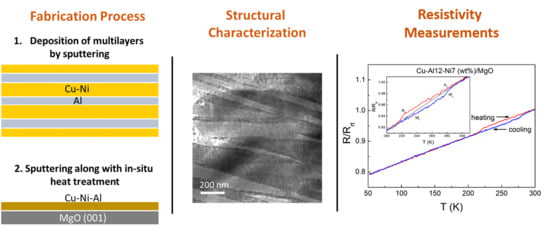
Epitaxial Versus Polycrystalline Shape Memory Cu-Al-Ni Thin Films
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- The microstructure of Cu-Al-Ni sputtered films is found to depend both on the alloy composition as well as the experimental procedure used to grow the films.
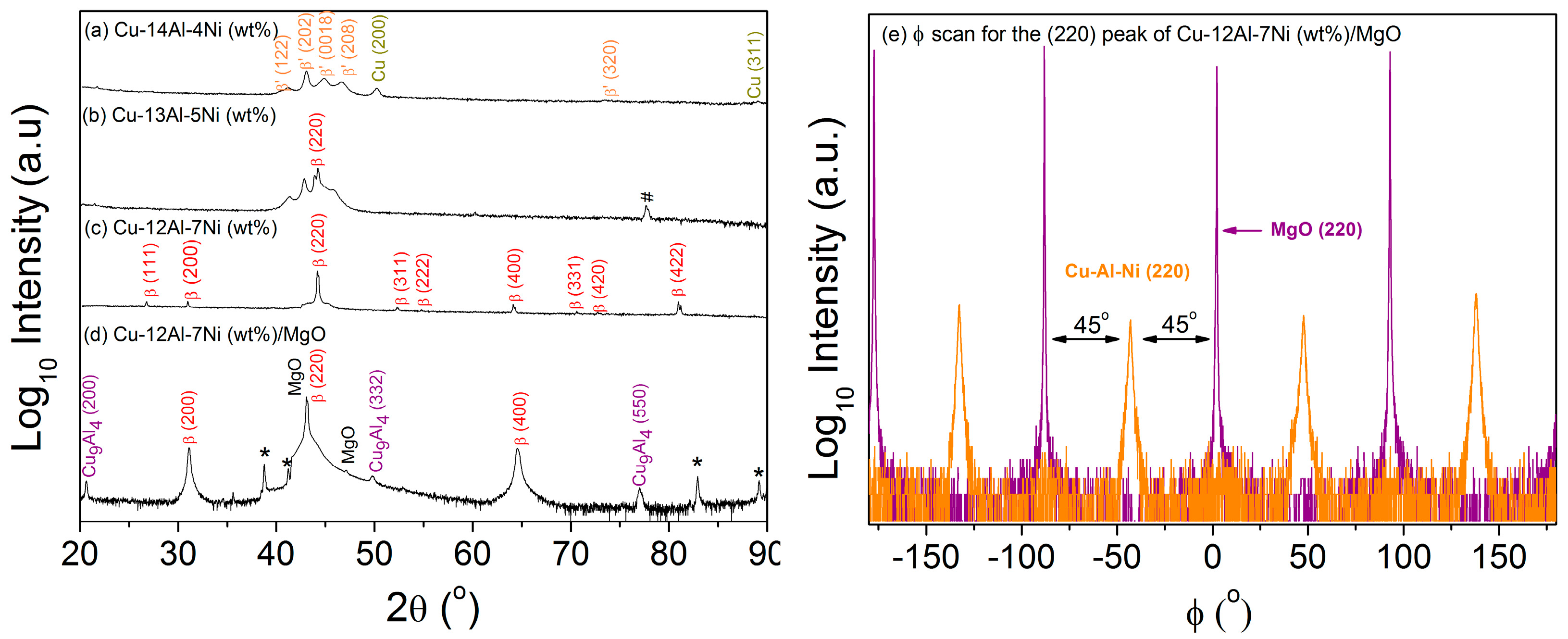
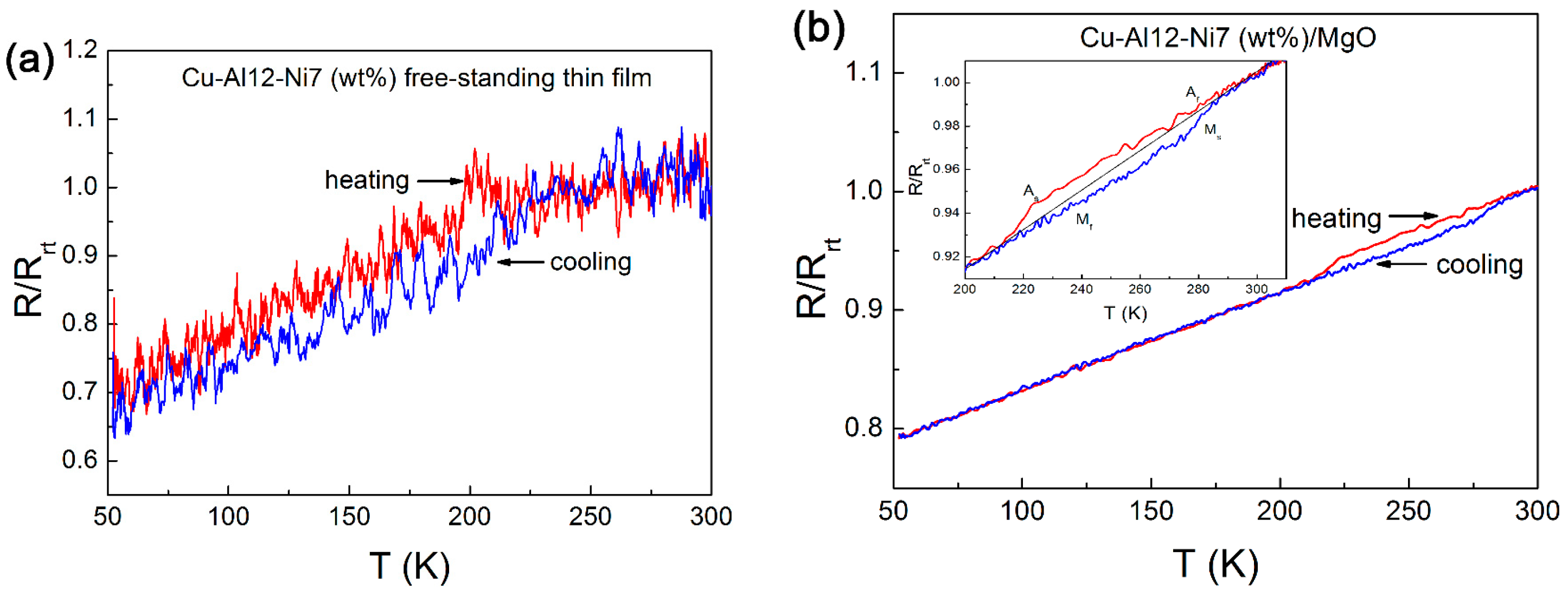
- A transition from martensite to austenite was observed as the Ni content increased and the Al content decreased in samples prepared by multilayer sputtering followed by quenching.
