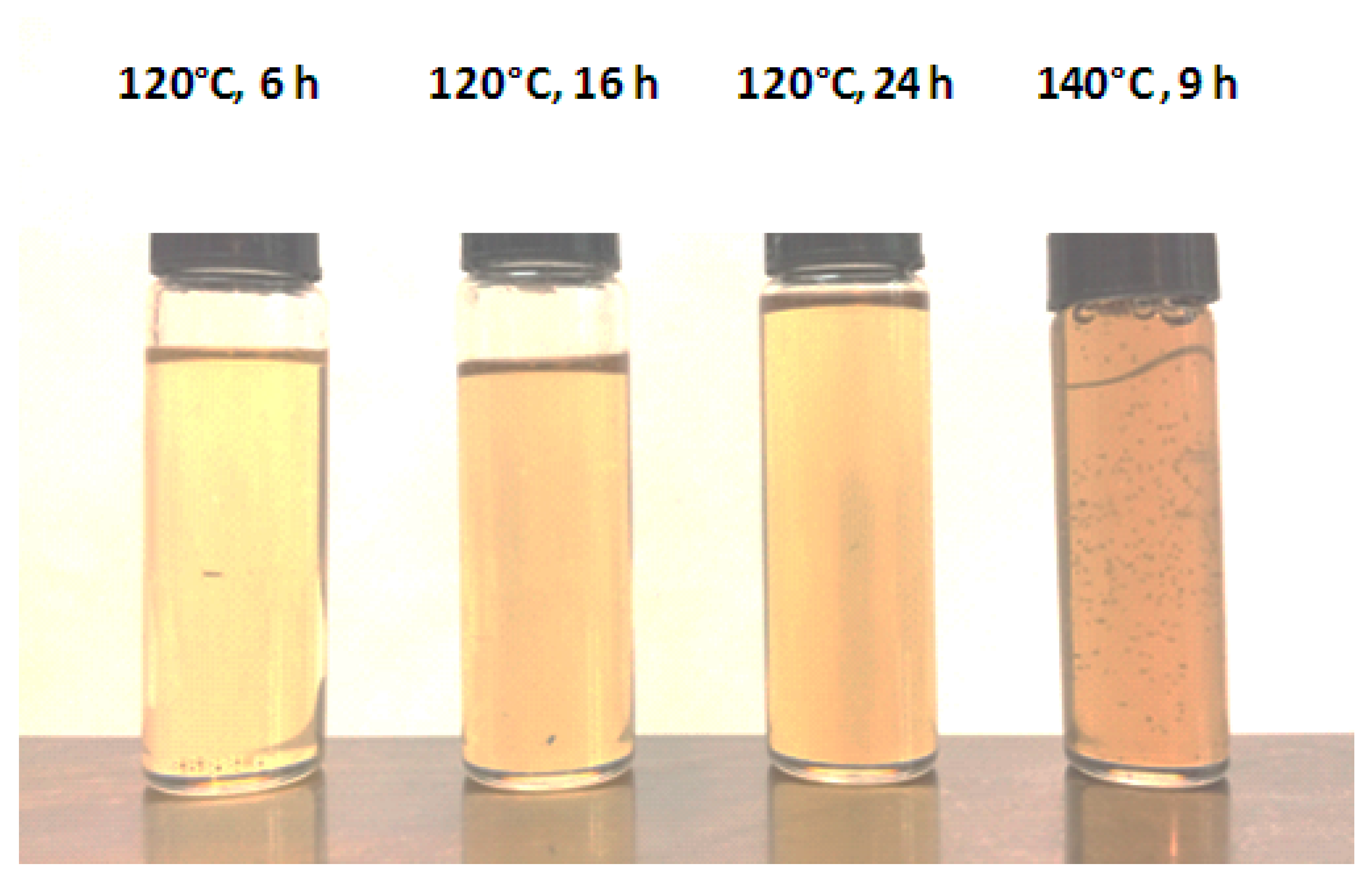
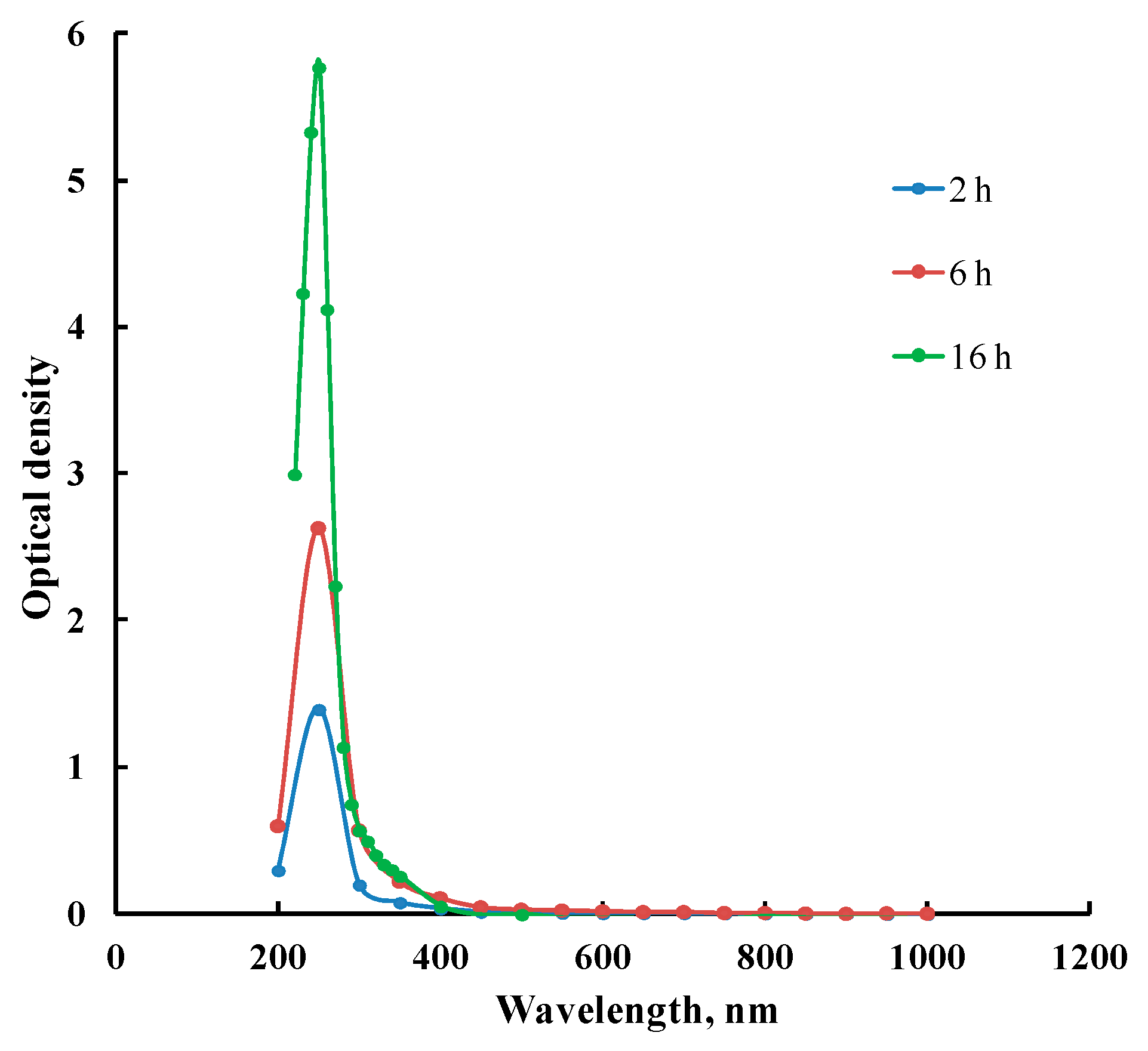
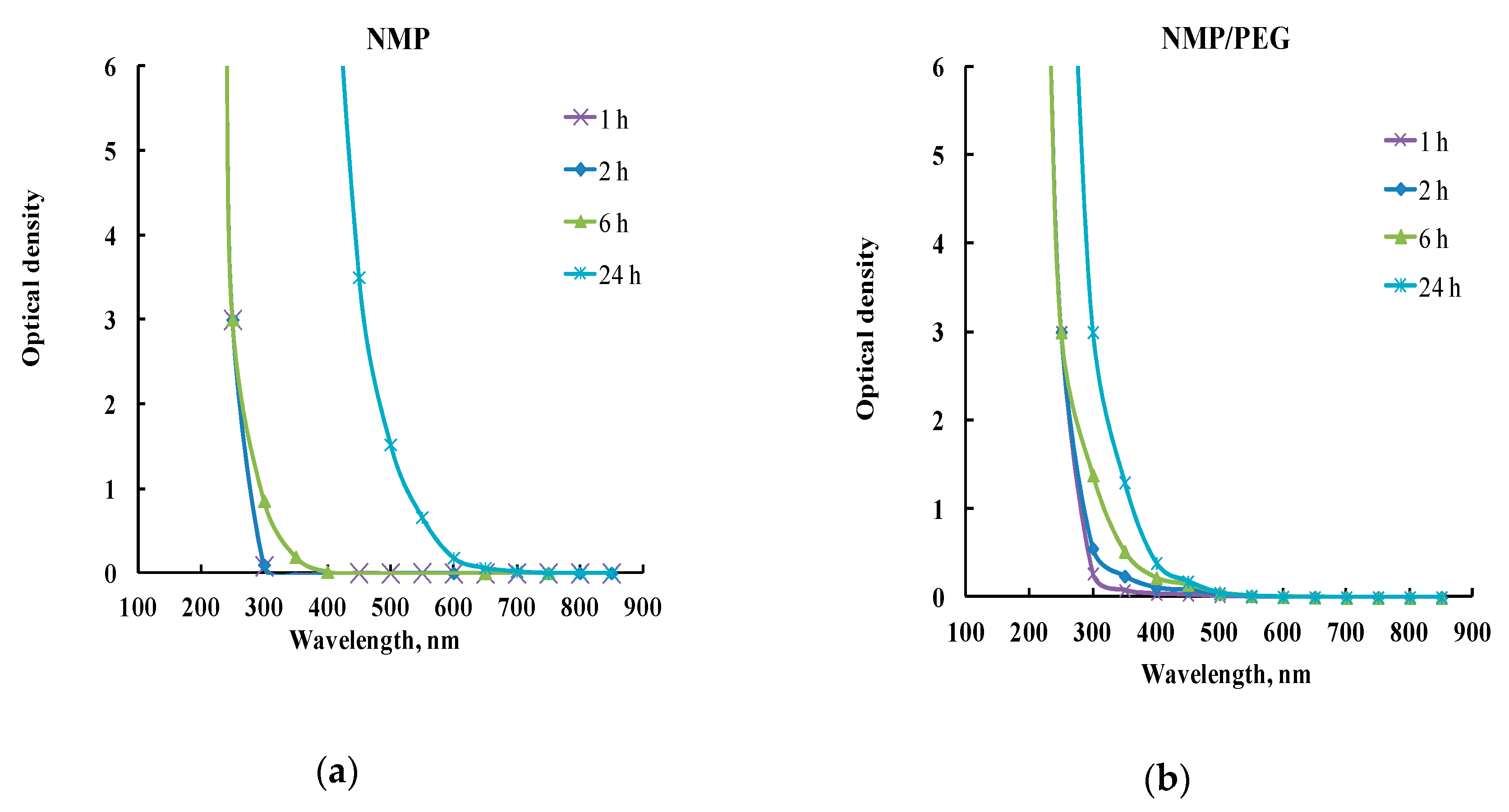
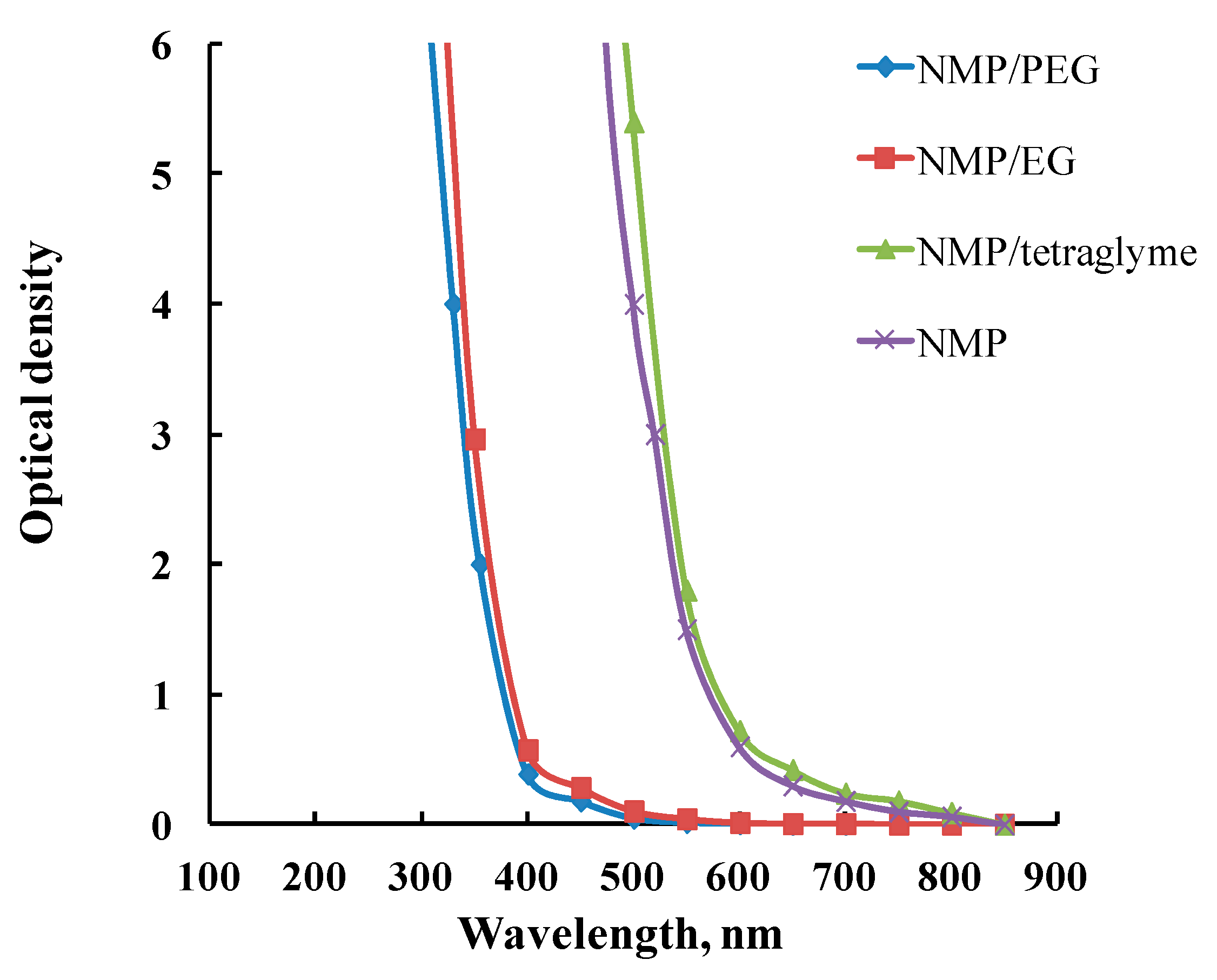
| Polymer | Pore-Forming Component | Solvent | Preparation Temperature, °C | Preparation Time, h | Ref. |
|---|---|---|---|---|---|
| PES | PEG-200, –400, –600 | DMFA | 80 | - | [29] |
| PVDF | PEG-600 | DMAA | 60 | 24 | [30] |
| PES | PEG-10000 | NMP | 60 | 48 | [31] |
| PSf | PEG-200 | NMP/water (95/5) | 25–27 | - | [32] |
| PSf | PEG-600, -6000, -20000, -35000, -150000 | NMP | 70 | 24 | [33] |
| PES | PEG-10000 | NMP | 45 | - | [34] |
