Atomic Layer Deposition of NiO to Produce Active Material for Thin-Film Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
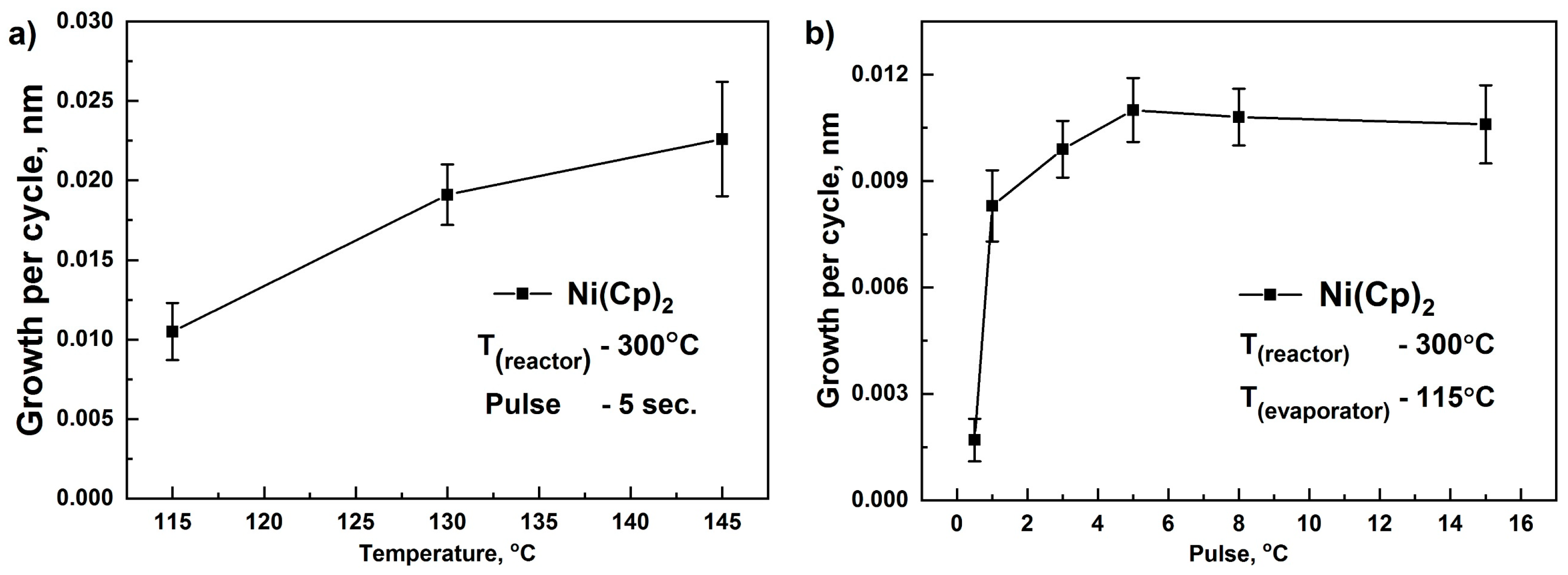
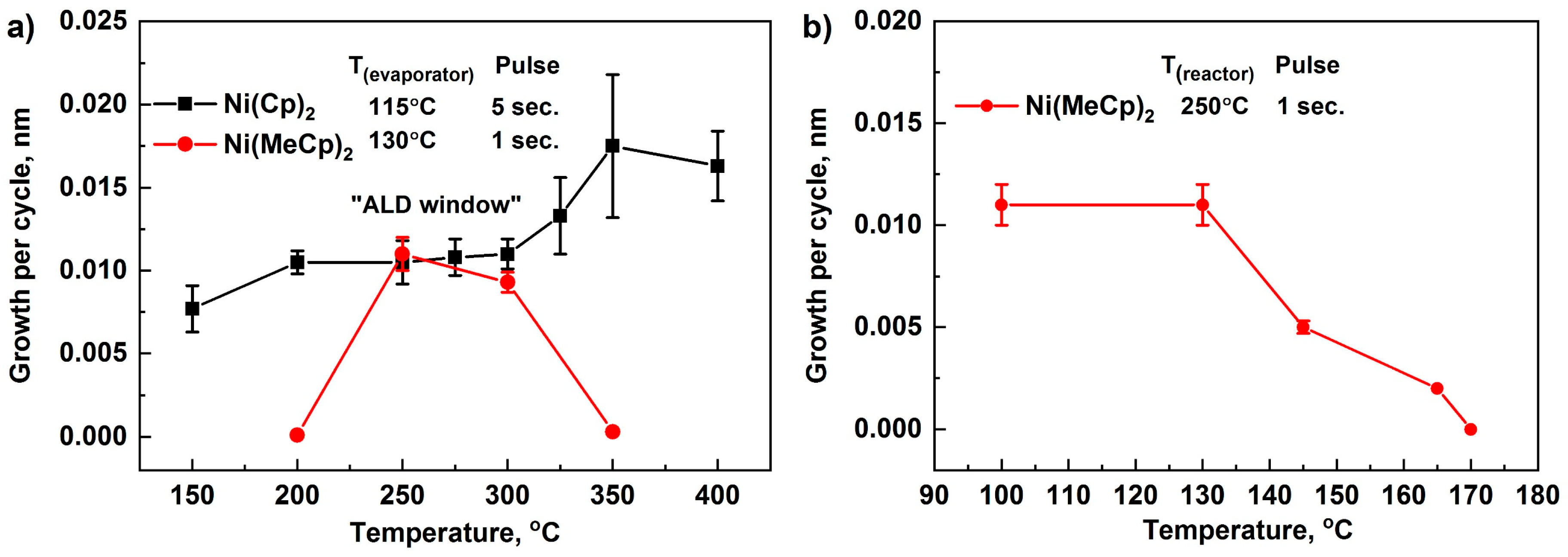
3.1. Atomic Layer Deposition of NiO Thin Films
