Elaboration of High Permeable Macrovoid Free Polysulfone Hollow Fiber Membranes for Air Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Asymmetric Hollow Fiber Spinning and Characterization
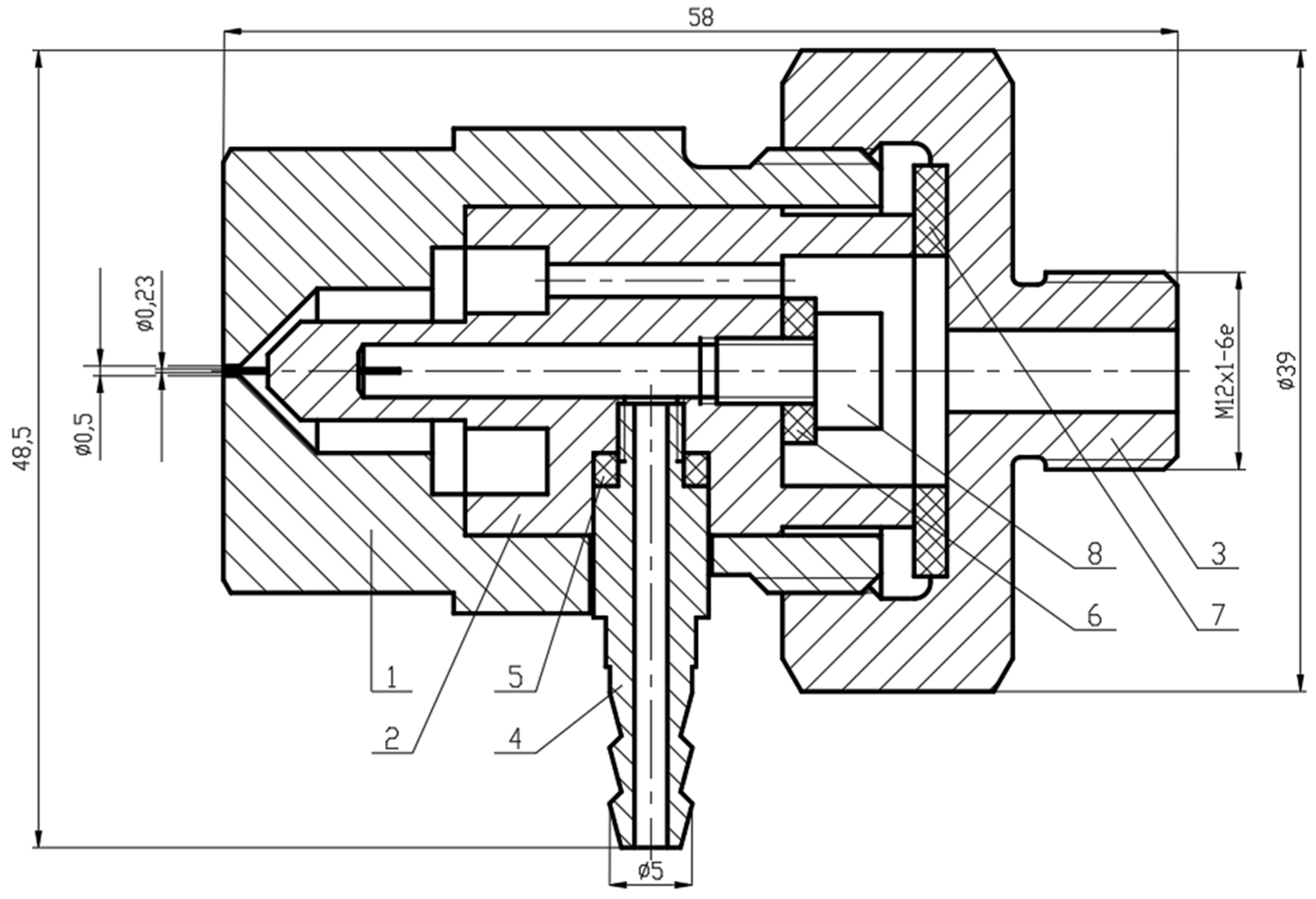
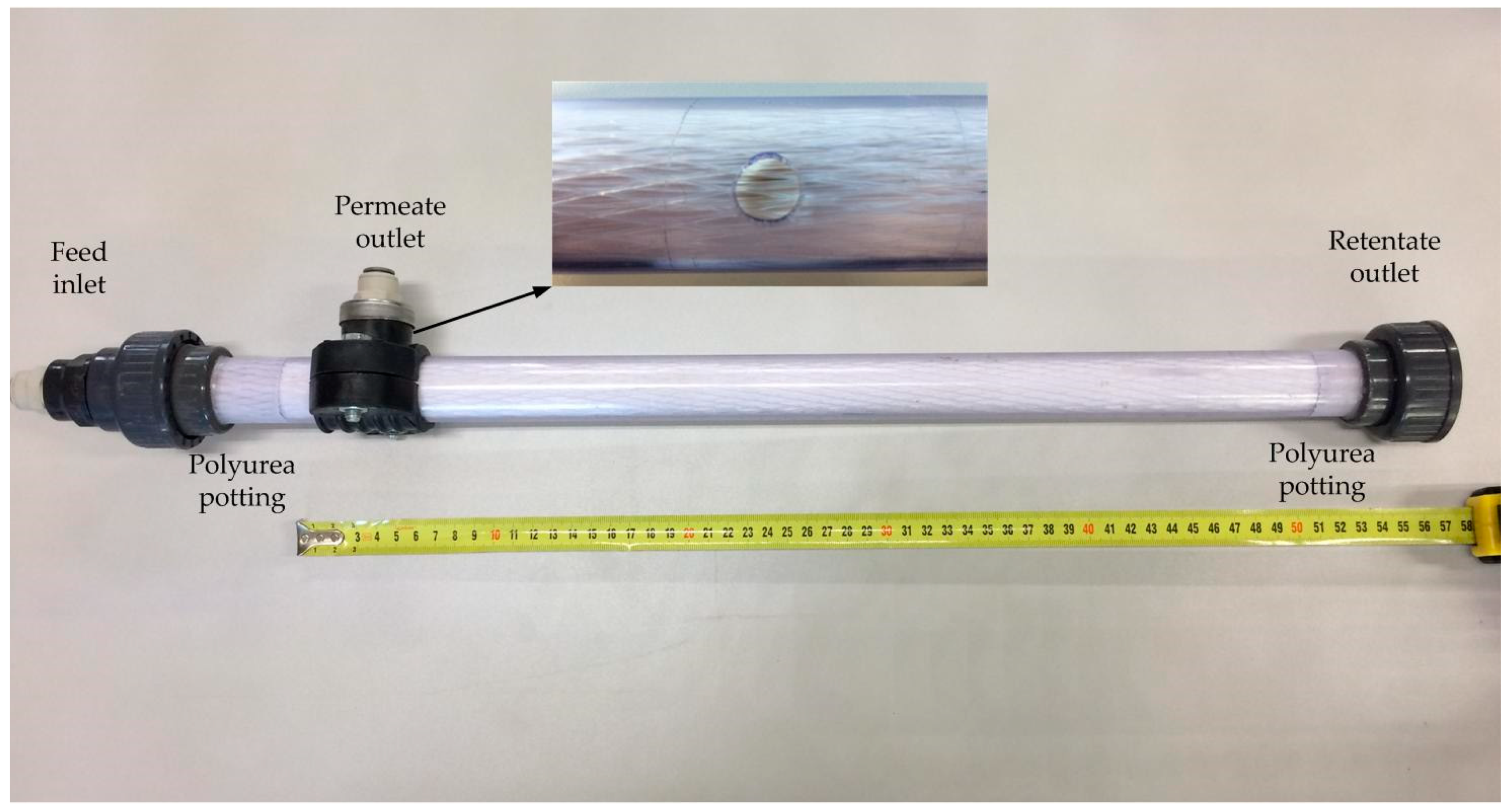
2.3. Membrane Element Preparation
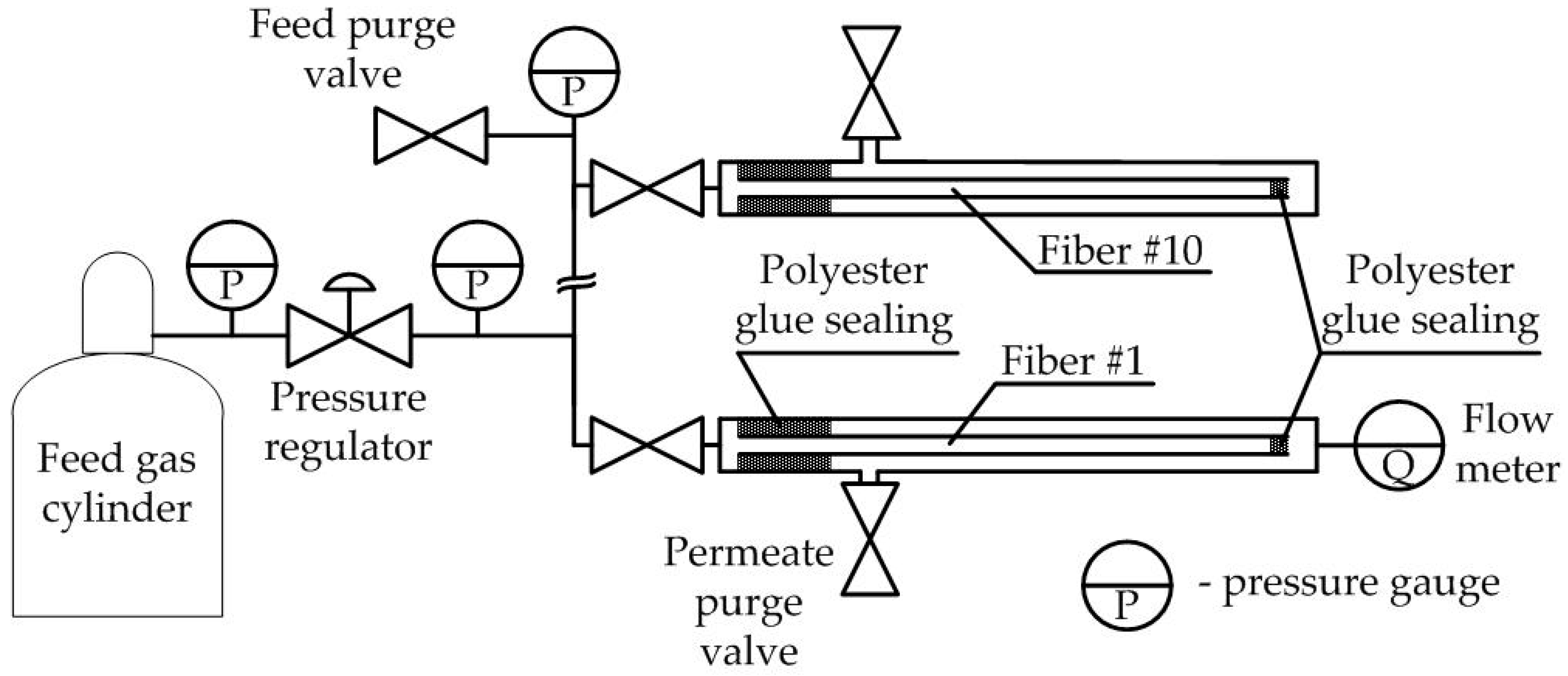
2.4. Membrane Element’s Pure and Mixed Gas Test
3. Results and Discussion
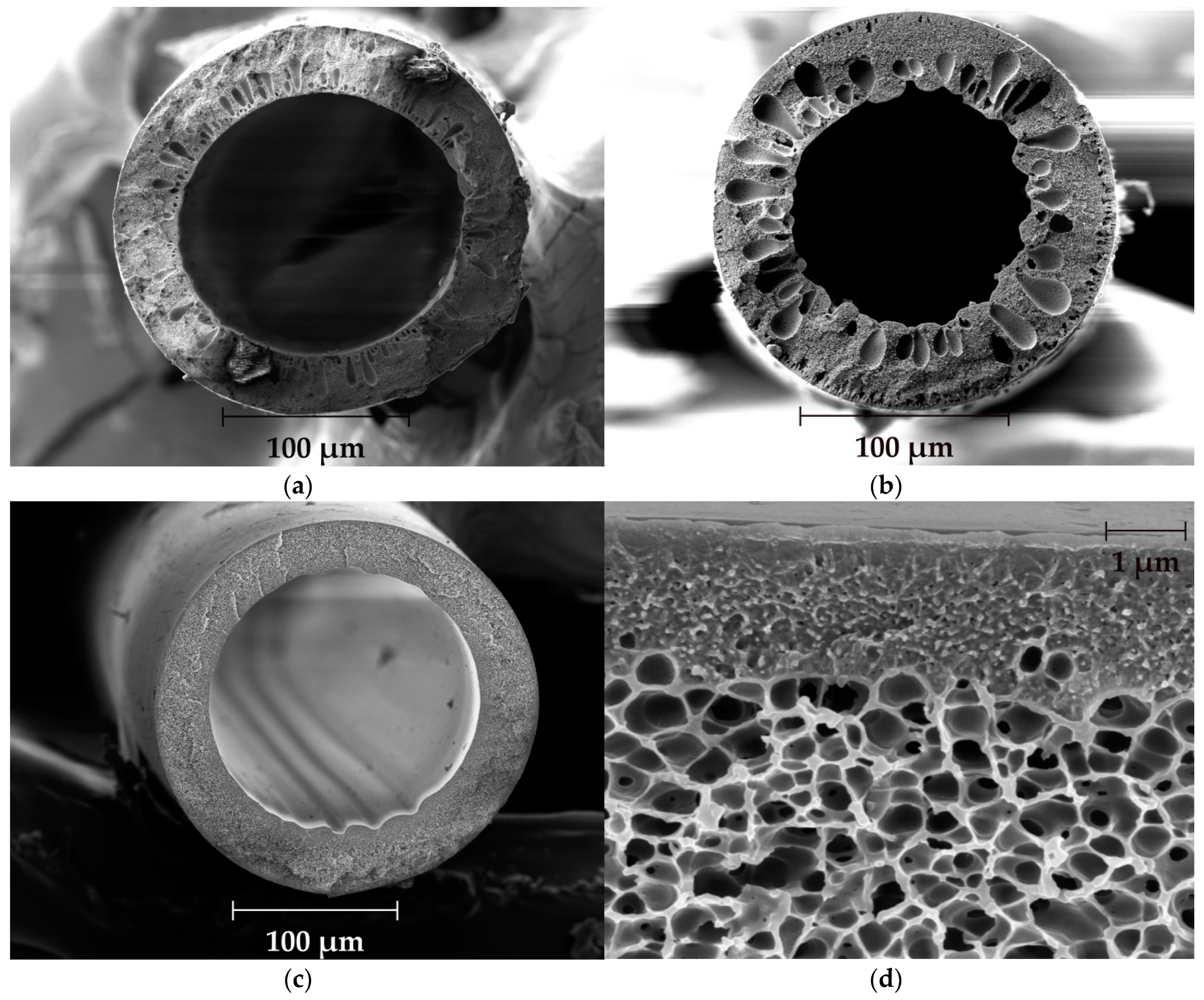
3.1. Hollow Fiber Spinning and Characterization
3.2. Membrane Element Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, X.; Ivory, J.; Rajan, V.S.V. Air separation by integrally asymmetric hollow-fiber membranes. AIChE J. 1999, 45, 2142–2152. [Google Scholar] [CrossRef]
- Dibrov, G.; Ivanov, M.; Semyashkin, M.; Sudin, V.; Kagramanov, G. High-Pressure Aging of Asymmetric Torlon® Hollow Fibers for Helium Separation from Natural Gas. Fibers 2018, 6, 83. [Google Scholar] [CrossRef]
- Rautenbach, R.; Struck, A.; Roks, M.F.M. A variation in fiber properties affects the performance of defect-free hollow fiber membrane modules for air separation. J. Membr. Sci. 1998, 150, 31–41. [Google Scholar] [CrossRef]
- Rautenbach, R.; Struck, A.; Melin, T.; Roks, M.F.M. Impact of operating pressure on the permeance of hollow fiber gas separation membranes. J. Membr. Sci. 1998, 146, 217–223. [Google Scholar] [CrossRef]
