The Effect of Zirconium Doping on Solution-Processed Indium Oxide Thin Films Measured by a Novel Nondestructive Testing Method (Microwave Photoconductivity Decay)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, W.; Hu, L.; Zhao, C.; Zhang, L.; Zhu, D.; Cao, P.; Liu, W.; Han, S.; Liu, X.; Jia, F.; et al. Low temperature solution-processed IGZO thin-film transistors. Appl. Surf. Sci. 2018, 455, 554–560. [Google Scholar] [CrossRef]
- Chen, R.; Lan, L. Solution-processed metal-oxide thin-film transistors: A review of recent developments. Nanotechnology 2019, 30, 312001. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.; Hori, T.; Yasuda, T.-O.; Kizu, T.; Tsukagoshi, K.; Nabatame, T.; Trinh, B.N.Q.; Fujiwara, A. Si-doping effect on solution-processed In-O thin-film transistors. Mater. Res. Express 2019, 6, 026410. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.-H.; Zhu, W.-Q.; Zhang, J.-H.; Zhang, Z.-L. Combustion synthesis of nontoxic water-induced InYO thin film and application in thin film transistor. Mater. Sci. Semicond. Process. 2019, 93, 201–207. [Google Scholar] [CrossRef]
- Kang, L.; An, H.; Jung, S.; Kim, S.; Nahm, S.; Kim, D.-G.; Lee, C.G. Low-voltage operating solution-processed CdS thin-film transistor with Ca2Nb3O10 nanosheets deposited using Langmuir-Blodgett method for a gate insulator. Appl. Surf. Sci. 2019, 476, 374–377. [Google Scholar] [CrossRef]