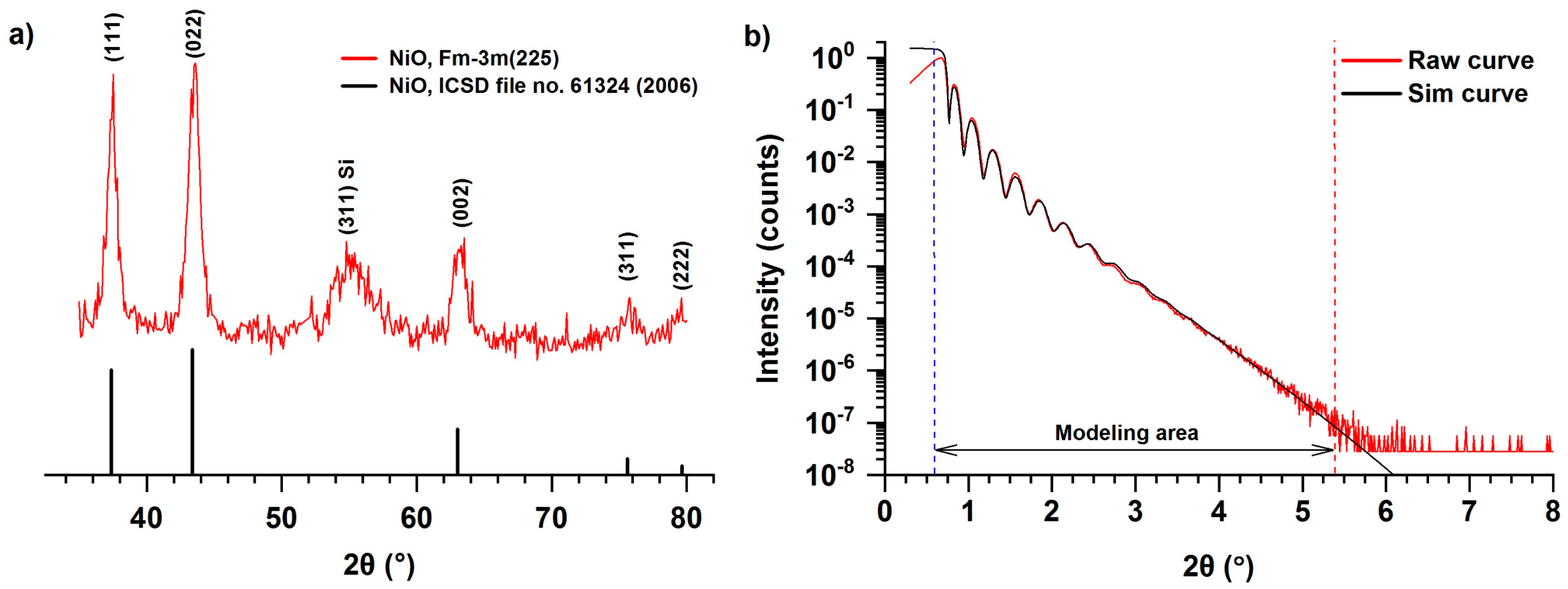
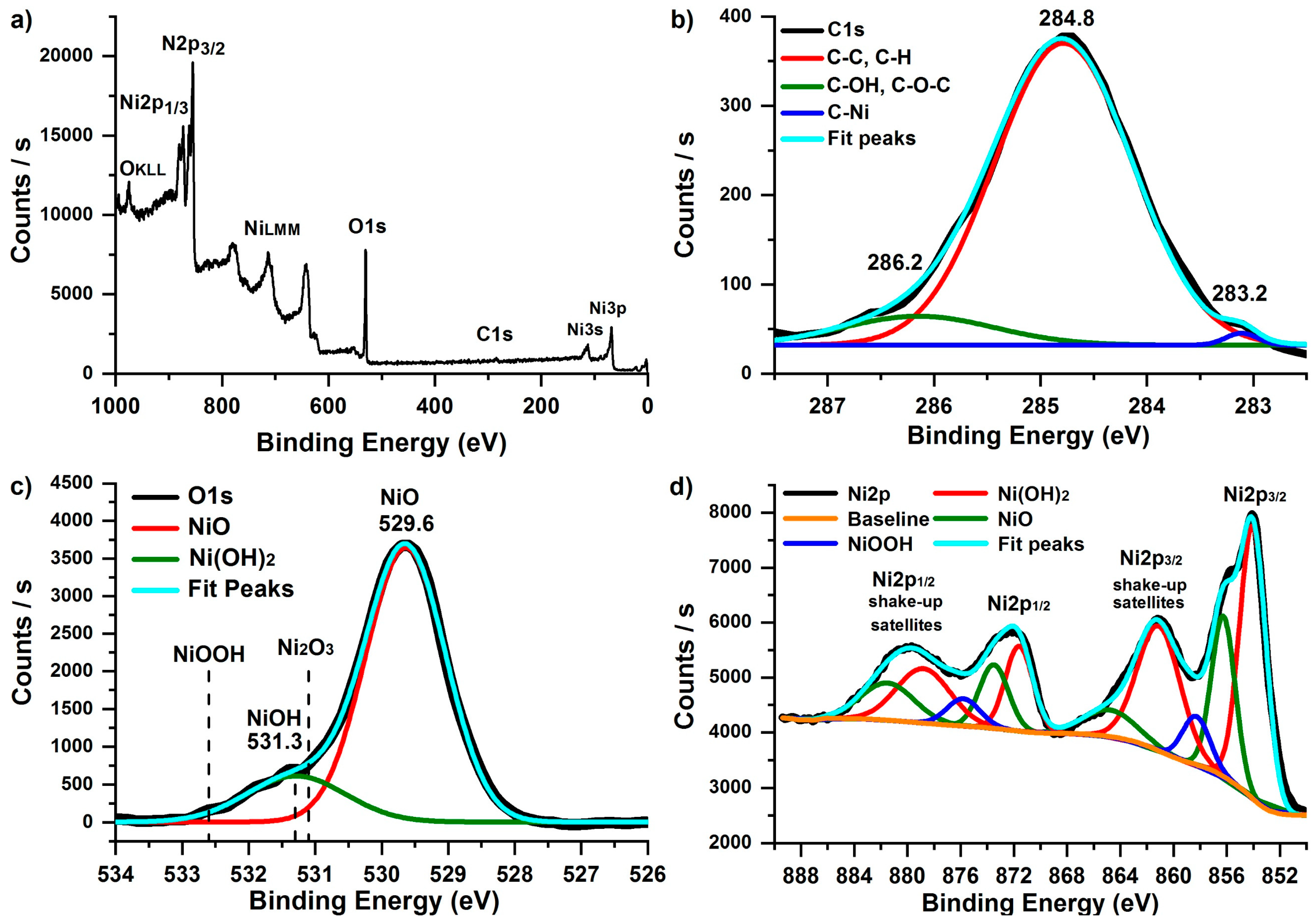
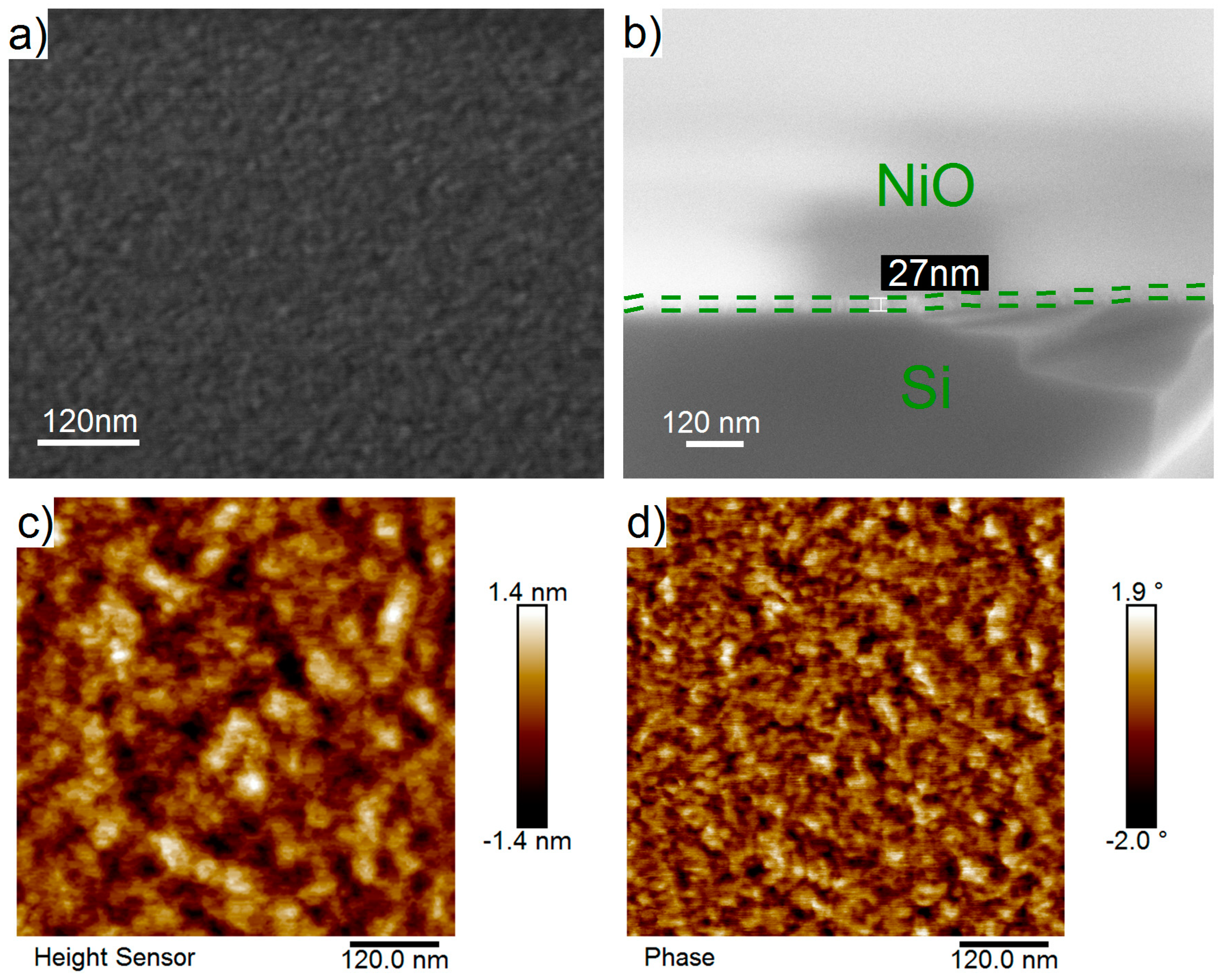