- Henis, J.M.S.; Tripodi, M.K. Composite Hollow Fiber Membranes for Gas Separation: The Resistance Model Approach. J. Membr. Sci. 1981, 8, 233–246. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Plisko, T.V.; Liubimova, A.S.; Volkov, V.V.; Usosky, V.V. Hydrophilization of polysulfone hollow fiber membranes via addition of polyvinylpyrrolidone to the bore fluid. J. Membr. Sci. 2017, 524, 537–549. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Usosky, V.V. Prevention of the capillary contraction of polysulfone based hollow fiber membranes. Pet. Chem. 2014, 54, 652–658. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Usosky, V.V.; Volkov, V.V. Influence of the concentration and molecular weight of polyethylene glycol on the structure and permeability of polysulfone hollow fiber membranes. Pet. Chem. 2016, 56, 321–329. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Plisko, T.V.; Usosky, V.V. The formation of polysulfone hollow fiber membranes by the free fall spinning method. Pet. Chem. 2016, 56, 379–400. [Google Scholar] [CrossRef]
- Kostyanaya, M.; Bazhenov, S.; Borisov, I.; Plisko, T.; Vasilevsky, V. Surface Modified Polysulfone Hollow Fiber Membranes for Ethane/Ethylene Separation Using Gas-Liquid Membrane Contactors with Ionic Liquid-Based Absorbent. Fibers 2019, 7, 4. [Google Scholar] [CrossRef]
- Kirsch, V.A.; Bildyukevich, A.V.; Bazhenov, S.D. Simulation of Convection–Diffusion Transport in a Laminar Flow Past a Row of Parallel Absorbing Fibers. Fibers 2018, 6, 90. [Google Scholar] [CrossRef]