- Ren, J.; Li, K.; Yang, J.; Lin, D.; Kang, H.; Shao, J.; Fu, R.; Zhang, Q. Solution-processed amorphous gallium-tin oxide thin film for low-voltage, high-performance transistors. Sci. China-Mater. 2019, 62, 803–812. [Google Scholar] [CrossRef]
- Ha, H.; Hori, T.; Yasuda, T.O.; Kizu, T.; Tsukagoshi, K.; Nabatame, T.; Trinh, B.N.Q.; Fujiwara, A. Investigation on solution-processed In-Si-O thin-film transistor via spin-coating method. In Proceedings of the 2018 25th International Workshop on Active-Matrix Flatpanel Displays and Devices (AM-FPD), Kyoto, Japan, 3–6 July 2018. [Google Scholar]
- Lee, J.; Lee, J.; Park, J.; Lee, S.-E.; Lee, E.G.; Im, C.; Lim, K.-H.; Kim, Y.S. Solution-grown homojunction oxide thin-film transistors. ACS Appl. Mater. Interfaces 2019, 11, 4103–4110. [Google Scholar] [CrossRef]
- Hwang, N.; Lim, Y.; Lee, S.; Lee, J.S.; Yi, M. Solution based indium zinc oxide thin film transistor with diffused aluminum oxide insulator layer. In Proceedings of the 2018 International Conference on Electronics, Information, and Communication (ICEIC), Honolulu, HI, USA, 24–27 January 2018; pp. 1–3. [Google Scholar]
- Lee, W.Y.; Jang, B.; Lee, S.; Kim, T.; Jang, J. High performance sol-gel processed SnO2 thin film transistor with sol-gel processed ZrO2 layers. In Proceedings of the 2018 25th International Workshop on Active-Matrix Flatpanel Displays and Devices (AM-FPD), Kyoto, Japan, 3–6 July 2018. [Google Scholar]