3.2. Structure and Composition of the Films Deposited on Silicon
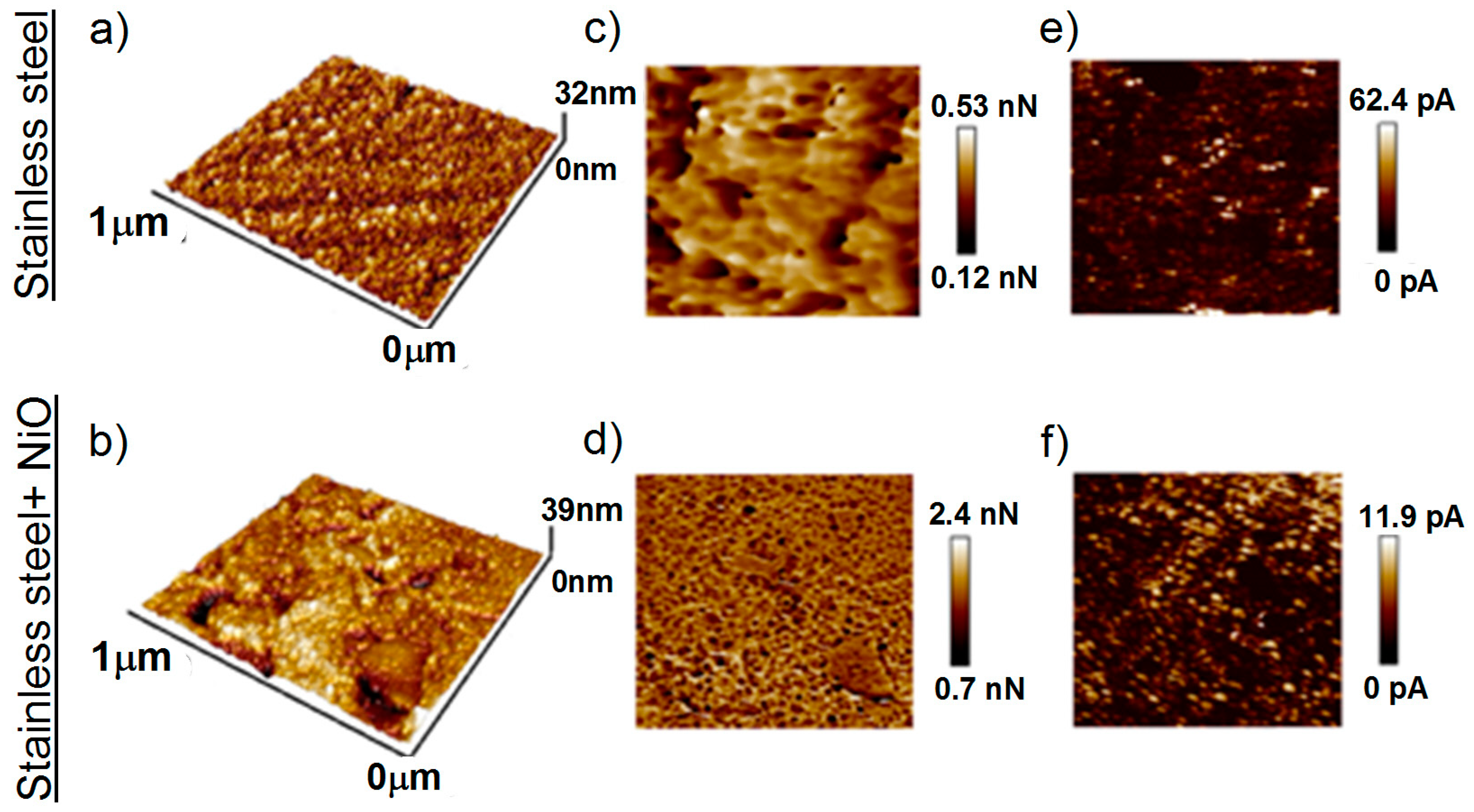
3.3. Morphology of the Films Deposited on Stainless Steel