- Bazhenov, S.D.; Bildyukevich, A.V.; Volkov, A.V. Gas-Liquid Hollow Fiber Membrane Contactors for Different Applications. Fibers 2018, 6, 76. [Google Scholar] [CrossRef]
- Borisov, I.; Ovcharova, A.; Bakhtin, D.; Bazhenov, S.; Volkov, A.; Ibragimov, R.; Gallyamov, R.; Bondarenko, G.; Mozhchil, R.; Bildyukevich, A.; et al. Development of Polysulfone Hollow Fiber Porous Supports for High Flux Composite Membranes: Air Plasma and Piranha Etching. Fibers 2017, 5, 6. [Google Scholar] [CrossRef]
- Volkov, V.V.; Bildukevich, A.V.; Dibrov, G.A.; Usoskiy, V.V.; Kasperchik, V.P.; Vasilevsk, V.P.; Novitsky, E.G. Elaboration of Composite Hollow Fiber Membranes with Selective Layer from Poly [1-(trimethylsylil). 1-propyne] for Regeneration of Aqueous Alkanolamine Solutions. Pet. Chem. 2013, 53, 619–626. [Google Scholar] [CrossRef]
- Ovcharova, A.; Vasilevsky, V.; Borisov, I.; Bazhenov, S.; Volkov, A.; Bildyukevich, A.; Volkov, V. Polysulfone porous hollow fiber membranes for ethylene-ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2017, 183, 162–172. [Google Scholar] [CrossRef]
- Malakhov, A.O.; Bazhenov, S.D.; Vasilevsky, V.P.; Borisov, I.L.; Ovcharova, A.A.; Bildyukevich, A.V.; Volkov, V.V.; Giorno, L.; Volkov, A.V. Thin-film composite hollow fiber membranes for ethylene/ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2019, 219, 64–73. