Atomic Layer Deposition of Inorganic Films for the Synthesis of Vertically Aligned Carbon Nanotube Arrays and Their Hybrids
Abstract
1. Introduction
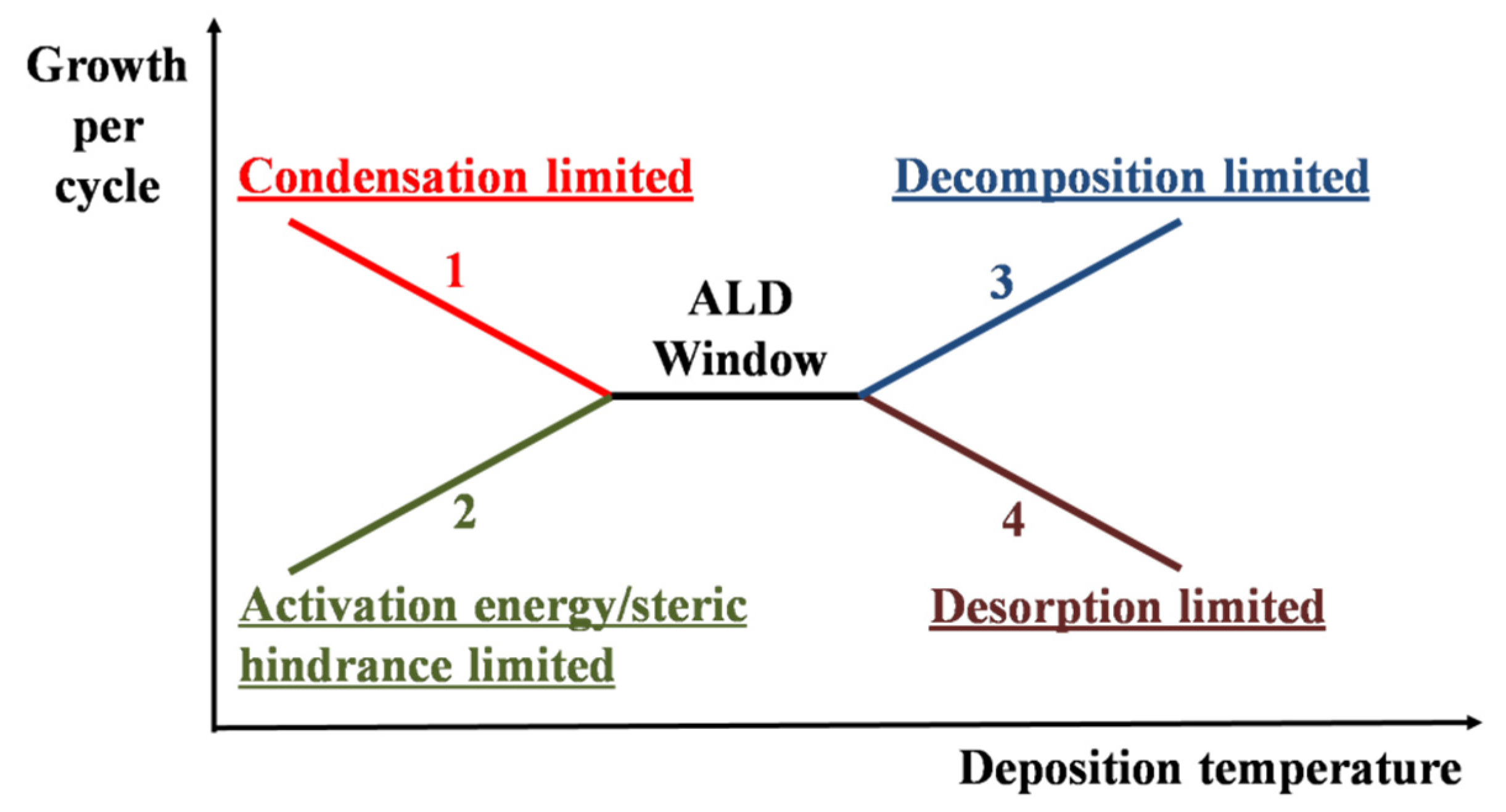
2. Fundamentals of ALD
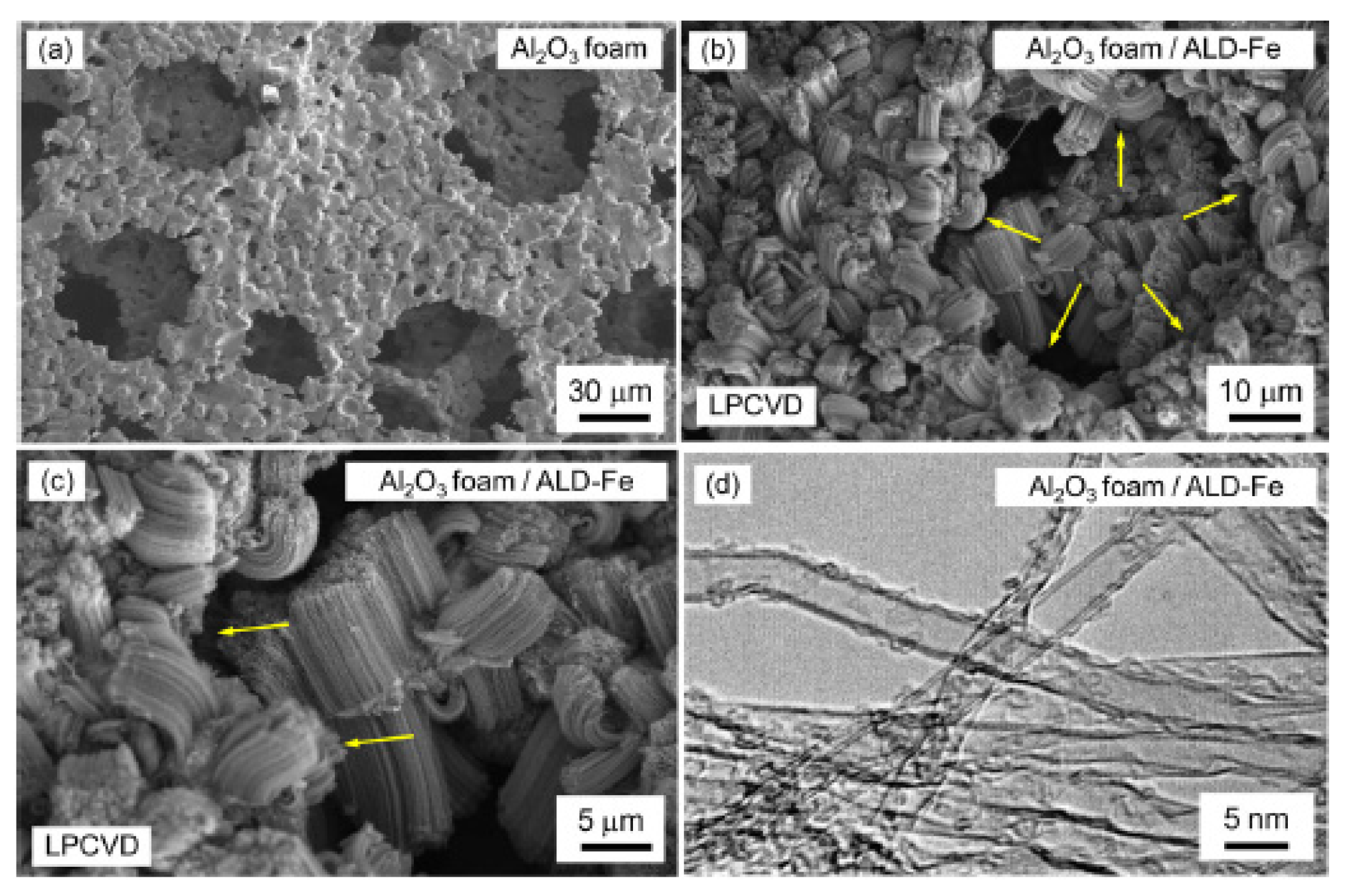
3. ALD of Catalyst Thin Films for the Synthesis of VACNTs
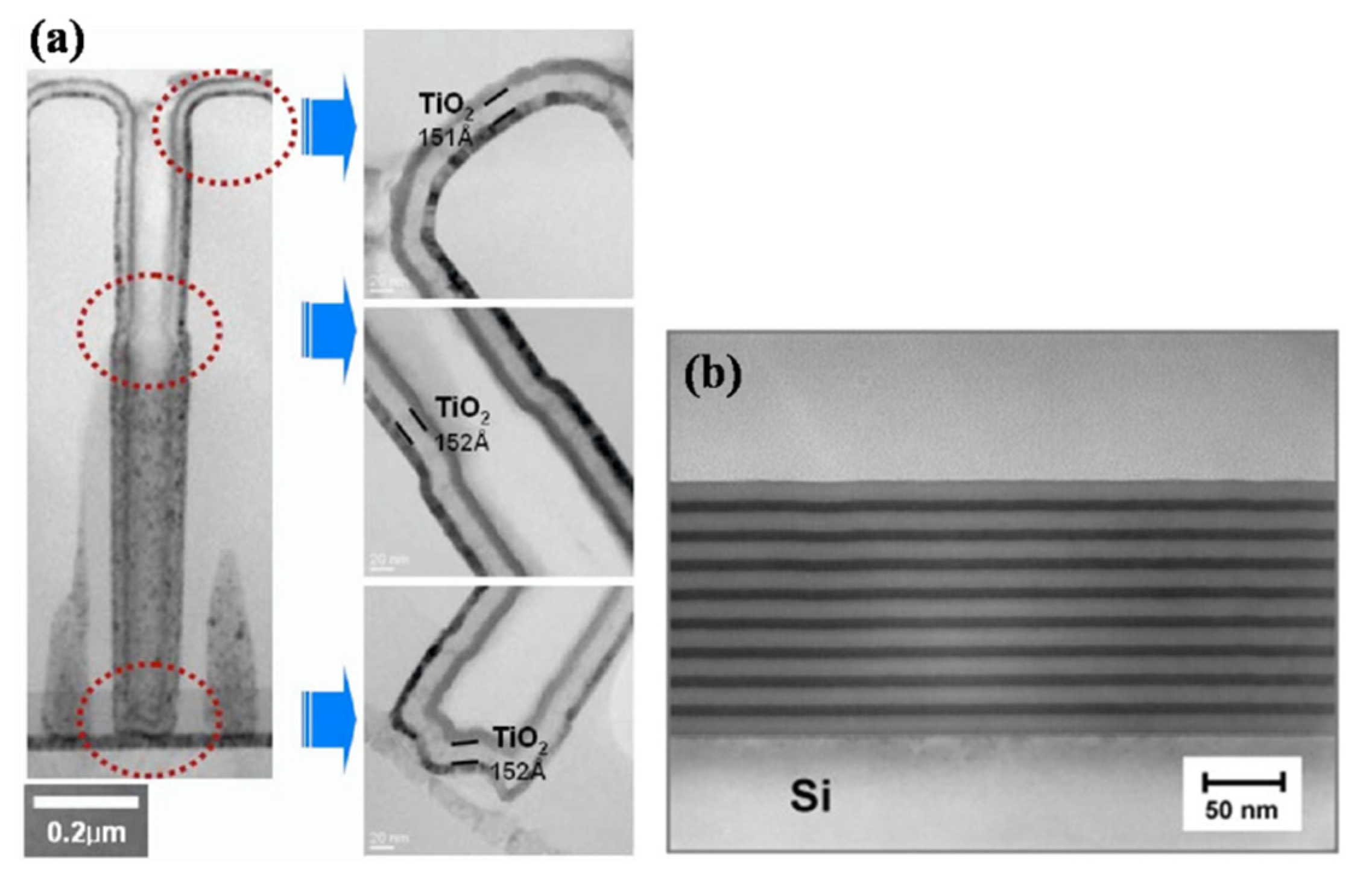
4. ALD for the Inorganic Coating of VACNTs
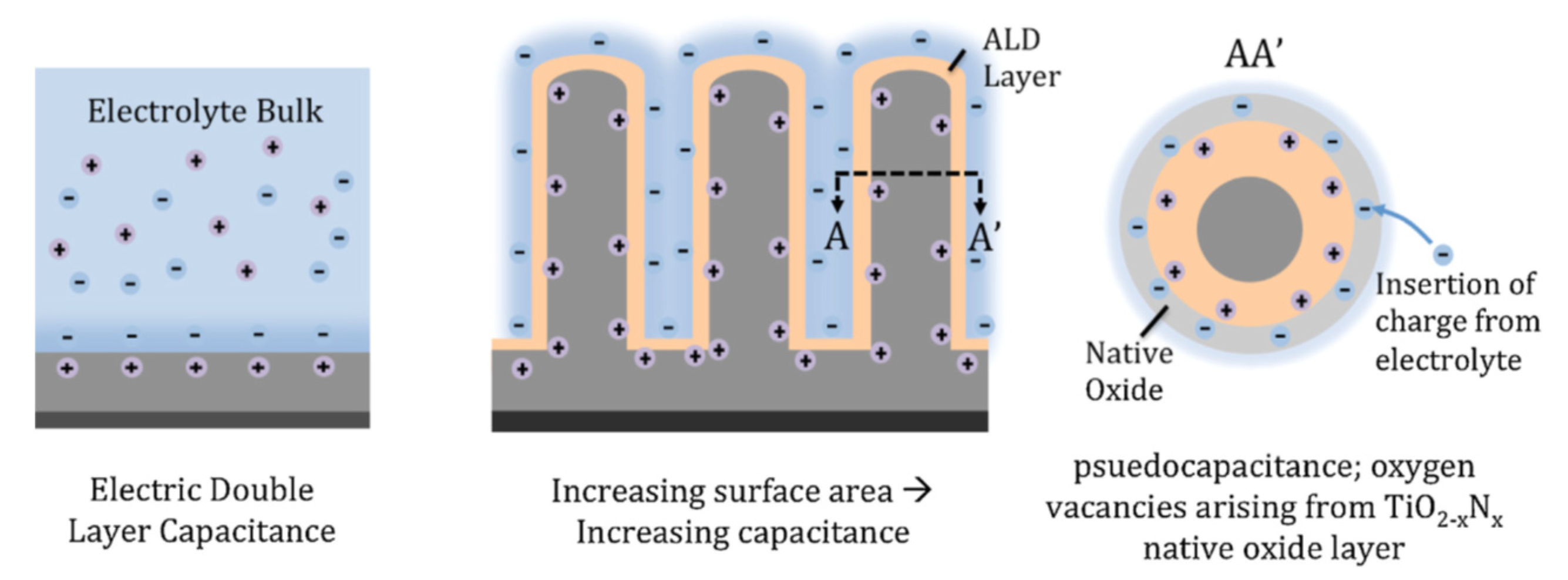
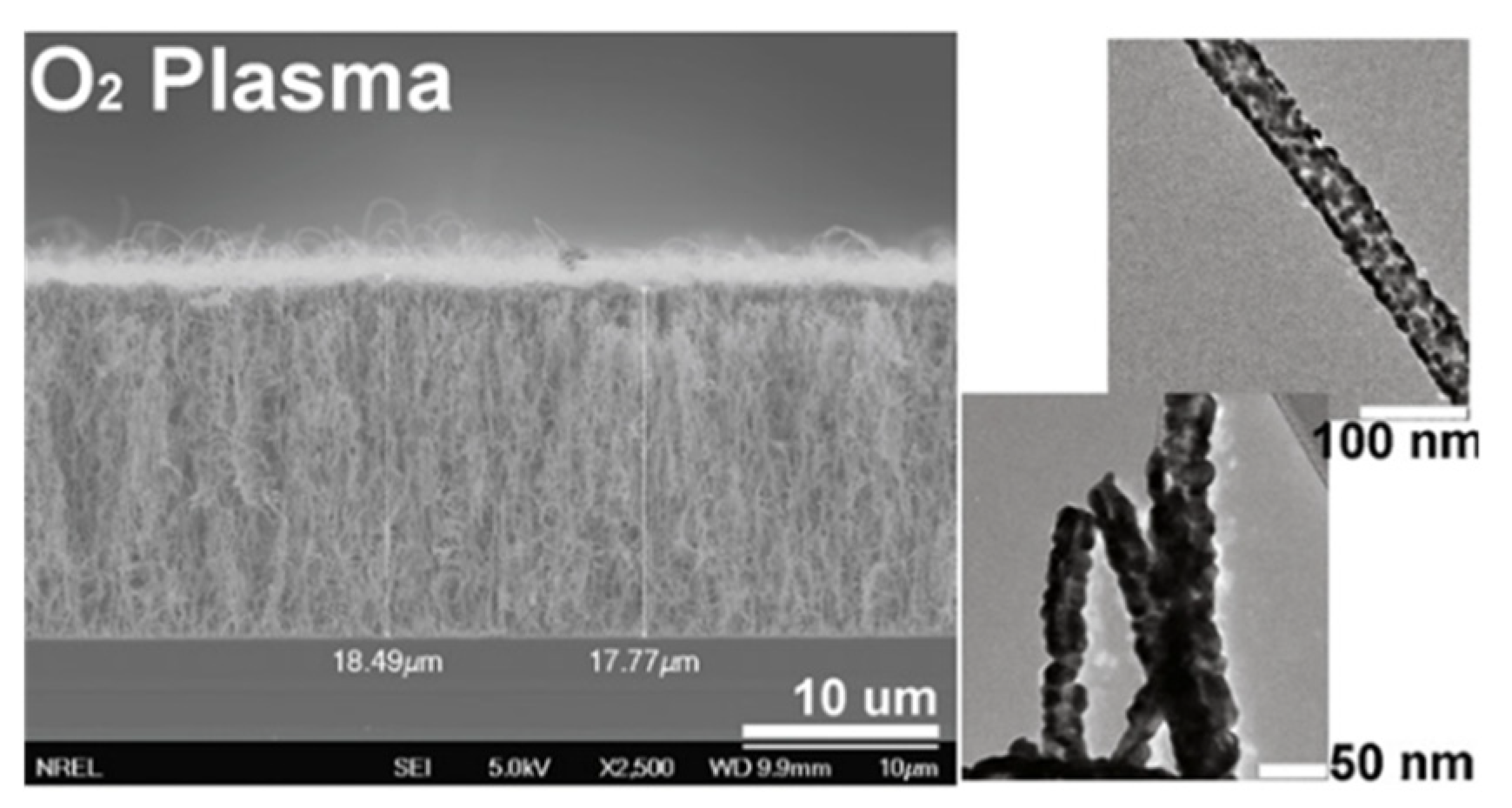
4.1. Supercapacitors
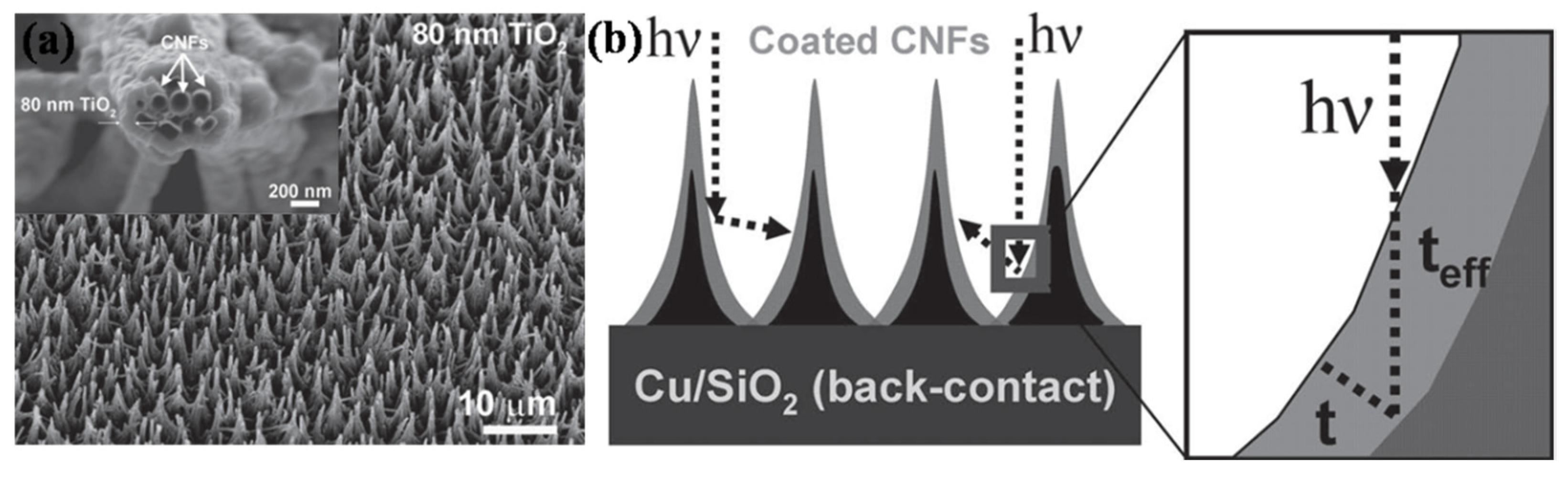
4.2. Solar Cells
4.3. Fuel Cells
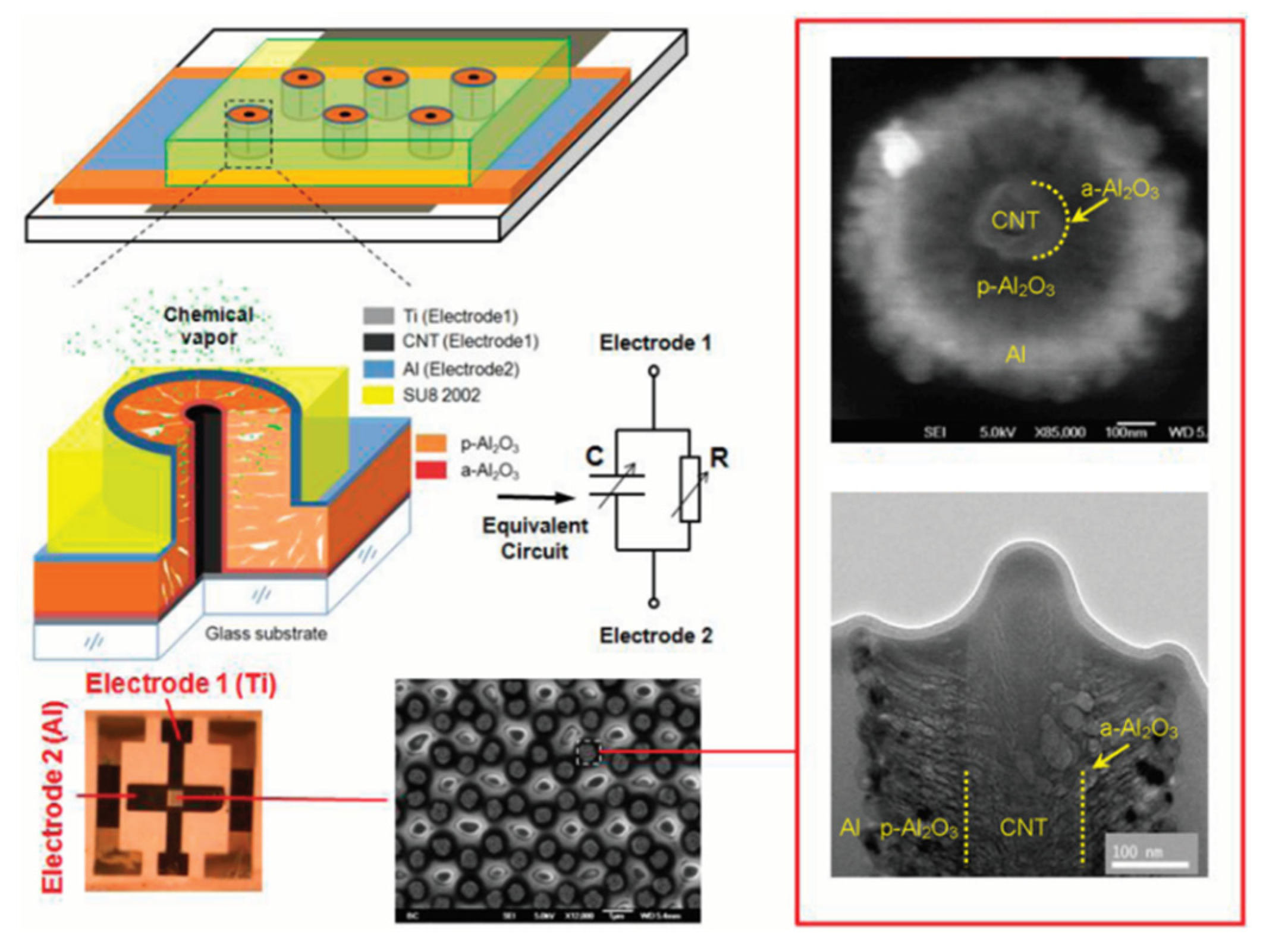
4.4. Sensors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ren, Z.F.; Huang, Z.P.; Xu, J.W.; Wang, J.H.; Bush, P.; Siegal, M.P.; Provencio, P.N. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science 1998, 282, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.Q.; Vajtai, R.; Jung, Y.; Ward, J.; Zhang, R.; Ramanath, G.; Ajayan, P.M. Organized assembly of carbon nanotubes. Nature 2002, 416, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Suhr, J.; Victor, P.; Ci, L.; Sreekala, S.; Zhang, X.; Nalamasu, O.; Ajayan, P.M. Fatigue resistance of aligned carbon nanotube arrays under cyclic compression. Nat. Nanotechnol. 2007, 2, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.T.; Dai, L.M.; Stone, M.; Xia, Z.H.; Wang, Z.L. Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off. Science 2008, 322, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.P.; He, P.G.; Lyu, Z.P.; Chen, T.F.; Huang, B.; Chen, L.; Fisher, T.S. Bioinspired leaves-on-branchlet hybrid carbon nanostructure for supercapacitors. Nat. Commun. 2018, 9, 790. [Google Scholar] [CrossRef]
- Gupta, B.K.; Kedawat, G.; Gangwar, A.K.; Nagpal, K.; Kashyap, P.K.; Srivastava, S.; Singh, S.; Kumar, P.; Suryawanshi, S.R.; Seo, D.M.; et al. High-performance field emission device utilizing vertically aligned carbon nanotubes-based pillar architectures. AIP Adv. 2018, 8, 015117. [Google Scholar] [CrossRef]
- Lin, P.H.; Sie, C.L.; Chen, C.A.; Chang, H.C.; Shih, Y.T.; Chang, H.Y.; Su, W.J.; Lee, K.Y. Field emission characteristics of the structure of vertically aligned carbon nanotube bundles. Nanoscale Res. Lett. 2015, 10, 297. [Google Scholar] [CrossRef]
- Maschmann, M.R.; Dickinson, B.; Ehlert, G.J.; Baur, J.W. Force sensitive carbon nanotube arrays for biologically inspired airflow sensing. Smart Mater. Struct. 2012, 21, 094024. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W.D.; Gunasekaran, S. An amperometric non-enzymatic glucose sensor by electrodepositing copper nanocubes onto vertically well-aligned multi-walled carbon nanotube arrays. Biosens. Bioelectron. 2010, 26, 279–284. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Wang, P.B.; Zang, X.N.; Yang, Y.; Kozinda, A.; Lin, L.W. Uniformly embedded metal oxide nanoparticles in vertically aligned carbon nanotube forests as pseudocapacitor electrodes for enhanced energy storage. Nano Lett. 2013, 13, 3524–3530. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Wang, X.N.; Pan, Z.H.; Sun, J.; Zhao, J.X.; Zhang, J.; Zhang, C.X.; Tang, L.; Luo, J.; Song, B.; et al. Wrapping aligned carbon nanotube composite sheets around vanadium nitride nanowire arrays for asymmetric coaxial fiber-shaped supercapacitors with ultrahigh energy density. Nano Lett. 2017, 17, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Raravikar, N.; Helms, B.A.; Prasher, R.; Ogletree, D.F. Enhanced thermal transport at covalently functionalized carbon nanotube array interfaces. Nat. Commun. 2014, 5, 3082. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.G.; Tey, J.N.; Li, Z.; Wei, J.; Bennett, K.; McNamara, A.; Joshi, Y.; Tan, R.L.S.; Ling, S.N.M.; Wong, C.P. High-quality vertically aligned carbon nanotubes for applications as thermal interface materials. IEEE Trans. Compon. Packag. Manuf. 2014, 4, 232–239. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; Heer, W.A.D. Carbon nanotubes-the route toward application. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhang, Q.; Qian, W.Z.; Xu, G.H.; Xiang, R.; Wen, Q.; Wang, Y.; Luo, G.H. Progress on aligned carbon nanotube aarays. New Carbon Mater. 2007, 22, 271–282. [Google Scholar]