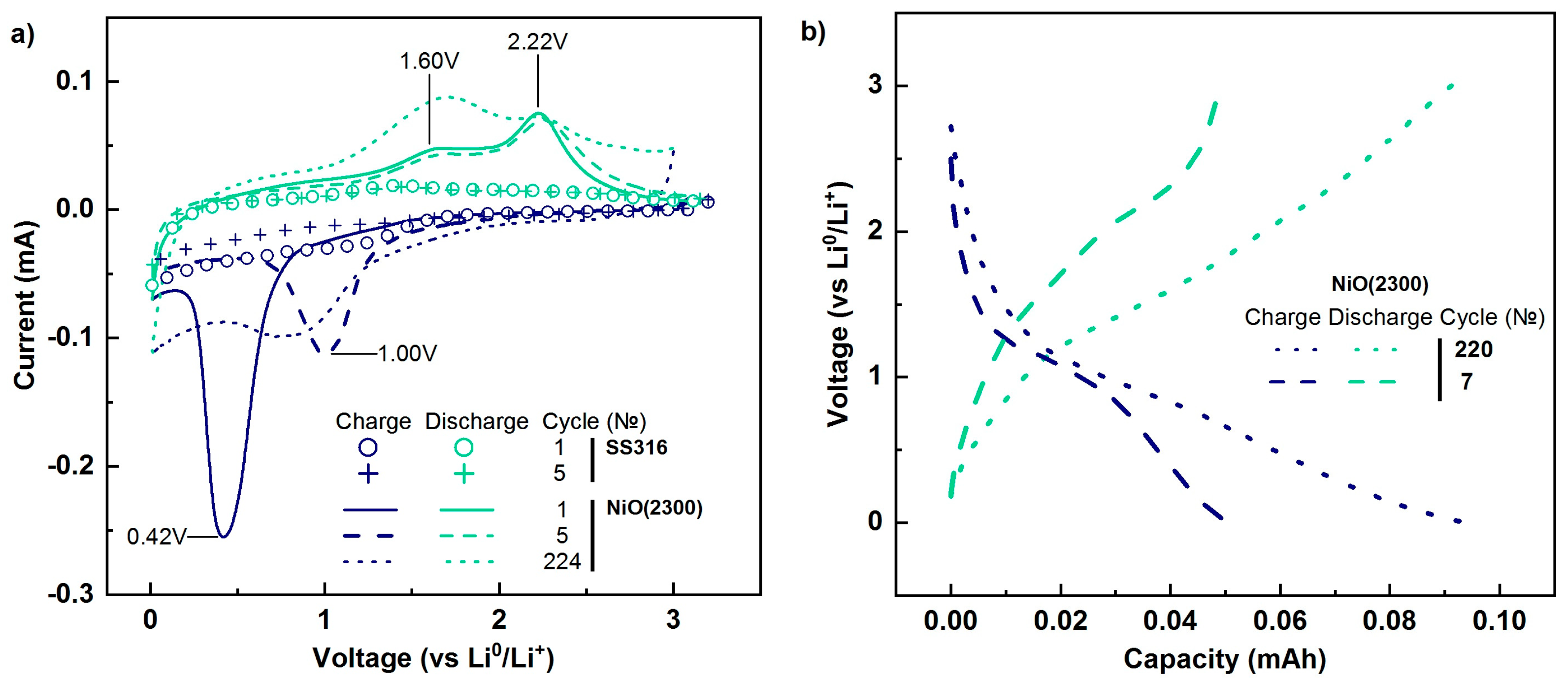
3.4. Electrochemical Properties of the Films Deposited on Stainless Steel
4. Conclusions
- Nanofilms of NiO were successfully obtained by ALD using Ni(MeCp)2, NiCp2, and oxygen plasma as counter-reagents.