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Montazer-Rahmati, M.M.; Matsuura, T. Morphology and permeation properties of polysulfone membranes for gas separation: Effects of non-solvent additives and co-solvent. Sep. Purif. Technol. 2010, 72, 194–202. [Google Scholar] [CrossRef]
- Tsai, H.A.; Kuo, C.Y.; Lin, J.H.; Wang, D.M.; Deratani, A.; Pochat-Bohatier, C.; Lee, K.R.; Lai, J.Y. Morphology control of polysulfone hollow fiber membranes via water vapor induced phase separation. J. Membr. Sci. 2006, 278, 390–400. [Google Scholar] [CrossRef]
- Wang, D.; Teo, W.K.; Li, K. Preparation and characterization of high-flux polysulfone hollow fibre gas separation membranes. J. Membr. Sci. 2002, 204, 247–256. [Google Scholar] [CrossRef]
- Fritzsche, A.K. Asymmetric Polysulfone Hollow Fiber Membranes for Gas Separations. In Applications of Polymers; Seymour, R.B., Mark, H.F., Eds.; Springer: Boston, MA, USA, 1988. [Google Scholar]
- Kesting, R.E.; Fritzsche, A.K.; Murphy, M.K.; Cruse, C.A.; Handermann, A.C.; Malon, R.F.; Moore, M.D. The second-generation polysulfone gas-separation membrane. I. The use of lewis acid: Base complexes as transient templates to increase free volume. J. Appl. Polym. Sci. 1990, 40, 1557–1574. [Google Scholar] [CrossRef]
- Ivanov, M.V.; Storozhuk, I.P.; Dibrov, G.A.; Semyashkin, M.P.; Pavlukovich, N.G.; Kagramanov, G.G. Elaboration of hollow fiber membrane from polyarylate-polyarylate block-copolymer for air separation. Pet. Chem. 2018, 8, 85–92. [Google Scholar]