- Li, H.H.; Yuan, G.J.; Shan, B.; Zhang, X.X.; Ma, H.P.; Tian, Y.Z.; Lu, H.L.; Liu, J. Chemical vapor deposition of vertically aligned carbon nanotube arrays: Critical effects of oxide buffer layers. Nanoscale Res. Lett. 2019, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Bekyarov, E.; Thostenson, E.T.; Yu, A.; Kim, H.; Gao, J.; Tang, J.; Hahn, H.T.; Chou, T.W.; Itkis, M.E.; Haddon, R.C. Multiscale Carbon nanotube−carbon fiber reinforcement for advanced epoxy composites. Langmuir 2007, 23, 3970–3974. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Huang, K.; Carnahan, D.; Xing, Y. Hybrid Li-air battery cathodes with sparse carbon nanotube arrays directly grown on carbon fiber papers. Energy Environ. Sci. 2013, 6, 3339–3345. [Google Scholar] [CrossRef]
- Su, D.S.; Schlogl, R. Nanostructured carbon and carbon nanocomposites for electrochemical energy storage applications. ChemSusChem 2010, 3, 136–168. [Google Scholar] [CrossRef]
- Su, D.S.; Chen, X.W.; Weinberg, G.; Hofmann, A.K.; Timpe, O.; Hamid, S.B.A.; Schlogl, R. Hierarchically structured carbon: Synthesis of carbon nanofibers nested inside or immobilized onto modified activated carbon. Angew. Chem. Int. Ed. 2005, 44, 5488–5492. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.Q.; Zhao, M.Q.; Qian, W.Z.; Wang, Y.; Wei, F. Radial growth of vertically aligned carbon nanotube arrays from ethylene on ceramic spheres. Carbon 2008, 46, 1152–1158. [Google Scholar] [CrossRef]
- Singh, C.; Shaffer, M.S.P.; Koziol, K.K.K.; Kinloch, I.A.; Windle, A.H. Towards the production of large-scale aligned carbon nanotubes. Chem. Phys. Lett. 2003, 372, 860–865. [Google Scholar] [CrossRef]
- Xiang, R.; Luo, G.H.; Qian, W.Z.; Wang, Y.; Wei, F.; Li, Q. Large area growth of aligned CNT arrays on spheres: Towards large scale and continuous production. Chem. Vapor Depos. 2007, 13, 533–536. [Google Scholar] [CrossRef]
- Ye, J.S.; Cui, H.F.; Liu, X.; Lim, T.M.; Zhang, W.D.; Sheu, F.S. Preparation and characterization of aligned carbon nanotube-ruthenium oxide nanocomposites for supercapacitors. Small 2005, 5, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Hsia, B.; Marschewski, J.; Wang, S.; In, J.B.; Carraro, C.; Poulikakos, D.; Grigoropoulos, C.P.; Maboudian, R. Highly flexible, all solid-state micro-supercapacitors from vertically aligned carbon nanotubes. Nanotechnology 2014, 25, 055401. [Google Scholar] [CrossRef]
- Liu, J.W.; Kuo, Y.T.; Klabunde, K.J.; Rochford, C.; Wu, J.; Li, J. Novel dye-sensitized solar cell architecture using TiO2-coated vertically aligned carbon nanofiber arrays. ACS Appl. Mater. Interfaces 2009, 1, 1645–1649. [Google Scholar] [CrossRef]
- Kilic, B.; Turkdogan, S.; Astam, A.; Ozer, O.C.; Asgin, M.; Cebeci, H.; Urk, D.; Mucur, S.P. Preparation of carbon nanotube/TiO2 mesoporous hybrid photoanode with iron pyrite (FeS2) thin films counter electrodes for dye-sensitized solar cell. Sci. Rep. 2016, 6, 27052. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Lim, S.H.; Poh, C.K.; Tang, Z.; Xia, Z.T.; Luo, Z.Q.; Shen, P.K.; Chua, D.; Feng, Y.P.; Shen, Z.X.; et al. A highly order-structured membrane electrode assembly with vertically aligned carbon nanotubes for ultra-low Pt loading PEM fuel cells. Adv. Energy Mater. 2011, 1, 1205–1214. [Google Scholar] [CrossRef]
- Zhang, W.M.; Minett, A.I.; Gao, M.; Zhao, J.; Razal, J.M.; Wallace, G.G.; Romeo, T.; Chen, J. Integrated high-efficiency Pt/carbon nanotube arrays for PEM fuel cells. Adv. Energy Mater. 2011, 1, 671–677. [Google Scholar] [CrossRef]