- Cai, W.; Wilson, J.; Zhang, J.; Park, S.; Majewski, L.; Song, A. Low-voltage, flexible InGaZnO thin-film transistors gated with solution-processed, ultra-thin AlxOy. IEEE Electron Device Lett. 2019, 40, 36–39. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Ha, T.-J. Improvement in interfacial characteristics of low-voltage carbon nanotube thin-film transistors with solution-processed boron nitride thin films. Appl. Surf. Sci. 2017, 413, 118–122. [Google Scholar] [CrossRef]
- Kang, J.K.; Park, S.P.; Na, J.W.; Lee, J.H.; Kim, D.; Choi, J.S.; Kim, H.J. Fabrication of eco-friendly solution-processed indium zinc oxide thin-film transistors through recycling based on photocatalytic reactions of TiO2. SID Symp. Dig. Tech. Pap. 2018, 49, 1264–1267. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Shin, K.-H.; Yoon, D.G.; Chin, B.D.; Kim, S.H. All-solution-processed, flexible thin-film transistor based on PANI/PETA as gate/gate insulator. RSC Adv. 2015, 5, 105785–105788. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.G.; Park, S.P.; Kim, D.; Kim, N.E.; Choi, J.S.; Kim, H.J. Self-pattern process of InZnO thin-film transistors using photosensitive precursors. SID Symp. Dig. Tech. Pap. 2017, 48, 180–182. [Google Scholar] [CrossRef]
- Rim, Y.S.; Keum, M.-J.; Baek, Y.; Lee, B.M.; Kim, K.H. Film Density Controlled-InGaZnO multi-stacked channel based thin-film transistors using a solution process. Sci. Adv. Mater. 2017, 9, 1578–1582. [Google Scholar] [CrossRef]
- Choi, H.-S.; Cho, W.-J. Controlling In-Ga-Zn-O thin-film resistance by vacuum rapid thermal annealing and application to transparent electrode. Phys. Status Solidi A 2019, 216, 1800653. [Google Scholar] [CrossRef]