- Ivanov, M.V.; Dibrov, G.A.; Loyko, A.V.; Varezhkin, A.V.; Kagramanov, G.G. Techniques to Manage Geometry Characteristics of Hollow Fiber Membranes. Theor. Found. Chem. Eng. 2016, 50, 316–324. [Google Scholar] [CrossRef]
- Roy, S.; Singha, N.R. Polymeric Nanocomposite Membranes for Next Generation Pervaporation Process: Strategies, Challenges and Future Prospects. Membranes 2017, 7, 53. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Bakhtin, D.S.; Legkov, S.A.; Bondarenko, G.N.; Volkov, A.V. Membrane material based on octyl-substituted polymethylsiloxane for separation of C3/C1 hydrocarbons. Pet. Chem. 2017, 57, 334–340. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Bakhtin, D.S.; Bondarenko, G.N.; Levin, I.S.; Volkov, A.V. Silicone rubbers with alkyl side groups for C3+ hydrocarbon separation. React. Funct. Polym. 2019, 134, 156–165. [Google Scholar] [CrossRef]
- Dibrov, G.A.; Volkov, V.V.; Vasilevsky, V.P.; Shutova, A.A.; Bazhenov, S.D.; Khotimsky, V.S.; van de Runstraat, A.; Goetheer, E.L.V.; Volkov, A.V. Robust high-permeance PTMSP composite membranes for CO2 membrane gas desorption at elevated temperatures and pressure. J. Membr. Sci. 2014, 470, 439–450. [Google Scholar] [CrossRef]
- Pan, C.Y. Gas Separation by Permeators with High-Flux Asymmetric Membranes. AIChE J. 1983, 29, 545–552. [Google Scholar] [CrossRef]
- Davis, R.; Sandall, O. A Simple Analysis for Gas Separation Membrane Experiments. Chem. Eng. Educ. 2003, 37, 74–80. [Google Scholar]