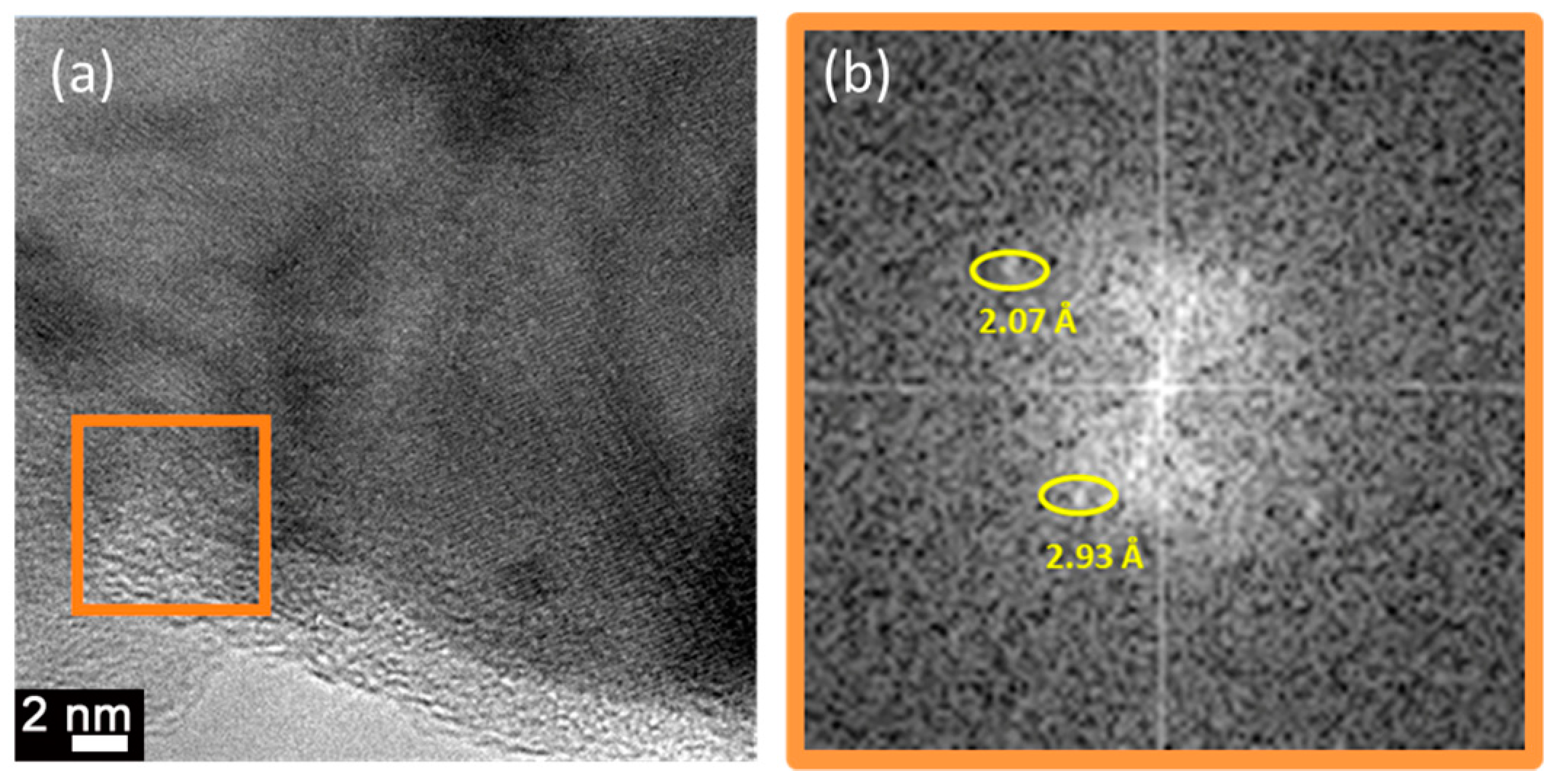
- Preferential growth along the (100) direction was observed in β-austenite Cu-12Al-7Ni (wt.%) grown on MgO at 700 °C due to the epitaxial relationship MgO(001)[100]/Cu-Al-Ni(001)[110].