- Nguyen, M.-C.; Nguyen, A.H.-T.; Ji, H.; Cheon, J.; Kim, J.-H.; Yu, K.-M.; Cho, S.-Y.; Kim, S.-W.; Choi, R. Application of single-pulse charge pumping method on evaluation of indium gallium zinc oxide thin-film transistors. IEEE Trans. Electron Devices 2018, 65, 3786–3790. [Google Scholar] [CrossRef]
- Xia, Z.; Lu, L.; Li, J.; Kwok, H.S.; Wong, M. The use of fluorination to enhance the performance and the reliability of elevated-metal metal-oxide thin-film transistors. SID Symp. Dig. Tech. Pap. 2018, 49, 1235–1238. [Google Scholar] [CrossRef]
- Kim, K.S.; Ahn, C.H.; Kang, W.J.; Cho, S.W.; Jung, S.H.; Yoon, D.H.; Cho, H.K. An all oxide-based imperceptible thin-film transistor with humidity sensing properties. Materials 2017, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, A.; Guo, Z.; Liu, G.; Shin, B.; Noh, Y.-Y.; Fortunato, E.; Martins, R.; Shan, F. Electronic devices based on oxide thin films fabricated by fiber-to-film process. ACS Appl. Mater. Interfaces 2018, 10, 18057–18065. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Abliz, A.; Su, M.; Liu, C.; Jiang, C.; Li, G.; Chen, H.; Guo, T.; Liu, X.; Liao, L. Low-frequency noise in high-mobility a-InGaZnO/InSnO nanowire composite thin-film transistors. IEEE Electron Device Lett. 2017, 38, 1540–1542. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, D.J.; Hwang, A.Y.; Park, J.W.; Jeong, J.K. High performance a-InZnSnO thin-film transistor with a self-diffusion-barrier formable copper contact. Thin Solid Films 2017, 637, 3–8. [Google Scholar] [CrossRef]