- Nagase, Y.; Naruse, A.; Matsui, K. Chemical modification of polysulphone: 2. Gas and liquid permeability of polysulphone/polydimethylsiloxane graft copolymer membranes. Polymer 1990, 31, 121–125. [Google Scholar] [CrossRef]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef]
- McKelvey, S.A.; Koros, W.J. Phase separation, vitrification, and the manifestation of macrovoids in polymeric asymmetric membranes. J. Membr. Sci. 1996, 112, 29–39. [Google Scholar] [CrossRef]
- Lemanski, J.; Lipscomb, G.G. Effect of fiber variation on the performance of countercurrent hollow fiber gas separation modules. J. Membr. Sci. 2000, 167, 241–252. [Google Scholar] [CrossRef]
| # | T 1, °C | Air Gap, cm | υ 2, m/min | OD, μm | ID, μm | P/l(O2) 3 | α(O2/N2) | P/l(O2) 3,4 | α(O2/N2) 4 | l 5, nm |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | 20 | 30 | 215 | 140 | 55 | 3.1 | 45 | 5.9 | 66 |
| 2 | 62 | 20 | 45 | 190 | 115 | 164 | 1.6 | 45 | 6.1 | 66 |
| 3 | 62 | 10 | 30 | 215 | 140 | 79 | 2.1 | 41 | 6.0 | 72 |
| 4 | 25 | 10 | 30 | 235 | 160 | 1122 | 1.0 | 265 | 1.8 | - |
| 5 | 25 | 20 | 30 | 230 | 155 | 450 | 1.1 | 70 | 6.0 | 42 |
| 6 | 25 | 25 | 30 | 225 | 150 | 284 | 1.4 | 59 | 6.1 | 50 |
| Work | P/l (O2) 1 | α(O2/N2) | l, nm | T, Δp 2 | Macrovoids |
|---|---|---|---|---|---|
| [17] | - | - | 400 | 25 °C, 8 bar | + |
| [19] | 55–80 | 5–6.5 | 36–50 | 25 °C, 5 bar | + |
| [20] | - | - | 500–700 | 50 °C | – |