- Resistance change with respect to temperature, suggesting martensitic transformation hysteresis, was observed in the preferentially oriented austenitic Cu-12Al-7Ni (wt.%) film, whereas martensitic transformation was completely suppressed in the polycrystalline austenitic sample produced by multilayer sputtering with the same composition.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Otsuka, K.; Wayman, C.M. Shape Memory Materials; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Ma, J.; Karaman, I. Expanding the repertoire of shape memory alloys. Science 2010, 327, 1468–1469. [Google Scholar] [CrossRef]
- Cingolani, E.; Ahlers, M.; Van Humbeeck, J. Stabilization and two-way shape memory effect in Cu-Al-Ni single crystals. Met. Mater. Trans. A 1999, 30, 493–499. [Google Scholar] [CrossRef]
- Ishibashi, M.; Tabata, N.; Suetake, T.; Omori, T.; Sutou, Y.; Kainuma, R.; Yamauchi, K.; Ishida, K. A simple method to treat an ingrowing toenail with a shape-memory alloy device. J. Dermatol. 2008, 19, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Seelecke, S.; Muller, I. Shape memory alloy actuators in smart structures: Modeling and simulation. Appl. Mech. Rev. 2004, 57, 23–46. [Google Scholar] [CrossRef]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Montero-Ocampo, C.; Lopez, H.; Salinas Rodriguez, A. Effect of compressive straining on corrosion resistance of a shape memory Ni-Ti alloy in ringer’s solution. J. Biomed. Mater. Res. 1993, 32, 583–591. [Google Scholar] [CrossRef]
- Bogue, R. Shape-memory materials: A review of technology and applications. Assem. Autom. 2009, 29, 214–219. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.S.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel-titanium biomedical devices. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Eggeler, G.; Hornbogen, E.; Yawny, A.; Heckmann, A.; Wagner, M. Structural and functional fatigue of NiTi fhape memory alloys. Mater. Sci. Eng. A 2004, 378, 24–33. [Google Scholar] [CrossRef]
- Ibrahim, H.; Jahadakbar, A.; Dehghan, A.; Moghaddam, N.S.; Amerinatanzi, A.; Elahinia, M. In vitro corrosion assessment of additively manufactured porous NiTi structures for bone fixation applications. Metals 2018, 8, 164. [Google Scholar] [CrossRef]
- Perkins, J.; Sponholz, R.O. Stress-induced martensitic transformation cycling and two-way shape memory training in Cu-Zn-Al alloys. Met. Mater. Trans. B 1984, 15, 313–321. [Google Scholar] [CrossRef]
- Ivanić, I.; Kožuh, S.; Grgurić, T.H.; Kosec, B.; Gojić, M. The influence of heat treatment on microstructure and phase transformation temperatures of Cu-Al-Ni shape memory alloys. Kem. Ind. 2019, 68, 111–118. [Google Scholar] [CrossRef]
- Recarte, V.; Pérez-Landazábal, J.I.; Ibarra, A.; Nó, M.L.; San Juan, J. High temperature β phase decomposition process in a Cu-Al-Ni shape memory alloy. Mater. Sci. Eng. A 2004, 378, 238–242. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Okotete, E.A. Reconciling viability and cost-effective shape memory alloy options–A review of copper and iron based shape memory metallic systems. Eng. Sci. Technol. Int. J. 2016, 19, 1582–1592. [Google Scholar] [CrossRef]