- Yick, S.; Han, Z.J.; Ostrikov, K. Atomspheric microplasma-functionalized 3D microfluidic strips within dense carbon nanotube arrays confine Au nanodots for SERS sensing. Chem. Commun. 2013, 49, 2861–2863. [Google Scholar] [CrossRef]
- Stano, K.L.; Carroll, M.; Padbury, R.; McCord, M.; Jur, J.S.; Bradford, P.D. Conformal atomic layer deposition of alumina on millimeter tall, vertically-aligned carbon nanotube arrays. ACS Appl. Mater. Interfaces 2014, 6, 19135–19143. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, N.; Chawla, V.; Edwards, E.; Wood, V.; Park, H.G.; Utke, I. Modeling and optimization of atomic layer deposition processes on vertically aligned carbon nanotubes. Beilstein J. Nanotechnol. 2014, 5, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Sakoda, K.; Momose, T.; Koshi, M.; Shimogaki, Y. Hot-wire assisted atomic layer deposition of a high quality cobalt film using cobaltocene: Elementary reaction analysis on NHx radical formation. J. Vac. Sci. Technol. A 2012, 20, 01A144. [Google Scholar] [CrossRef]
- Gordon, R.G.; Hausmann, D.; Kim, E.; Shepard, J. A kinetic model for step coverage by atomic layer deposition in narrow holes or trenches. Chem. Vap. Depos. 2003, 9, 73–78. [Google Scholar] [CrossRef]
- Knez, M.; Nielsh, K.; Niinisto, L. Synthesis and surface engineering of complex nanostructures by atomic layer deposition. Adv. Mater. 2007, 19, 3425–3438. [Google Scholar] [CrossRef]
- Detavernier, C.; Dendooven, J.; Sree, S.P.; Ludwig, K.F.; Martens, J.A. Tailoring nanoporous materials by atomic layer deposition. Chem. Soc. Rev. 2011, 40, 5242–5253. [Google Scholar] [CrossRef]
- Zazpe, R.; Knaut, M.; Sopha, H.; Hromadko, L.; Albert, M.; Prikryl, J.; Gartnerova, V.; Bartha, J.W.; Macak, J.M. Atomic layer deposition for coating high aspect ratio TiO2 nanotube layers. Langmuir 2016, 32, 10551–10558. [Google Scholar] [CrossRef]
- Weber, M.; Julbe, A.; Ayral, A.; Miele, P.; Bechelany, M. Atomic layer deposition for membranes: Basics, challenges, and opportunities. Chem. Mater. 2018, 30, 7368–7390. [Google Scholar] [CrossRef]
- Graniel, O.; Weber, M.; Balme, S.; Miele, P.; Bechelany, M. Atomic layer deposition for biosensing applications. Biosens. Bioelectron. 2018, 122, 147–159. [Google Scholar] [CrossRef]
- Marichy, C.; Pinna, N. Carbon-nanostructures coated/decorated by atomic layer deposition: Growth and applications. Coord. Chem. Rev. 2013, 257, 3232–3253. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.H.; Ahn, J.H.; Park, P.K.; Kang, S.W. Applicability of step-coverage modeling to TiO2 thin films in atomic layer deposition. J. Electrochem. Soc. 2007, 154, H1008–H1013. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yun, S.J. Nanolaminated Al2O3-TiO2 thin films grown by atomic layer deposition. J. Cryst. Growth 2005, 274, 585–593. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Miikkulainen, V.; Leskela, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Ponraj, J.S.; Attolini, G.; Bosi, M. Review on atomic layer deposition and applications of oxide thin films. Crit. Rev. Solid State 2013, 38, 203–233. [Google Scholar] [CrossRef]
- Puurunen, R.L.; Root, A.; Haukka, S.; Iiskola, E.I.; Lindblad, M.; Krause, A.O.I. IR and NMR study of the chemisorptions of ammonia on trimethylaluminum-modified silica. J. Phys. Chem. B 2000, 104, 6599–6609. [Google Scholar] [CrossRef]
- Puurunen, R.L. Growth per cycle in atomic layer deposition: Real application examples of a theoretical model. Chem. Vap. Depos. 2003, 9, 327–332. [Google Scholar] [CrossRef]
- Muneshwar, T.; Cadien, K. AxBAxB… pulsed atomic layer deposition: Numerical growth model and experiments. J. Appl. Phys. 2016, 119, 085306. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Bobrysheva, N.P.; Osmolovskaya, O.M.; Osmolovsky, M.G.; Smirnov, V.M. Atomic layer deposition of TiN dioxide nanofilms: A review. Rev. Adv. Mater. Sci. 2015, 40, 262–275. [Google Scholar]