- Pan, T.-M.; Peng, B.-J.; Her, J.-L.; Lou, B.-S. Effect of In and Zn content on structural and electrical properties of InZnSnO thin-film transistors using an Yb2TiO5 gate dielectric. IEEE Trans. Electron Devices 2017, 64, 2233–2238. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Dai, S.; Li, R.; Ma, Y.; Wang, Q.; Tian, L.; Ning, K.; Zhang, X. Double active layer InZnO:N/InZnSnO thin film transistors with high mobility at low annealing temperature. J. Mater. Sci.-Mater. Electron. 2019, 30, 1496–1499. [Google Scholar] [CrossRef]
- Yang, J.-W.; Na, Y.-B.; Shin, J.-H.; Hong, C.-H.; Seo, W.-H.; Kim, K.-H.; Song, C.-W.; Song, S.-H.; Kwon, H.-I.; Cheong, W.-S. Effects of Er-doping on amorphous InZnSnO/InZnSnO: Er double-channel thin-film transistors. J. Nanosci. Nanotechnol. 2017, 17, 3415–3419. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Hung, C.-C.; Hsu, W.-C. Deposition of oxide thin films by ultrasonic spray pyrolysis deposition for InGaZnO thin-film transistor applications. IEEE Electron Device Lett. 2018, 39, 1520–1523. [Google Scholar] [CrossRef]
- Takechi, K.; Kuwahara, Y.; Tanaka, J.; Tanabe, H. Depth-profiling XPS analysis of He-plasma treated amorphous InGaZnO thin films for use in top-gate coplanar thin-film transistors. Jpn. J. Appl. Phys. 2019, 58, 038005. [Google Scholar] [CrossRef]