| [21] | 116 | 5–5.2 | - | 50 °C, 13.6 bar | – |
| This work | 70 | 6.0 | 42 | 22 °C, 5 bar | – |
| ΔP, bar | xr,% | qr, m3/h | qp, m3/h | θ | P/l (O2) 1 | α(O2/N2) |
|---|---|---|---|---|---|---|
| 5 | 6.2 | 0.29 | 0.36 | 0.55 | 74.4 | 4.1 |
| 5 | 4.8 | 0.21 | 0.33 | 0.61 | 74.2 | 4.0 |
| 5 | 0.9 | 0.06 | 0.31 | 0.83 | 81.1 | 3.6 |
| 7 | 4.9 | 0.4 | 0.53 | 0.57 | 87.6 | 4.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibrov, G.; Ivanov, M.; Semyashkin, M.; Sudin, V.; Fateev, N.; Kagramanov, G. Elaboration of High Permeable Macrovoid Free Polysulfone Hollow Fiber Membranes for Air Separation. Fibers 2019, 7, 43. https://doi.org/10.3390/fib7050043
Dibrov G, Ivanov M, Semyashkin M, Sudin V, Fateev N, Kagramanov G. Elaboration of High Permeable Macrovoid Free Polysulfone Hollow Fiber Membranes for Air Separation. Fibers. 2019; 7(5):43. https://doi.org/10.3390/fib7050043
Chicago/Turabian StyleDibrov, George, Mikhail Ivanov, Mikhail Semyashkin, Vladislav Sudin, Nikita Fateev, and George Kagramanov. 2019. "Elaboration of High Permeable Macrovoid Free Polysulfone Hollow Fiber Membranes for Air Separation" Fibers 7, no. 5: 43. https://doi.org/10.3390/fib7050043
APA StyleDibrov, G., Ivanov, M., Semyashkin, M., Sudin, V., Fateev, N., & Kagramanov, G. (2019). Elaboration of High Permeable Macrovoid Free Polysulfone Hollow Fiber Membranes for Air Separation. Fibers, 7(5), 43. https://doi.org/10.3390/fib7050043