- Austin, D.Z.; Jenkins, M.A.; Allman, D.; Hose, S.; Price, D.; Dezelah, C.L.; Conley, J.F. Atomic layer deposition of ruthenium and ruthenium oxide using a zero-oxidation state precursor. Chem. Mater. 2017, 29, 1107–1115. [Google Scholar] [CrossRef]
- Parsons, G.N.; George, S.M.; Knez, M.; Editors, G. Progress and future directions for atomic layer deposition and ALD-based chemistry. MRS Bull. 2011, 36, 865–871. [Google Scholar] [CrossRef]
- Niinisto, J.; Mantymaki, M.; Kukli, K.; Costelle, L.; Puukilainen, E.; Ritala, M.; Leskela, M.J. Growth and phase stabilization of HfO2 thin films by ALD using novel precursors. J. Cryst. Growth 2010, 312, 245–249. [Google Scholar]
- Putkonen, M.; Sajavaara, T.; Niinisto, L.; Keinonen, J. Analysis of ALD-processed thin films by ion-beam techniques. Anal. Bioanal. Chem. 2005, 382, 1792–1799. [Google Scholar] [CrossRef]
- Puurunen, R.L.; Root, A.; Sarv, P.; Viitanen, M.M.; Brongersma, H.H.; Lindblad, M.; Krause, A.O.I. Growth of aluminum nitride on porous alumina and silica through separate saturated gas-solid reactions of trimethylaluminum and ammonia. Chem. Mater. 2002, 14, 720–729. [Google Scholar] [CrossRef]
- Puurunen, R.L.; Airaksinen, S.M.K.; Krause, A.O.I. Chromium (III) supported on aluminum-nitride-surfaced alumina: Characteristics and dehydrogenation activity. J. Catal. 2003, 213, 281–290. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, J.; Huang, G.S.; Yuan, G.L.; Mei, Y.F. Effect of physisorption and chemisorptions of water on resonant modes of rolled-up tubular microcavities. Nanoscale Res. Lett. 2013, 8, 531. [Google Scholar] [CrossRef]
- Jeong, S.J.; Kim, H.W.; Heo, J.; Lee, M.H.; Song, H.J.; Ku, J.; Lee, Y.; Cho, Y.; Jeon, W.; Suh, H.; et al. Physisorbed-precursor-assisted atomic layer deposition of reliable ultrathin dielectric films on inert graphene surfaces for low-power electronics. 2D Mater. 2016, 3, 035327. [Google Scholar] [CrossRef]
- Teng, H.; Hsieh, C.T. Activation energy for oxygen chemisorptions on carbon at low temperatures. Ind. Eng. Chem. Res. 1999, 38, 292–297. [Google Scholar] [CrossRef]
- Sudan, P.; Zuttel, A.; Mauron, P.; Emmenegger, C.; Wenger, P.; Schlapbach, L. Physisorption of hydrogen in single-walled carbon nanotubes. Carbon 2003, 41, 2377–2383. [Google Scholar] [CrossRef]
- Ritala, M.; Leskela, M.; Rauhala, E.; Haussalo, P. Atomic layer epitaxy growth of TiN thin films. J. Electrochem. Soc. 1995, 142, 2731–2737. [Google Scholar] [CrossRef]
- Becker, J.B.; Suh, S.; Wang, S.L.; Gorden, R.G. Highly conformal thin films of tungsten nitride prepared by atomic layer deposition from a novel precursor. Chem. Mater. 2003, 15, 2969–2976. [Google Scholar] [CrossRef]
- Ott, A.W.; Klaus, J.W.; Johnson, J.M.; George, S.M. Al2O3 thin film growth on Si(100) using binary reaction sequence chemistry. Thin Solid Films 1997, 292, 135–144. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-temperature Al2O3 atomic layer deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Ham, S.Y.; Ko, D.H.; Shin, S.; Jin, Z.Y.; Min, Y.S. A kinetic study of ZnO atomic layer deposition: Effect of surface hydroxyl concentration and steric hindrance. Appl. Surf. Sci. 2019, 469, 804–810. [Google Scholar] [CrossRef]
- Leskela, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Ebbesen, T.W.; Ichihashi, T.; Iijima, S.; Tanigaki, K.; Hiura, H. Opening carbon nanotubes with oxygen and implications for filling. Nature 1993, 362, 522–525. [Google Scholar] [CrossRef]
- Penza, M.; Rossi, R.; Alvisi, M.; Serra, E. Metal-modified and vertically aligned carbon nanotube sensors arrays for landfill gas monitoring applications. Nanotechnology 2010, 21, 105501. [Google Scholar] [CrossRef]
- Kong, J.; Cassell, A.M.; Dai, H. Chemical vapor deposition of methane for single-walled carbon nanotubes. Chem. Phys. Lett. 1998, 292, 567–574. [Google Scholar] [CrossRef]