- The optimal temperature range for ALD using NiCp2 was 200–300 °C, while using Ni(MeCp)2, the range is much narrower and is 250–300 °C. Growth per cycle for both precursors was 0.011–0.012 nm. Thus, NiCp2 is more thermally stable than Ni(MeCp)2, has a wider ALD window, and as a result is more suitable for the deposition of NiO films.
- The films deposited using NiCp2 (300 °C, 2300 ALD cycles) are continuous and uniform, consist of cubic modification Fm3m of NiO, and have low roughness (0.3–0.63 nm) and high density (6.6 g/cm3).
- The film contains predominantly NiO. However, carbon as cyclopentadienyl residues and Ni(OH)2/NiOOH oxide are also present in small amounts.
- The specific discharge capacity (1336–1379 mAh/g) of nickel oxide films deposited on steel substrates significantly exceeded the theoretical capacity of bulk NiO (718 mAh/g). Moreover, with repeated cycling, capacity increased. Based on the experimental data, we assume that such a high capacity is formed by the conversion capacity (reduction and oxidation of NiO) and pseudo-capacity due to the formation of a gel-like SEI film.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nardi, K.L.; Yang, N.Y.; Dickens, C.F.; Strickler, A.L.; Bent, S.F. Creating highly active atomic layer deposited nio electrocatalysts for the oxygen evolution reaction. Adv. Energy Mater. 2015, 5, 10. [Google Scholar] [CrossRef]
- Patella, B.; Sunseri, C.; Inguanta, R. Nanostructured based electrochemical sensors. J. Nanosci. Nanotechnol. 2019, 19, 3459–3470. [Google Scholar] [CrossRef]
- Kerli, S.; Alver, U. Preparation and characterisation of ZnO/NiO nanocomposite particles for solar cell applications. J. Nanotechnol. 2016, 5. [Google Scholar] [CrossRef]
- Sugiyama, I.; Shibata, N.; Wang, Z.C.; Kobayashi, S.; Yamamoto, T.; Ikuhara, Y. Ferromagnetic dislocations in antiferromagnetic nio. Nat. Nanotechnol. 2013, 8, 266–270. [Google Scholar] [CrossRef]
- Nilsen, O.; Miikkulainen, V.; Gandrud, K.B.; Ostreng, E.; Ruud, A.; Fjellvag, H. Atomic layer deposition of functional films for Li-ion microbatteries. Phys. Status Solidi A Appl. Mater. Sci. 2014, 211, 357–367. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metaloxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Maximov, M.Y.; Novikov, P.A.; Nazarov, D.V.; Rymyantsev, A.M.; Silin, A.O.; Zhang, Y.; Popovich, A.A. Characterization and electrochemical performance at high discharge rates of tin dioxide thin films synthesized by atomic layer deposition. J. Electron. Mater. 2017, 46, 6571–6577. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Chen, J.S.; Lou, X.W. SnO2-based nanomaterials: Synthesis and application in lithium-ion batteries. Small 2013, 9, 1877–1893. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, N.Q.; Sun, K.N. Facile ammonia-induced fabrication of nanoporous nio films with enhanced lithium-storage properties. Electrochem. Commun. 2012, 20, 137–140. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, B.; Li, Q.Y.; Cartmell, S.; Ferrara, S.; Deng, Z.Q.D.; Xiao, J. Lithium and lithium ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Ukoba, K.O.; Eloka-Eboka, A.C.; Inambao, F.L. Review of nanostructured nio thin film deposition using the spray pyrolysis technique. Renew. Sust. Energ. Rev. 2018, 82, 2900–2915. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskela, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 101. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-assisted atomic layer deposition: Basics, opportunities, and challenges. J. Vac. Sci. Technol. A 2011, 29, 26. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, H.; Shiraz, M.H.A. Toward 3D solid-state batteries via atomic layer deposition aproach. Front. Energy Res. 2018, 6, 10. [Google Scholar]