- Wang, W.-H.; Heredia, E.; Lyu, S.-R.; Liu, S.-H.; Liao, P.-Y.; Chang, T.-C.; Jiang, P.-H. Weak localization and weak antilocalization in double-gate a-InGaZnO thin-film transistors. IEEE Electron Device Lett. 2018, 39, 212–215. [Google Scholar] [CrossRef]
- Yang, J.; Chang, T.-C.; Chen, B.-W.; Liao, P.-Y.; Chiang, H.-C.; Zhang, Q. Effects of mechanical stress on flexible dual-gate a-InGaZnO thin-film transistors. Phys. Status Solidi A 2018, 215, 1700426. [Google Scholar] [CrossRef]
- Xiao, P.; Dong, T.; Lan, L.; Lin, Z.; Song, W.; Song, E.; Sun, S.; Li, Y.; Gao, P.; Luo, D. High-mobility flexible thin-film transistors with a low-temperature zirconium-doped indium oxide channel layer. Phys. Status Solidi (RRL) Rapid Res. Lett. 2016, 10, 493–497. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Ning, H.; Yuan, W.; Deng, Y.; Zhang, X.; Wang, S.; Wang, J.; Yao, R.; Peng, J. Characterization studies of the structure and properties of Zr-doped SnO2 thin films by spin-coating technique. Superlattices Microstruct. 2018, 123, 330–337. [Google Scholar] [CrossRef]
- Liu, X.; Cai, W.; Chen, J.; Fang, Z.; Ning, H.; Hu, S.; Tao, R.; Zeng, Y.; Zheng, Z.; Yao, R.; et al. A novel nondestructive testing method for amorphous Si–Sn–O films. J. Phys. D Appl. Phys. 2016, 49, 505102. [Google Scholar] [CrossRef]
- Yao, R.; Zheng, Z.; Xiong, M.; Zhang, X.; Ning, H.; Fang, Z.; Xie, W.; Lu, X.; Peng, J. Low-temperature fabrication of sputtered high-k HfO2 gate dielectric for flexible a-IGZO thin film transistors. Appl. Phys. Lett. 2018, 112, 103503. [Google Scholar] [CrossRef]