- Wal, R.L.V.; Ticich, T.M. Comparative flame and furnace synthesis of single-walled carbon nanotubes. Chem. Phys. Lett. 2001, 336, 24–32. [Google Scholar]
- Laplaze, D.; Bernier, P.; Maser, W.K.; Flamant, G.; Guillard, T.; Loiseau, A. Cabron nanotubes: The solar approach. Carbon 1998, 36, 685–688. [Google Scholar] [CrossRef]
- De, I.A.T.; Garnier, M.G.; Seo, J.K.; Oelhafen, P.; Thommen, V.; Mathys, D. The influence of catalyst chemical state and morphology on carbon nanotube growth. J. Phys. Chem. B 2004, 108, 7728–7734. [Google Scholar]
- Fiawoo, M.F.C.; Bonnot, A.M.; Amara, H.; Bichara, C.; Thibault-Penisson, J.; Loiseau, A. Evidence of correalation between catalyst particles and the single-wall carbon nanotube diameter: A first step towards chirality control. Phys. Rev. Lett. 2012, 108, 195503. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Yuan, G.J.; Shan, B.; Zhang, X.X.; Ma, H.P.; Tian, Y.Z.; Lu, H.L.; Liu, J. Atomic layer deposition of buffer layers for the growth of vertically aligned carbon nanotube arrays. Nanoscale Res. Lett. 2019, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Thissen, N.F.W.; Verheijen, M.A.; Houben, R.G.; Marel, C.V.D.; Kessels, W.M.M.; Bol, A.A. Synthesis of single-walled carbon nanotubes from atomic-layer-deposited Co3O4 and Co3O4/Fe2O3 catalyst films. Carbon 2017, 121, 389–398. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Bae, J.C.; Baik, H.K.; Cho, S.; Lee, S.J.; Song, K.M.; Myung, N.S. Growth control of single and multi-walled carbon nanotubes by thin film catalyst. Chem. Phys. Lett. 2002, 366, 109–114. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Fouquet, M.; Bayer, B.C.; Ducati, C.; Smajda, R.; Hofmann, S.; Robertson, J. Growth of ultrahigh density vertically aligned carbon nanotube forests for interconnects. ACS Nano 2010, 4, 7431–7436. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Q.C.; Li, C.W.; Wang, X.N.; Zhang, K.; Xu, Y.C.; Li, T.T.; Fang, J.H.; Yao, Y.G. Atomic layer deposition of Al2O3 catalysts for narrow diameter distributed single-walled carbon nanotube arrays growth. Carbon 2017, 114, 224–229. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Arato, D.; Li, R.Y.; Merel, P.; Sun, X.L. Aligned multi-walled carbon nanotubes on different substrates by floating catalyst chemical vapor deposition: Critical effects of buffer layer. Surf. Coat. Technol. 2008, 202, 4114–4120. [Google Scholar] [CrossRef]
- Zhou, K.; Huang, J.Q.; Zhang, Q.; Wei, F. Multi-directional growth of aligned carbon nanotubes over catalyst film prepared by atomic layer deposition. Nanoscale Res. Lett. 2010, 5, 1555–1560. [Google Scholar] [CrossRef]
- Chen, B.A.; Zhang, C.; Esconjauregui, S.; Xie, R.S.; Zhong, G.F.; Bhardwaj, S.; Cepek, C.; Robertson, J. Carbon nanotube forests growth using catalysts from atomic layer deposition. J. Appl. Phys. 2014, 115, 144303. [Google Scholar] [CrossRef]
- Eder, D. Carbon nanotube-inorganic hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Li, C.; Zhang, Y.; Chu, D.P.; Milne, W.I.; Fan, H.J. Atomic layer deposition of ZnO on multi-walled carbon nanotubes and its use for synthesis of CNT-ZnO heterostructures. Nanoscale Res. Lett. 2010, 5, 1836–1840. [Google Scholar] [CrossRef] [PubMed]
- Haremza, J.M.; Hahn, M.A.; Krauss, T.D.; Chen, S.; Calcines, J. Attachment of single CdSe nanocrystals to individual single-walled carbon nanotubes. Nano Lett. 2002, 2, 1253–1258. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Mandar, H.; Uustare, T. Atomic layer deposition of titanium dioxide from TiCl4 and H2O: Investigation of growth mechanism. Appl. Surf. Sci. 2001, 172, 148–158. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Uustare, T.; Ritala, M.; Leskela, M. Titanium isopropoxide as a precursor for atomic layer deposition: Characterization of titanium dioxide growth process. Appl. Surf. Sci. 2000, 161, 385–395. [Google Scholar] [CrossRef]