- Pan, Q.; Cho, C. The investigation of a shape memory alloy micro-damper for MEMS applications. Sensors 2007, 7, 1887–1900. [Google Scholar] [CrossRef]
- Olson, G.B.; Cohen, M. A general mechanism of martensitic nucleation: Part I. General concepts and the FCC → HCP transformation. Metall. Mater. Trans. A 1976, 7, 1897–1904. [Google Scholar]
- Sugimura, Y.; Cohen-Karni, I.; McCluskey, P.; Vlassak, J. Stress evolution in sputter-deposited Fe–Pd shape-memory thin films. J. Mater. Res. 2005, 20, 2279–2287. [Google Scholar] [CrossRef]
- Chu, J.P.; Lai, Y.W.; Lin, T.N.; Wang, S.F. Deposition and characterization of TiNi-base thin films by sputtering. Mater. Sci. Eng. A 2000, 277, 11–17. [Google Scholar] [CrossRef]
- Torres, C.E.; Condo, A.; Haberkorn, N.; Zelaya, E.; Schryvers, D.; Guimpel, J.; Lovey, F. Structures in textured Cu–Al–Ni shape memory thin films grown by sputtering. Mater. Charact. 2014, 96, 256–262. [Google Scholar] [CrossRef]
- Tolstova, Y.; Omelchenko, S.T.; Shing, A.M.; Atwater, H.A. Heteroepitaxial growth of Pt and Au thin films on MgO single crystals by bias-assisted sputtering. Sci. Rep. 2016, 6, 23232. [Google Scholar] [CrossRef]
- Gu, H.; You, L.; Leung, K.; Chung, C.; Chan, K.; Lai, J. Growth of TiNiHf shape memory alloy thin films by laser ablation of composite targets. Appl. Surf. Sci. 1998, 127, 579–583. [Google Scholar] [CrossRef]
- Dong, J.W.; Chen, L.C.; Palmstro/m, C.J.; James, R.D.; McKernan, S. Molecular beam epitaxy growth of ferromagnetic single crystal (001) Ni2MnGa on (001) GaAs. Appl. Phys. Lett. 1999, 75, 1443–1445. [Google Scholar] [CrossRef]
- Shih, T.C.; Xie, J.Q.; Dong, J.W.; Dong, X.Y.; Srivastava, S.; Adelmann, C.; McKernan, S.; James, R.D.; Palmstrøm, C.J. Epitaxial growth and characterization of single crystal ferromagnetic shape memory Co2NiGa films. Ferroelectrics 2006, 342, 35–42. [Google Scholar] [CrossRef]
- Kühnemund, L.; Edler, T.; Kock, I.; Seibt, M.; Mayr, S.G. Epitaxial growth and stress relaxation of vapor-deposited Fe-Pd magnetic shape memory films electron beam evaporation. New J. Phys. 2009, 11, 113054. [Google Scholar] [CrossRef]
- Gisser, K.R.C.; Busch, J.D.; Johnson, A.D.; Ellis, A.B. Oriented nickel-titanium shape memory alloy films prepared by annealing during deposition. Appl. Phys. Lett. 1992, 61, 1632–1634. [Google Scholar] [CrossRef]
- Jenkins, C.A.; Ramesh, R.; Huth, M.; Eichhorn, T.; Pörsch, P.; Elmers, H.J.; Jakob, G. Growth and magnetic control of twinning structure in thin films of Heusler shape memory compound Ni2MnGa. Appl. Phys. Lett. 2008, 93, 234101. [Google Scholar] [CrossRef]
- Niemann, R.; Backen, A.; Kauffmann-Weiss, S.; Behler, C.; Rößler, U.; Seiner, H.; Heczko, O.; Nielsch, K.; Schultz, L.; Fähler, S. Nucleation and growth of hierarchical martensite in epitaxial shape memory films. Acta Mater. 2017, 132, 327–334. [Google Scholar] [CrossRef]