- Wei, J.; Fang, Z.; Peng, J.; Cai, W.; Zhu, Z.; Yao, R.; Zhou, S.; Yuan, W.; Lu, K.; Ning, H. High-performance spin-coated aluminum oxide dielectric fabricated by a simple oxygen plasma-treatment process. J. Phys. D Appl. Phys. 2018, 51, 365101. [Google Scholar] [CrossRef]
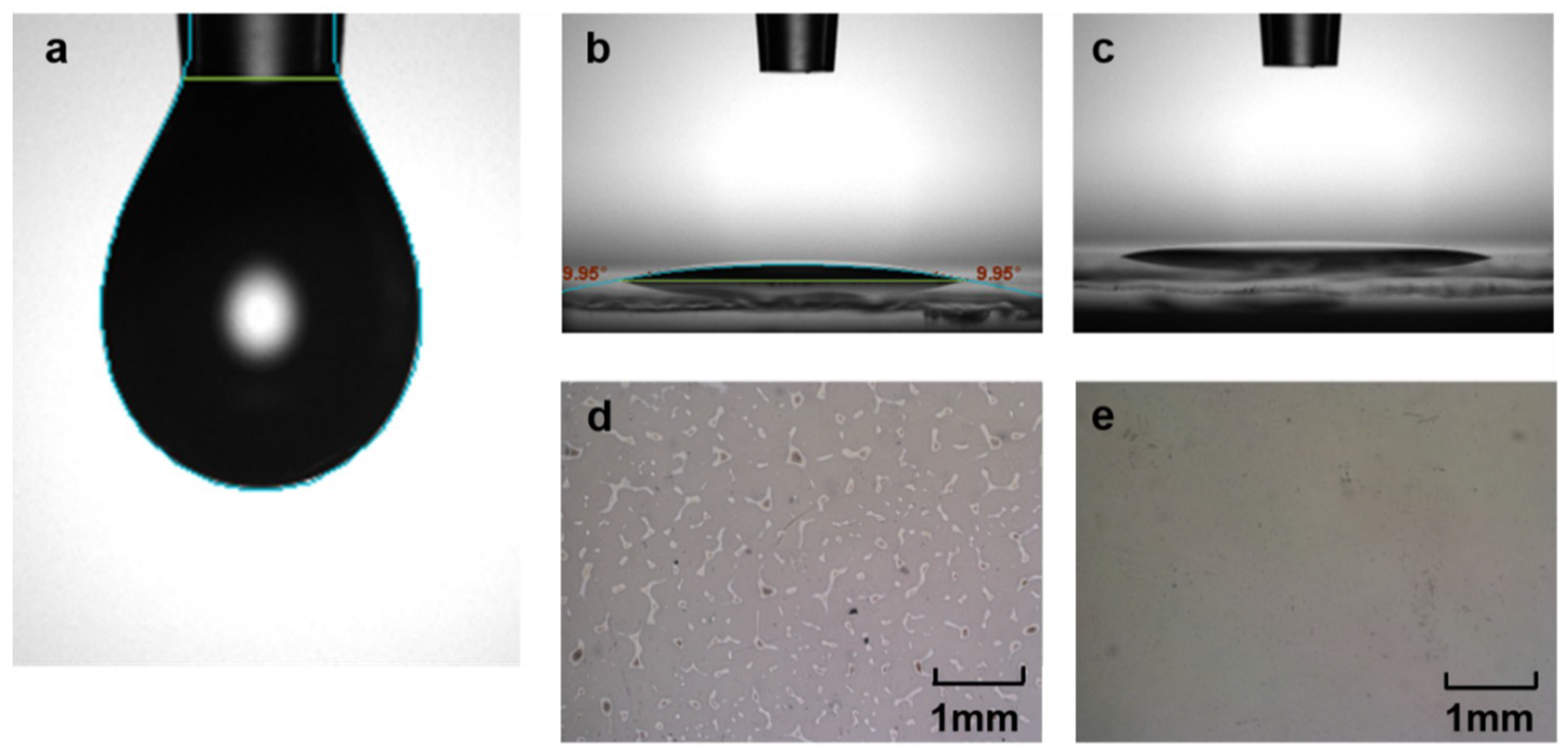
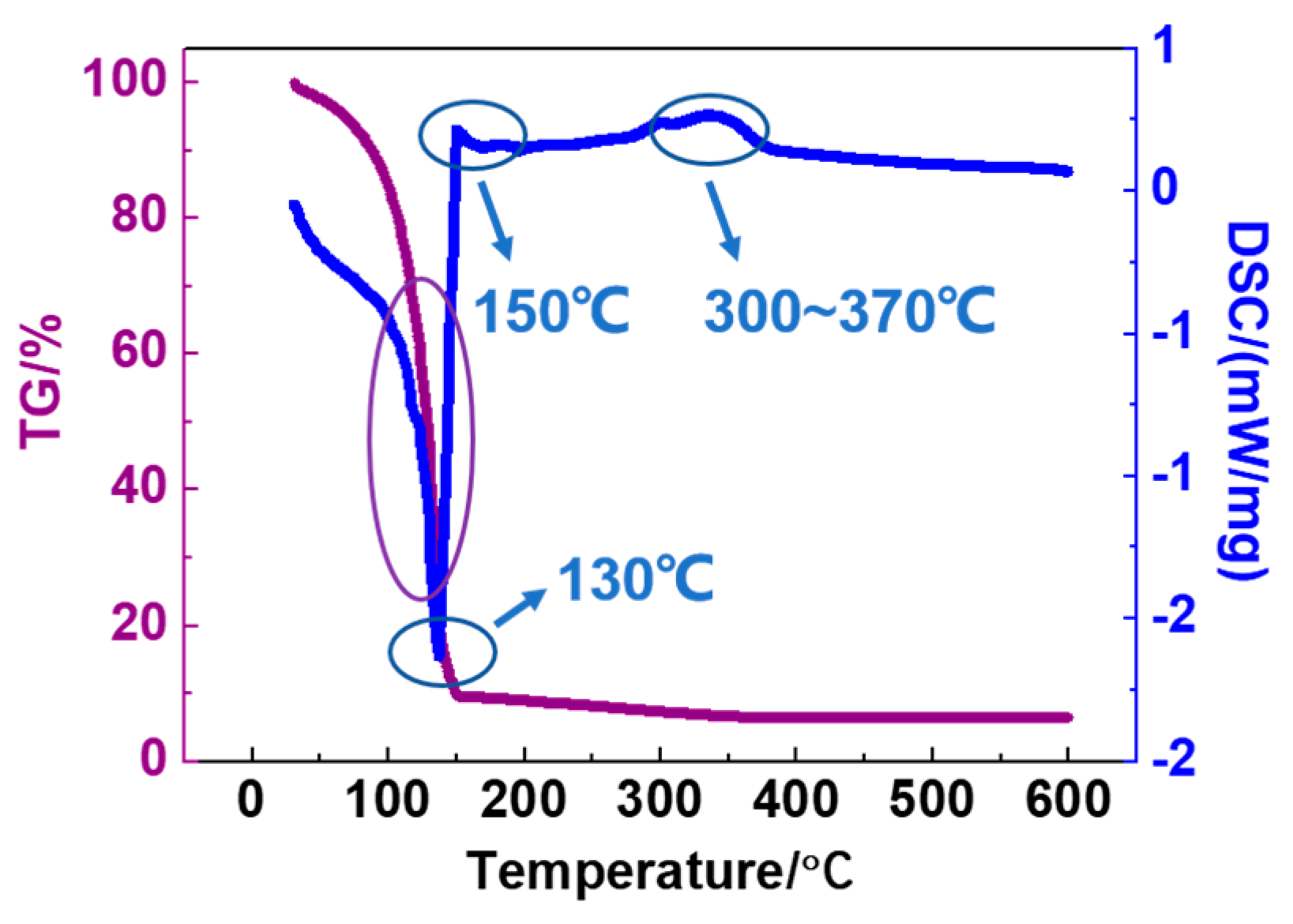
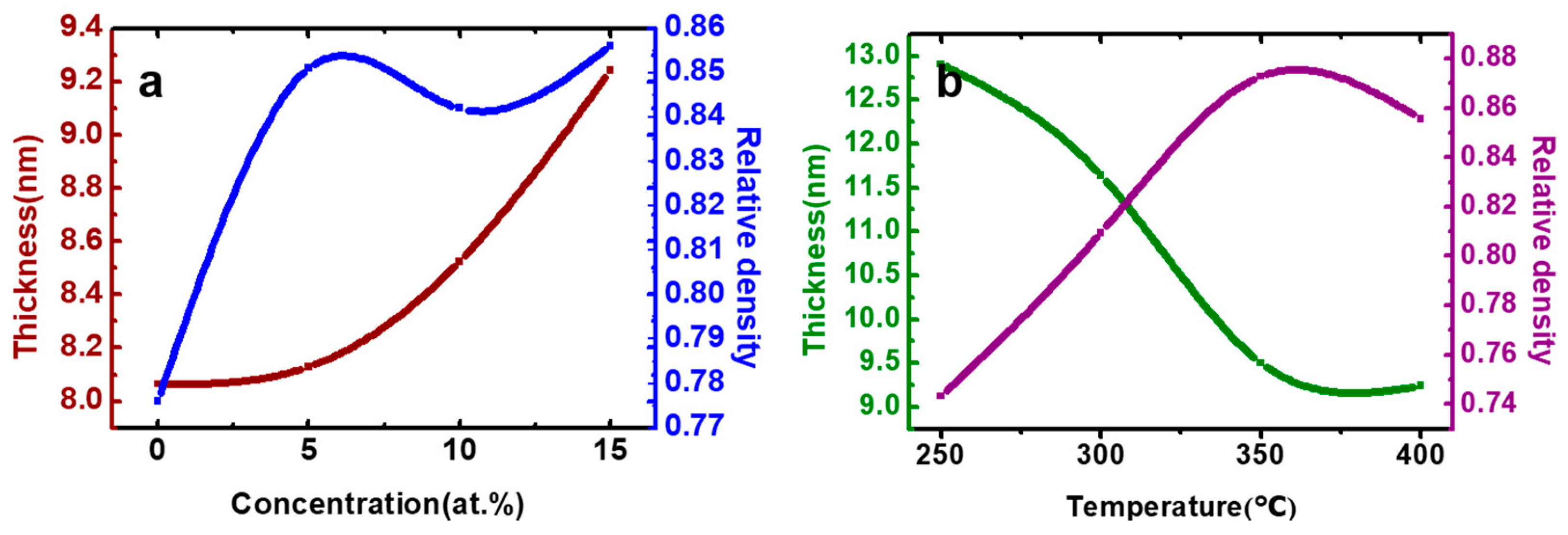
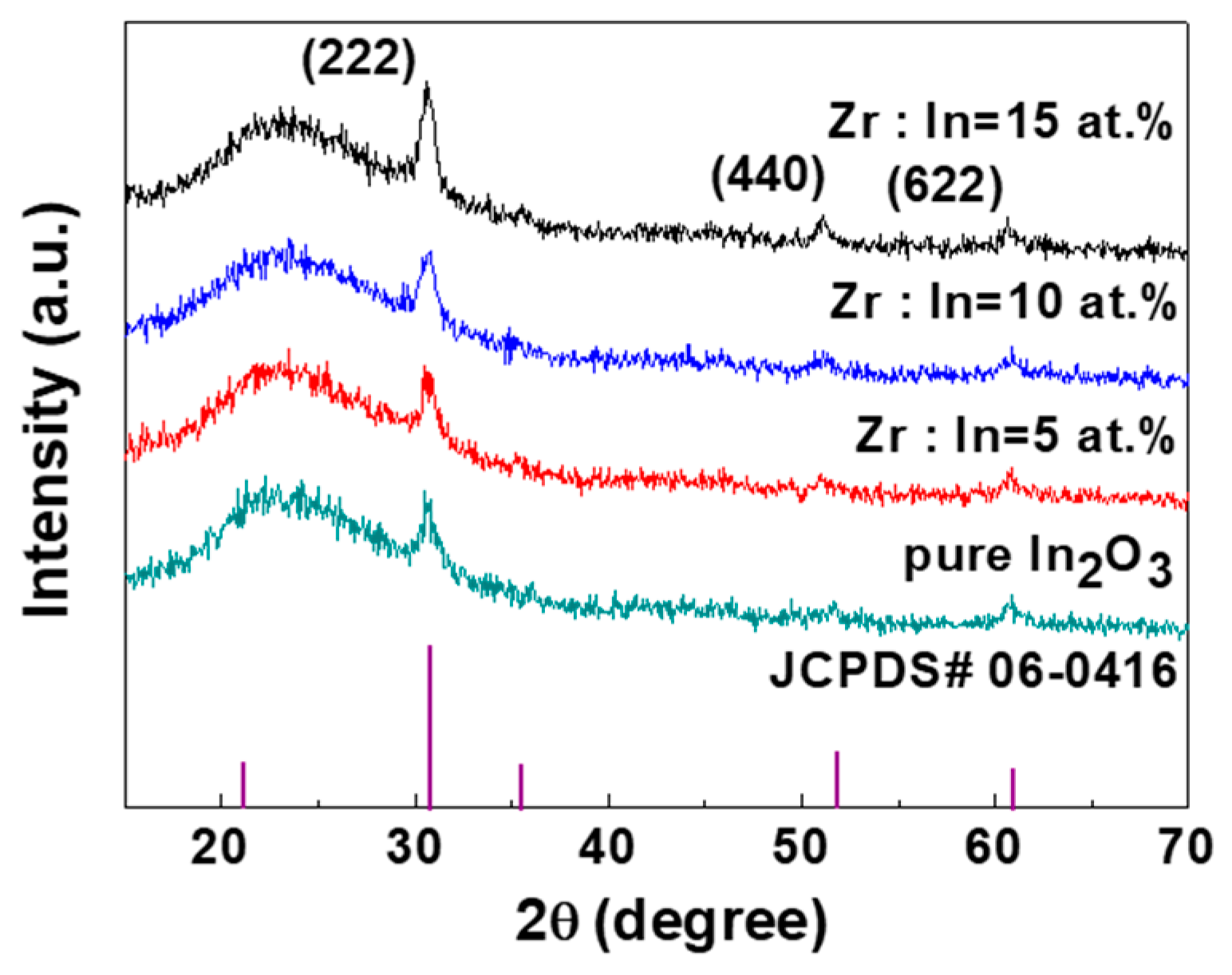
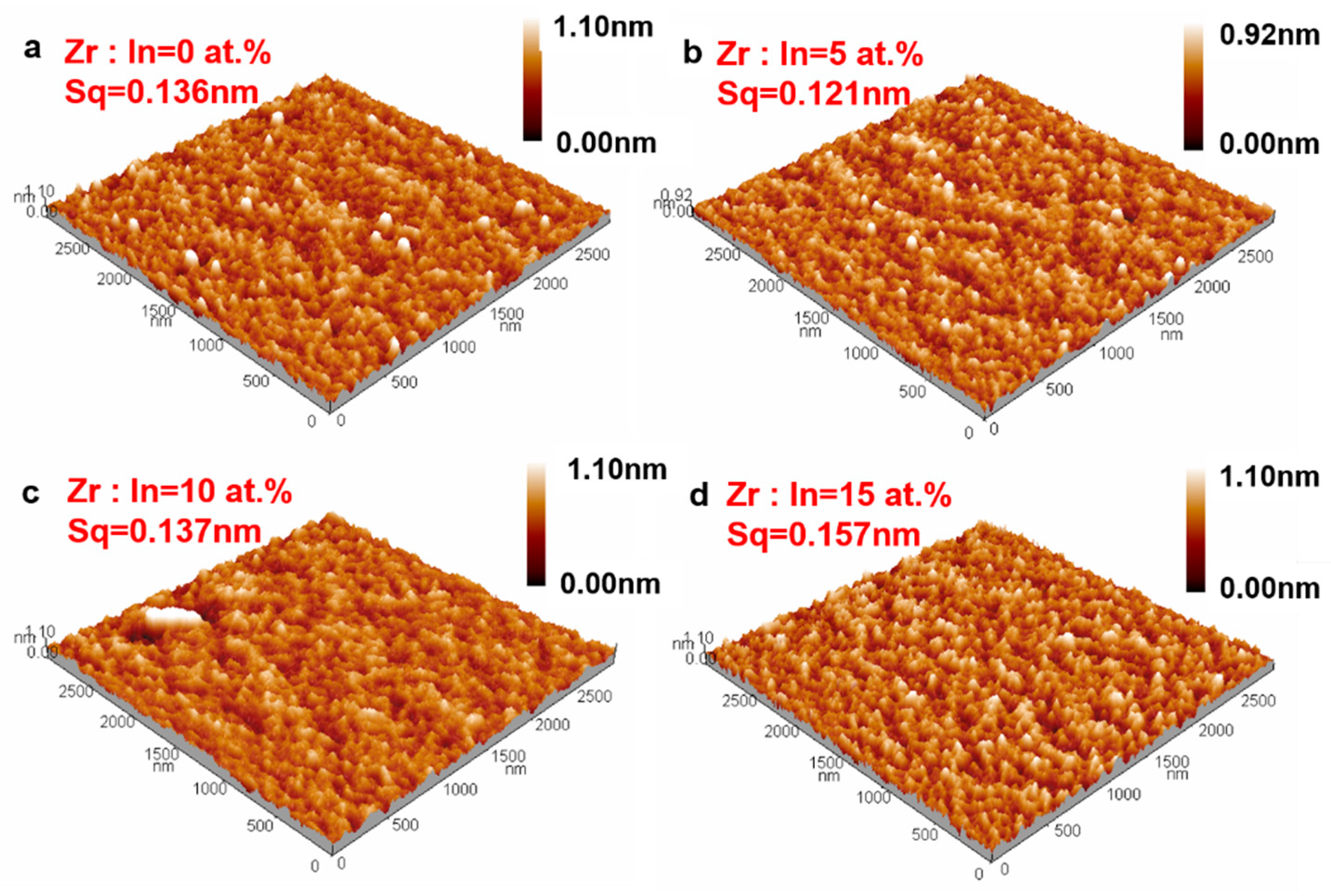
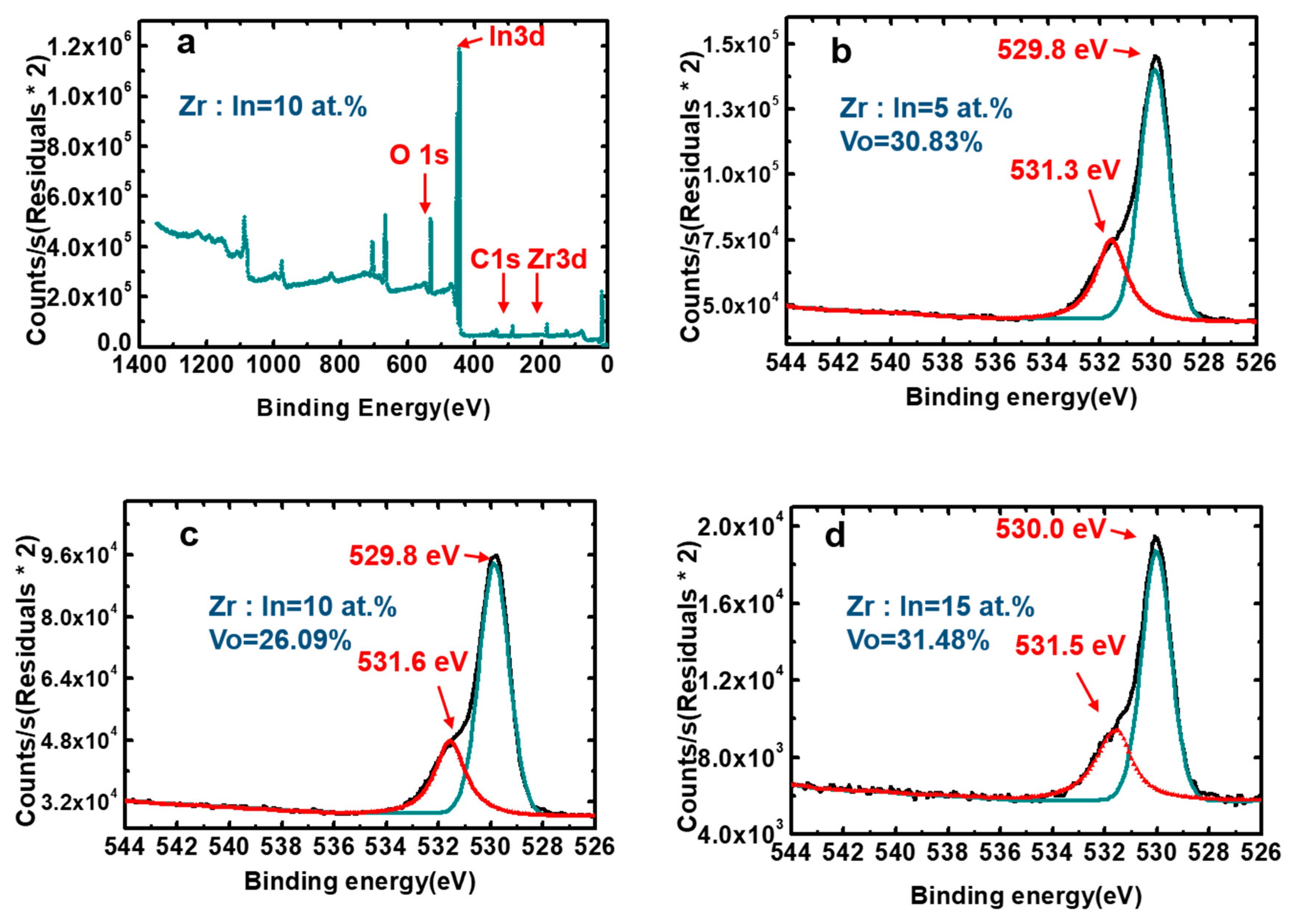
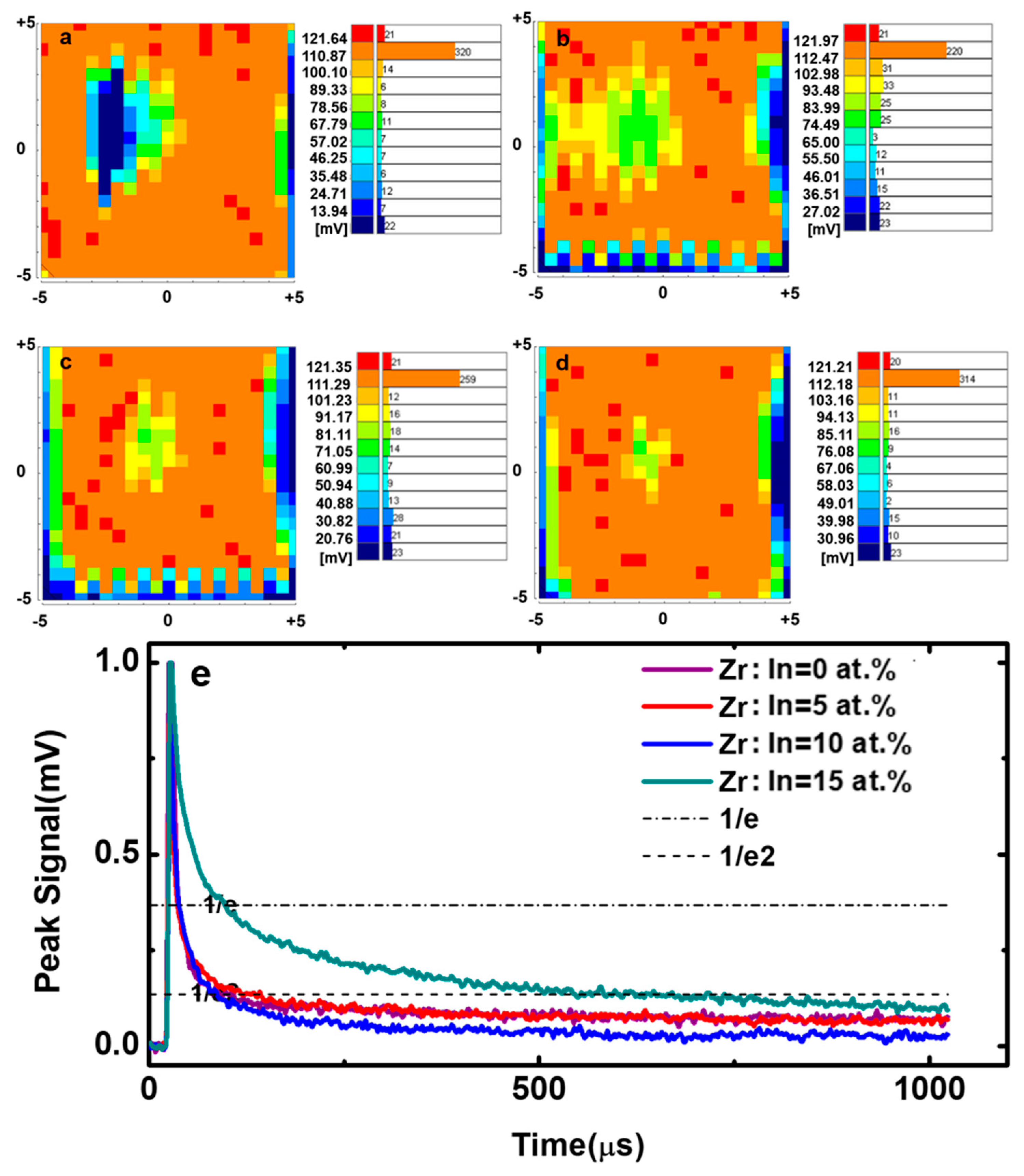

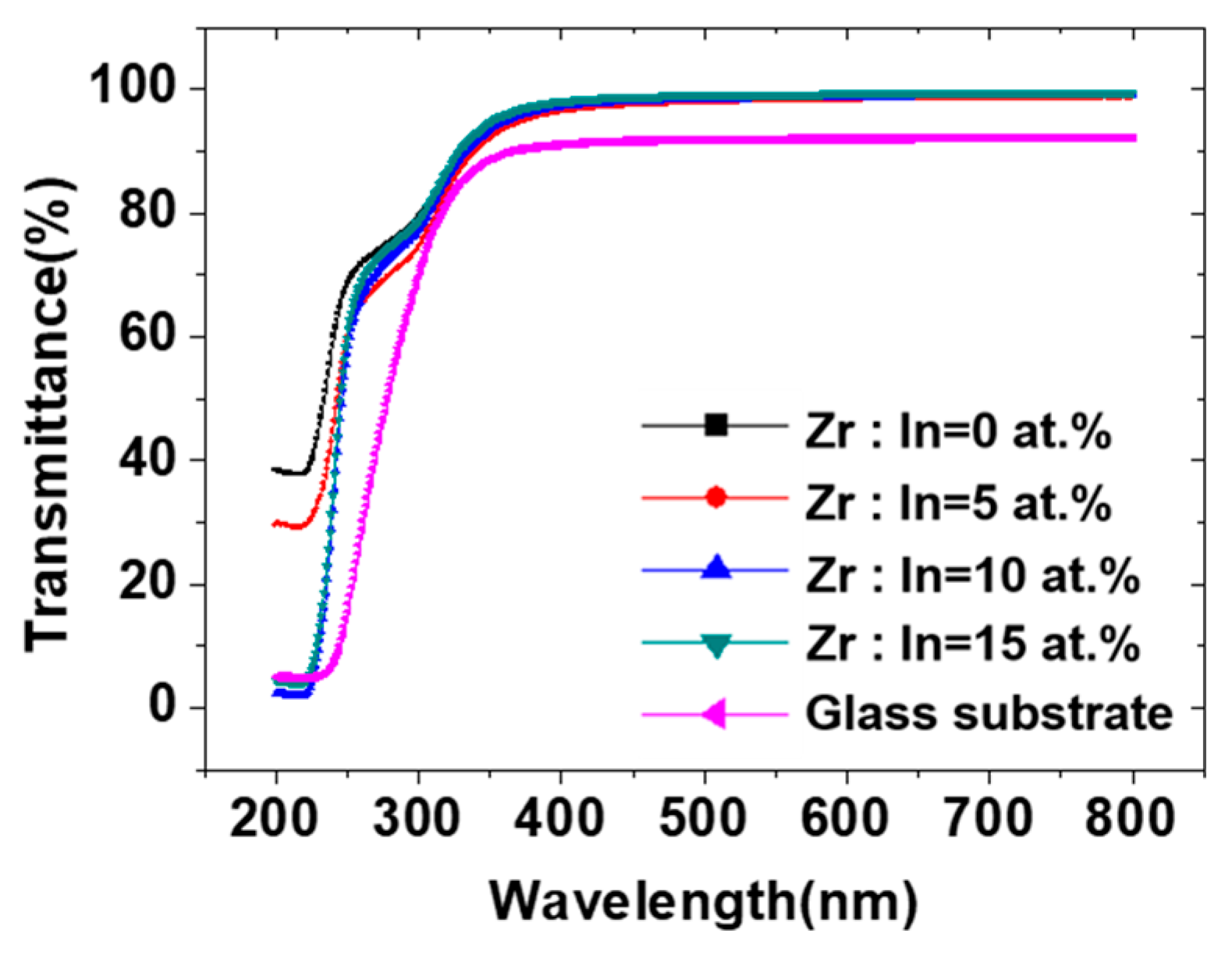
| Zr Doping Concentration (at.%) | Temperature (°C) | Thickness (nm) | Density | Bulk Density | Relative Density (q) |
|---|---|---|---|---|---|
| 0 | 400 | 8.065 | 5.574 | 7.179 | 0.776 |
| 5 | 8.130 | 6.051 | 7.113 | 0.851 | |
| 10 | 8.525 | 5.930 | 7.046 | 0.842 | |
| 15 | 9.244 | 5.972 | 6.980 | 0.856 | |
| 15 | 250 | 12.910 | 5.187 | 6.980 | 0.743 |
| 300 | 11.642 | 5.650 | 0.809 | ||
| 350 | 9.504 | 6.093 | 0.873 | ||
| 400 | 9.244 | 5.972 | 0.856 |
| Zr Doping Concentration (at.%) | Relative Quantity of Oxygen Vacancies (%) |
|---|---|
| 0 | 41.78 |
| 5 | 30.83 |
| 10 | 26.09 |
| 15 | 31.48 |
| Zr Doping Concentration (at.%) | Peak Mean (mV) | D Value | τ1 (μs) | τ2 (μs) |
|---|---|---|---|---|
| 0 | 104.7 | 0.3702 | 0.0593 | 0.4207 |
| 5 | 95.4 | 0.3925 | 0.0562 | 0.6472 |