- Herrmann, C.F.; Fabreguette, F.H.; Finch, D.S.; Geiss, R.; George, S.M. Multilayer and functional coatings on carbon nanotubes using atomic layer deposition. Appl. Phys. Lett. 2005, 87, 123110. [Google Scholar] [CrossRef]
- Lu, Y.R.; Bangsaruntip, S.; Wang, X.R.; Zhang, L.; Nishi, Y.; Dai, H.J. DNA functionalization of carbon nanotubes for ultrathin atomic layer deposition of high k dielectrics for nanotube transistors with 60 mV/decade switching. J. Am. Chem. Soc. 2006, 128, 3518–3519. [Google Scholar] [CrossRef]
- Wang, X.R.; Tabakman, S.; Dai, H.J. Atomic layer deposition of metal oxides on pristine and functionalized graphene. J. Am. Chem. Soc. 2008, 130, 8152–8153. [Google Scholar] [CrossRef]
- Xuan, Y.; Wu, Y.Q.; Shen, T.; Qi, M.; Capano, M.A.; Cooper, J.A.; Ye, P.D. Atomic-layer-deposited nanostructures for graphene-based nanoelectronics. Appl. Phys. Lett. 2008, 92, 013101. [Google Scholar] [CrossRef]
- Cavanagh, A.S.; Wilson, C.A.; Weimer, A.W.; George, S.M. Atomic layer deposition on gram quantities of multi-walled carbon nanotubes. Nanotechnology 2009, 20, 255602. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Park, H.S.; Kang, S.W. Kinetic modeling of film growth rate in atomic layer deposition. J. Appl. Phys. 2001, 148, C403–C408. [Google Scholar] [CrossRef]
- Yazdani, N.; Bozyigit, D.; Utke, I.; Buchheim, J.; Youn, S.K.; Patscheider, J.; Wood, V.; Park, H.G. Enhanced charge transport kinetics in anisotropic, stratified photoanodes. ACS Appl. Mater. Interfaces 2014, 6, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.S.; Lee, I.H.; Lee, Y.H.; Hwang, C.S. Botryoidal growth of crystalline ZnO nanoparticles on a forest of single-walled carbon nanotubes by atomic layer deposition. CrystEngComm 2011, 13, 3451–3454. [Google Scholar] [CrossRef]
- Lee, J.S.; Min, B.; Cho, K.; Kim, S.; Park, J.; Lee, Y.T.; Kim, N.S.; Lee, M.S.; Park, S.O.; Moon, J.T. Al2O3 nanotubes and nanorods fabricated by coating and filling of carbon nanotubes with atomic-layer deposition. J. Cryst. Growth 2003, 254, 443–448. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, S.M.; Scholz, R.; Knez, M.; Gosele, U.; Fallert, J.; Kalt, H.; Zacharias, M. Synthesis and optical properties of ZnO and carbon nanotube based coaxial heterostructures. Appl. Phys. Lett. 2008, 93, 103108. [Google Scholar] [CrossRef]
- Lahiri, I.; Oh, S.M.; Hwang, J.Y.; Kang, C.; Choi, M.; Jeon, H.; Banerjee, R.; Sun, Y.K.; Choi, W. Ultrathin alumina-coated carbon nanotubes as an anode for high capacity Li-ion batteries. J. Mater. Chem. 2011, 21, 13621–13626. [Google Scholar] [CrossRef]
- Farmer, D.B.; Gordon, R.G. ALD of high-k dielectrics on suspended functionalized SWNTs. Electrochem. Solid State Lett. 2005, 8, G89–G91. [Google Scholar] [CrossRef]
- Zhan, G.D.; Du, X.H.; King, D.M.; Hakim, L.F.; Liang, X.H.; McCormick, J.A.; Weimer, A.W. Atomic layer deposition on bulk quantities of surfactant-modified single-walled carbon nanotubes. J. Am. Ceram. Soc. 2008, 91, 831–835. [Google Scholar] [CrossRef]
- Liyanage, L.S.; Cott, D.J.; Delabie, A.; Elshocht, S.V.; Bao, Z.N.; Wong, H.S.P. Atomic layer deposition of high-k dielectrics on single-walled carbon nanotubes: A Raman study. Nanotechnology 2013, 24, 245703. [Google Scholar] [CrossRef]
- Li, M.Y.; Zu, M.; Yu, J.S.; Cheng, H.F.; Li, Q.W.; Li, B. Controllable synthesis of core-sheath structured aligned carbon nanotube/titanium dioxide hybrid fibers by atomic layer deposition. Carbon 2017, 123, 151–157. [Google Scholar] [CrossRef]
- Boukhalfa, S.; Evanoff, K.; Yushin, G. Atomic layer deposition of vanadium oxide on carbon nanotubes for high-power supercapacitor electrodes. Energy Environ. Sci. 2012, 5, 6872–6879. [Google Scholar] [CrossRef]
- Zhang, Y.; Guerra-Nunez, C.; Utke, I.; Michler, J.; Rossell, M.D.; Erni, R. Understanding and controlling nucleation and growth of TiO2 deposited on multiwalled carbon nanotubes by atomic layer deposition. J. Phys. Chem. C 2015, 119, 3379–3387. [Google Scholar] [CrossRef]