| PVDF + HFP | PEG-200, -600, -6000 | NMP | 60 | 72 | [35] |
| PSf + HBPE | PEG-400 | NMP | 25 | 24 | [36] |
| PES | PEG-200, -600, -6000, -10000 | NMP | 25 | - | [37] |
| PES | DEG | NMP | 25 | - | [38] |
| PSf | PEG-200, -400, -600, -4000, -20000, -35000 | DMFA | 60 | 12 | [39] |
| Polylactide | PEG-6000 | DMSO, NMP | 130/90 | 3/- | [40] |
| PES | DEG | NMP | 80 | 24 | [41] |
| PSf | PEG-400 | DMAA | 120 | 4 | [42] |
| PPSU | PEG-400, -2000, -6000, -20000, -35000, -40000 | NMP | 120 | 4 | [43] |
| Time of Treatment, h | Viscosity, cP | Membrane Thickness, μm | Selective Layer Thickness, μm | P/l (He), m3/(m2∙h∙atm) | P/l (CO2), m3/(m2∙h∙atm) | α (He/CO2) |
|---|---|---|---|---|---|---|
| 6 | 31,200 | 102 | 3 | 41.1 | 15.9 | 2.6 |
| 11 | 37,000 | 103 | 10 | 22.2 | 7.9 | 2.8 |
| 16 | 39,900 | 99 | 11 | 19.3 | 6.6 | 3.0 |
| 24 | 46,900 | 102 | 15 | 8.7 | 2.9 | 3.0 |
| Treatment Time, h | Viscosity, cP | Coag. Value, g/g | Density, g/cm3 | P/l (He), m3/(m2∙h∙atm) | P/l (CO2), m3/(m2∙h∙atm) | α (He/CO2) |
|---|---|---|---|---|---|---|
| 6 | 31,100 | 0.182 | 0.253 | 580 | 240 | 2.4 |
| 11 | 37,300 | 0.183 | 0.276 | 54 | 18 | 3.0 |