- Goryczka, T. Effect of wheel velocity on texture formation and shape memory in Cu-Al-Ni melt-spun ribbons. Arch. Metall. Mater. 2009, 54, 755–763. [Google Scholar]
- Delaey, L. Diffusionless transformations. In Phase Tranformation in Materials; Kostorz, G., Ed.; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Morán, M.; Condó, A.; Soldera, F.; Sirena, M.; Haberkorn, N. Martensitic transformation in freestanding and supported Cu–Al–Ni thin films obtained at low deposition temperatures. Mater. Lett. 2016, 184, 177–180. [Google Scholar] [CrossRef]
- Niedbalski, S.; Durán, A.; Walczak, M.; Ramos-Grez, J.A. Laser-assisted synthesis of Cu-Al-Ni shape memory alloys: Effect of inert gas pressure and Ni content. Materials 2019, 12, 794. [Google Scholar] [CrossRef]
- Recarte, V.; Pérez-Sáez, R.B.; Juan, J.S.; Bocanegra, E.H.; Nó, M.L. Influence of Al and Ni concentration on the Martensitic transformation in Cu-Al-Ni shape-memory alloys. Met. Mater. Trans. A 2002, 33, 2581–2591. [Google Scholar] [CrossRef]
- Braga, F.D.O.; Matlakhov, A.N.; Matlakhova, L.A.; Monteiro, S.N.; De Araújo, C.J. Martensitic transformation under compression of a plasma processed polycrystalline shape memory CuAlNi Alloy. Mater. Res. 2017, 20, 1579–1592. [Google Scholar] [CrossRef]
- Haidar, M.A.; Saud, S.N.; Hamzah, E. Microstructure, mechanical properties, and shape memory effect of annealed Cu-Al-Ni-xCo shape memory alloys. Metallogr. Microstruct. Anal. 2018, 7, 57–64. [Google Scholar] [CrossRef]
- Saud, S.N.; Bakar, T.A.A.; Hamzah, E.; Ibrahim, M.K.; Bahador, A. Effect of quarterly element addition of cobalt on phase transformation Cu-Al-Ni shape memory alloys. Metall. Mater. Trans. A 2015, 46, 3528–3542. [Google Scholar] [CrossRef]
- Sharma, M.; Vajpai, S.K.; Dube, R.K. Processing and characterization of Cu-Al-Ni shape memory alloy strips prepared via a novel powder metallurgy route. Metall. Mater. Trans. A 2010, 41, 2905–2913. [Google Scholar] [CrossRef]
- Gómez-Cortés, J.; Juan, J.S.; Lopez, G.A.; Nó, M. Synthesis and characterization of Cu–Al–Ni shape memory alloy multilayer thin films. Thin Solid Films 2013, 544, 588–592. [Google Scholar] [CrossRef]
- Canbay, C.A.; Tekatas, A.; Ozkul, I. Fabrication of Cu-Al-Ni shape memory thin film by thermal evaporation. Turk. J. Eng. 2017, 1, 27–32. [Google Scholar]
- Minemura, T.; Andoh, H.; Kita, Y.; Ikuta, I. Shape memory effect and microstructures of sputter-deposited Cu-Al-Ni films. J. Mater. Sci. Lett. 1985, 4, 793–796. [Google Scholar] [CrossRef]
- Agafonov, V.; Naudot, P.; Dubertret, A.; Dubois, B. Influence of the aluminium content on the appearance and stability of martensites in the Cu Al Ni system. Scr. Met. 1988, 22, 489–494. [Google Scholar] [CrossRef]
- Suresh, N.; Ramamurty, U. Aging response and its effect on the functional properties of Cu–Al–Ni shape memory alloys. J. Alloy. Compd. 2008, 449, 113–118. [Google Scholar] [CrossRef]