- Fiorentino, G.; Vollebregt, S.; Tichelaar, F.D.; Ishihara, R.; Sarro, P.M. Impact of the atomic layer deposition precursors diffusion on solid-state carbon nanotube based supercapacitors performances. Nanotechnology 2015, 26, 064002. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.G.; Zhang, J.L.; Meyer, M.K.; Lu, X.C.; Choi, D.; Lemmon, J.P.; Liu, J.G. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatti, T.S. A review on electrochemical double-layer capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Jiang, Y.; Lin, L. A two-stage, self-aligned vertical densification process for as-grown CNT forests in supercapacitor applications. Sens. Actuators A Phys. 2012, 188, 261–267. [Google Scholar] [CrossRef]
- Jiang, Y.; Kozinda, A.; Lin, L. Flexible energy storage devices based on carbon nanotube forests with built-in metal electrodes. Sens. Actuators A Phys. 2013, 195, 224–230. [Google Scholar] [CrossRef]
- Futaba, D.N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef]
- Turano, S.P.; Ready, W.J. Chemical vapor deposition synthesis of self-aligned carbon nanotube arrays. J. Electron. Mater. 2006, 35, 192–194. [Google Scholar] [CrossRef]
- Reit, R.; Nguyen, J.; Ready, W.J. Growth time performance dependence of vertically aligned carbon nanotube supercapacitors grown on aluminum substrates. Electrochim. Acta 2013, 91, 96–100. [Google Scholar] [CrossRef]
- Kao, E.; Yang, C.; Warren, R.; Kozinda, A.; Lin, L.W. ALD titanium nitride on vertically aligned carbon nanotube forests for electrochemical supercapacitors. Sens. Actuators A Phys. 2016, 240, 160–166. [Google Scholar] [CrossRef]
- Pint, C.L.; Nicholas, N.W.; Xu, S.; Sun, Z.Z.; Tour, J.M.; Schmidt, H.K.; Gordon, R.G.; Hauge, R.H. Three dimensional solid-state supercapacitors from aligned single-walled carbon nanotube array templates. Carbon 2011, 49, 4890–4897. [Google Scholar] [CrossRef]
- Fisher, R.A.; Watt, M.R.; Konjeti, R.; Ready, W.J. Atomic layer deposition of titanium oxide for pseudocapacitive functionalization of vertically-aligned carbon nanotube supercapacitor electrodes. ECS J. Solid State 2015, 4, M1–M5. [Google Scholar] [CrossRef]
- Warren, R.; Sammoura, F.; Tounsi, F.; Sanghadasa, M.; Lin, L.W. Highly active ruthenium oxide coating via ALD and electrochemical activation in supercapacitor applications. J. Mater. Chem. A 2015, 3, 15568–15575. [Google Scholar] [CrossRef]
- Fan, Z.Y.; Razavi, H.; Do, J.W.; Moriwaki, A.; Ergen, O.; Chueh, Y.L.; Leu, P.W.; Ho, J.C.; Takahashi, T.; Reichertz, L.A.; et al. Three-dimensional nanopillar-array photovoltaics on low-cost and flexible substrates. Nat. Mater. 2009, 8, 648–653. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- Fan, Z.Y.; Kapadia, R.; Leu, P.W.; Zhang, X.B.; Chueh, Y.L.; Takei, K.; Yu, K.; Jamshidi, A.; Rathore, A.A.; Ruebusch, D.J.; et al. Ordered arrays of dual-diameter nanopillars for maximized optical absorption. Nano Lett. 2010, 10, 3823–3827. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chattopadhyay, S.; Jen, Y.J.; Peng, C.Y.; Liu, T.A.; Hsu, Y.K.; Pan, C.L.; Lo, H.C.; Hsu, C.H.; Chang, Y.H.; et al. Improved broadband and quasi-omnidirectional anti-reflection properties with biomimetic silicon nanostructures. Nat. Nanothechnol. 2007, 2, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, R.; Fan, Z.Y.; Javery, A. Design constraints and guidelines for CdS/SdTe nanopillar based photovoltaics. Appl. Phys. Lett. 2010, 96, 103116. [Google Scholar] [CrossRef]
- Lin, Y.J.; Yuan, G.B.; Liu, R.; Zhou, S.; Sheehan, S.W.; Wang, D.W. Semiconductor nanostructure-based photoelectrochemical water splitting: A brief review. Chem. Phys. Lett. 2011, 507, 209–215. [Google Scholar] [CrossRef]