| 16 | 40,100 | 0.183 | 0.284 | 42 | 13 | 3.3 |
| 24 | 46,800 | 0.185 | 0.313 | 30 | 10 | 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisov, I.; Vasilevsky, V.; Matveev, D.; Ovcharova, A.; Volkov, A.; Volkov, V. Effect of Temperature Exposition of Casting Solution on Properties of Polysulfone Hollow Fiber Membranes. Fibers 2019, 7, 110. https://doi.org/10.3390/fib7120110
Borisov I, Vasilevsky V, Matveev D, Ovcharova A, Volkov A, Volkov V. Effect of Temperature Exposition of Casting Solution on Properties of Polysulfone Hollow Fiber Membranes. Fibers. 2019; 7(12):110. https://doi.org/10.3390/fib7120110
Chicago/Turabian StyleBorisov, Ilya, Vladimir Vasilevsky, Dmitry Matveev, Anna Ovcharova, Alexey Volkov, and Vladimir Volkov. 2019. "Effect of Temperature Exposition of Casting Solution on Properties of Polysulfone Hollow Fiber Membranes" Fibers 7, no. 12: 110. https://doi.org/10.3390/fib7120110
APA StyleBorisov, I., Vasilevsky, V., Matveev, D., Ovcharova, A., Volkov, A., & Volkov, V. (2019). Effect of Temperature Exposition of Casting Solution on Properties of Polysulfone Hollow Fiber Membranes. Fibers, 7(12), 110. https://doi.org/10.3390/fib7120110