| 10 | 87.0 | 0.5263 | 0.0586 | 0.4034 |
| 15 | 99.7 | 0.5012 | 0.3761 | 1.1260 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fu, X.; Zhou, S.; Ning, H.; Wang, Y.; Guo, D.; Cai, W.; Liang, Z.; Yao, R.; Peng, J. The Effect of Zirconium Doping on Solution-Processed Indium Oxide Thin Films Measured by a Novel Nondestructive Testing Method (Microwave Photoconductivity Decay). Coatings 2019, 9, 426. https://doi.org/10.3390/coatings9070426
Zhang J, Fu X, Zhou S, Ning H, Wang Y, Guo D, Cai W, Liang Z, Yao R, Peng J. The Effect of Zirconium Doping on Solution-Processed Indium Oxide Thin Films Measured by a Novel Nondestructive Testing Method (Microwave Photoconductivity Decay). Coatings. 2019; 9(7):426. https://doi.org/10.3390/coatings9070426
Chicago/Turabian StyleZhang, Jingying, Xiao Fu, Shangxiong Zhou, Honglong Ning, Yiping Wang, Dong Guo, Wei Cai, Zhihao Liang, Rihui Yao, and Junbiao Peng. 2019. "The Effect of Zirconium Doping on Solution-Processed Indium Oxide Thin Films Measured by a Novel Nondestructive Testing Method (Microwave Photoconductivity Decay)" Coatings 9, no. 7: 426. https://doi.org/10.3390/coatings9070426
APA StyleZhang, J., Fu, X., Zhou, S., Ning, H., Wang, Y., Guo, D., Cai, W., Liang, Z., Yao, R., & Peng, J. (2019). The Effect of Zirconium Doping on Solution-Processed Indium Oxide Thin Films Measured by a Novel Nondestructive Testing Method (Microwave Photoconductivity Decay). Coatings, 9(7), 426. https://doi.org/10.3390/coatings9070426