- Avouris, P.; Chen, Z.; Perebeinos, V. Carbon-based electronics. Nat. Nanotechnol. 2007, 2, 605–615. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Pint, C.L.; Takei, K.; Kapadia, R.; Zheng, M.; Ford, A.C.; Zhang, J.J.; Jamshidi, A.; Bardhan, R.; Urban, J.J.; Wu, M.; et al. Rationally designed, three-dimensional carbon nanotube back-contacts for efficient solar devices. Adv. Energy Mater. 2011, 1, 1040–1045. [Google Scholar] [CrossRef]
- Yun, D.J.; Ra, H.; Kim, J.M.; Oh, E.; Lee, J.; Jeong, M.H.; Jeong, Y.J.; Yang, H.; Jang, J. Multi-walled carbon nanotube forests covered with atomic-layer-deposited ruthenium layers for high-performance counter electrodes of dye-sensitized solar cells. Org. Electron. 2019, 65, 349–356. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Dameron, A.A.; Pylypenko, S.; Bult, J.B.; Neyerlin, K.C.; Engtrakul, C.; Bochert, C.; Leong, G.J.; Frisco, S.L.; Simpson, L.; Dinh, H.N.; et al. Aligned carbon nanotube array functionalization for enhanced atomic layer deposition of platinum electrocatalysts. Appl. Surf. Sci. 2012, 258, 5212–5221. [Google Scholar] [CrossRef]
- Hu, X.G.; Dong, S.J. Metal nanomaterials and carbon nanotubes-synthesis, functionalization and potential applications towards electrochemistry. J. Mater. Chem. 2008, 18, 1279–1295. [Google Scholar] [CrossRef]
- Zhang, W.M.; Sherrell, P.; Minett, A.I.; Razal, J.M.; Chen, J. Carbon nanotube architectures as catalyst supports for proton exchange membrane fuel cells. Energy Environ. Sci. 2010, 3, 1286–1293. [Google Scholar] [CrossRef]
- Li, W.; Zie, S.; Qian, X.; Chang, L.; Zou, B.; Zhou, W.; Zhou, R.; Wang, G. Large-scale synthesis of aligned carbon nanotubes. Science 1996, 274, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.R.F.; Bard, A.J. Electrochemical detection of single molecules. Science 1995, 267, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Gu, J.; Brandin, E.; Kim, Y.R.; Wang, Q.; Branton, D. Probing single DNA molecule transport using fabricated nanopores. Nano Lett. 2004, 4, 2293–2298. [Google Scholar] [CrossRef]
- Shekhawat, G.; Tark, S.H.; Dravid, V.P. Mosfet-embedded microcantilevers for measuring deflection in biomolecular sensors. Science 2006, 311, 1592–1595. [Google Scholar] [CrossRef]
- Armani, A.M.; Kulkarni, R.P.; Fraser, S.E.; Flagan, R.C.; Vahala, K.J. Label-free, single-molecule detection with optical microcavities. Science 2007, 317, 783–787. [Google Scholar] [CrossRef]
- Snow, E.S.; Perkins, F.K.; Houser, E.J.; Badescu, S.C.; Reinecke, T.L. Chemical detection with a single-walled carbon nanotube capacitor. Science 2005, 307, 1942–1945. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Rizal, B.; McMahon, G.; Wang, H.Z.; Dhakal, P.; Kirkpatrick, T.; Ren, Z.F.; Chiles, T.C.; Naughton, M.J.; Cai, D. Ultrasensitive chemical detection using a nanocoax sensor. ACS Nano 2012, 6, 3171–3178. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, G.-J.; Xie, J.-F.; Li, H.-H.; Lu, H.-L.; Tian, Y.-Z. Atomic Layer Deposition of Inorganic Films for the Synthesis of Vertically Aligned Carbon Nanotube Arrays and Their Hybrids. Coatings 2019, 9, 806. https://doi.org/10.3390/coatings9120806
Yuan G-J, Xie J-F, Li H-H, Lu H-L, Tian Y-Z. Atomic Layer Deposition of Inorganic Films for the Synthesis of Vertically Aligned Carbon Nanotube Arrays and Their Hybrids. Coatings. 2019; 9(12):806. https://doi.org/10.3390/coatings9120806
Chicago/Turabian StyleYuan, Guang-Jie, Jie-Fei Xie, Hao-Hao Li, Hong-Liang Lu, and Ying-Zhong Tian. 2019. "Atomic Layer Deposition of Inorganic Films for the Synthesis of Vertically Aligned Carbon Nanotube Arrays and Their Hybrids" Coatings 9, no. 12: 806. https://doi.org/10.3390/coatings9120806
APA StyleYuan, G.-J., Xie, J.-F., Li, H.-H., Lu, H.-L., & Tian, Y.-Z. (2019). Atomic Layer Deposition of Inorganic Films for the Synthesis of Vertically Aligned Carbon Nanotube Arrays and Their Hybrids. Coatings, 9(12), 806. https://doi.org/10.3390/coatings9120806