- Sharma, M.; Vajpai, S.K.; Dube, R.K.; Vajpai, S. Synthesis and properties of Cu–Al–Ni shape memory alloy strips prepared via hot densification rolling of powder preforms. Power Metall. 2011, 54, 620–627. [Google Scholar] [CrossRef]
- Araujo, A.P.M.; Simões, J.B.; Araújo, C.J. Analysis of compositional modification of commercial aluminum bronzes to obtain functional shape memory properties. Mater. Res. 2017, 20, 331–341. [Google Scholar] [CrossRef][Green Version]
- Malygin, G.A. Nanoscopic size effects on martensitic transformations in shape memory alloys. Phys. Solid State 2008, 50, 1538–1543. [Google Scholar] [CrossRef]
- Waitz, T.; Karnthaler, H. Martensitic transformation of NiTi nanocrystals embedded in an amorphous matrix. Acta Mater. 2004, 52, 5461–5469. [Google Scholar] [CrossRef]
- Shi, X.; Cui, L.; Jiang, D.; Yu, C.; Guo, F.; Yu, M.; Ren, Y.; Liu, Y. Grain size effect on the R-phase transformation of nanocrystalline NiTi shape memory alloys. J. Mater. Sci. 2014, 49, 4643–4647. [Google Scholar] [CrossRef]
- Wan, D.; Komvopoulos, K. Thickness effect on thermally induced phase transformations in sputtered titanium-nickel shape-memory films. J. Mater. Res. 2005, 20, 1606–1612. [Google Scholar] [CrossRef]
- Chen, Y.; Schuh, C.A. Size effects in shape memory alloy microwires. Acta Mater. 2011, 59, 537–553. [Google Scholar] [CrossRef]
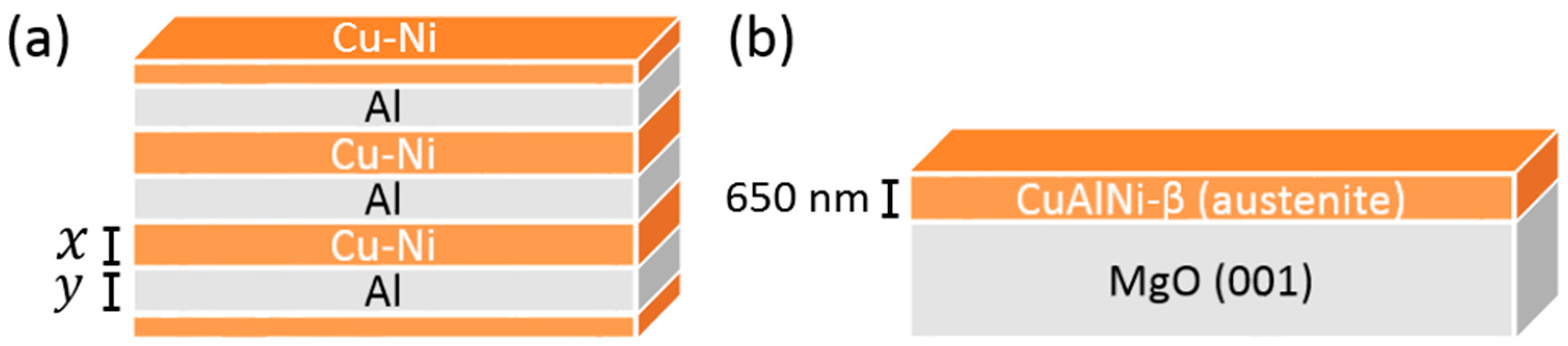

| Composition | Layer Thickness (nm) | Total Thin Film Thickness (nm) | |
|---|---|---|---|
| Cu-Ni (x) | Al (y) | Cu-Al-Ni | |
| Cu-14Al-4Ni (wt.%) | 230 | 58 | 864 |
| Cu-13Al-5Ni (wt.%) | 242 | 63 | 915 |
| Cu-12Al-7Ni (wt.%) | 347 | 70 | 1250 |
| Composition | Cu-Ni | Al | |||
|---|---|---|---|---|---|
| P (W) | t (min) | P (W) | t (min) | ||
| Cu | Ni | ||||
| Cu-14Al-4Ni (wt.%) | 200 | 60 | 10 | 200 | 14 |
| Cu-13Al-5Ni (wt.%) | 200 | 75 | 9 | 200 | 15.5 |
| Cu-12Al-7Ni (wt.%) | 200 | 85 | 10 | 200 | 17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilican, D.; Kurdi, S.; Zhu, Y.; Solsona, P.; Pellicer, E.; Barber, Z.H.; Greer, A.L.; Sort, J.; Fornell, J. Epitaxial Versus Polycrystalline Shape Memory Cu-Al-Ni Thin Films. Coatings 2019, 9, 308. https://doi.org/10.3390/coatings9050308
Bilican D, Kurdi S, Zhu Y, Solsona P, Pellicer E, Barber ZH, Greer AL, Sort J, Fornell J. Epitaxial Versus Polycrystalline Shape Memory Cu-Al-Ni Thin Films. Coatings. 2019; 9(5):308. https://doi.org/10.3390/coatings9050308
Chicago/Turabian StyleBilican, Doga, Samer Kurdi, Yi Zhu, Pau Solsona, Eva Pellicer, Zoe H. Barber, Alan Lindsay Greer, Jordi Sort, and Jordina Fornell. 2019. "Epitaxial Versus Polycrystalline Shape Memory Cu-Al-Ni Thin Films" Coatings 9, no. 5: 308. https://doi.org/10.3390/coatings9050308
APA StyleBilican, D., Kurdi, S., Zhu, Y., Solsona, P., Pellicer, E., Barber, Z. H., Greer, A. L., Sort, J., & Fornell, J. (2019). Epitaxial Versus Polycrystalline Shape Memory Cu-Al-Ni Thin Films. Coatings, 9(5), 308. https://doi.org/10.3390/coatings9050308