- Lindblad, M.; Lindfors, L.P.; Suntola, T. Preparation of Ni/Al2O3 catalysts from vapor-phase by atomic layer epitaxy. Catal. Lett. 1994, 27, 323–336. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Lindfors, L.P.; Reintjes, J.G.H.; Jylha, O.; Brongersma, H.H. The growth-mechanism of nickel in the preparation of Ni/Al2O3 catalysts studied by LEIS, XPS and catalytic activity. Catal. Lett. 1994, 25, 315–324. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Tiainen, L.P.; Lindblad, M.; Demirkan, K.; Kumar, N.; Sjoholm, R.; Ollonqvist, T.; Vayrynen, J.; Salmi, T.; Murzin, D.Y. Liquid-phase hydrogenation of citral for production of citronellol: Catalyst selection. Appl. Catal. A Gen. 2003, 241, 271–288. [Google Scholar] [CrossRef]
- Utriainen, M.; Kroger-Laukkanen, M.; Niinisto, L. Studies of nio thin film formation by atomic layer epitaxy. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 1998, 54, 98–103. [Google Scholar] [CrossRef]
- Bratvold, J.E.; Fjellvag, H.; Nilsen, O. Atomic layer deposition of oriented nickel titanate (NiTiO3). Appl. Surf. Sci. 2014, 311, 478–483. [Google Scholar] [CrossRef]
- Shim, J.W.; Fuentes-Hernandez, C.; Dindar, A.; Zhou, Y.H.; Khan, T.M.; Kippelen, B. Polymer solar cells with NiO hole-collecting interlayers processed by atomic layer deposition. Org. Electron. 2013, 14, 2802–2808. [Google Scholar] [CrossRef]
- Nam, W.J.; Gray, Z.; Stayancho, J.; Plotnikov, V.; Kwon, D.; Waggoner, S.; Shenai-Khatkhatec, D.V.; Pickering, M.; Okano, T.; Compaan, A.; et al. ALD NiO thin films as a hole transport-electron blocking layer material for photo-detector and solar cell devices. Ecs Trans. 2015, 66, 275–279. [Google Scholar] [CrossRef]
- Li, H.; Perera, T.; Shenai, D.V.; Li, Z.; Gordon, R.G. New Ni amidinate source for ALD/CVD of NiNx, NiO and Ni. In Proceedings of the 11th International Conference on Atomic Layer Deposition, Cambridge, MA, USA, 26–29 June 2011. [Google Scholar]
- Chae, J.; Park, H.S.; Kang, S.W. Atomic layer deposition of nickel by the reduction of preformed nickel oxide. Electrochem. Solid State Lett. 2002, 5, C64–C66. [Google Scholar] [CrossRef]
- Kim, D.H.; Sim, J.K.; Lee, J.; Seo, H.O.; Jeong, M.G.; Kim, Y.D.; Kim, S.H. Carbon dioxide reforming of methane over mesoporous Ni/SiO2. Fuel 2013, 112, 111–116. [Google Scholar] [CrossRef]
- Jeong, M.G.; Kim, D.H.; Lee, S.K.; Lee, J.H.; Han, S.W.; Park, E.J.; Cychosz, K.A.; Thommes, M.; Hwang, Y.K.; Chang, J.S.; et al. Decoration of the internal structure of mesoporous chromium terephthalate MIL-101 with NiO using atomic layer deposition. Microporous Mesoporous Mat. 2016, 221, 101–107. [Google Scholar] [CrossRef]
- Wang, G.Z.; Gao, Z.; Wan, G.P.; Lin, S.W.; Yang, P.; Qin, Y. High densities of magnetic nanoparticles supported on graphene fabricated by atomic layer deposition and their use as efficient synergistic microwave absorbers. Nano Res. 2014, 7, 704–716. [Google Scholar] [CrossRef]
- Lu, H.L.; Scarel, G.; Wiemer, C.; Perego, M.; Spiga, S.; Fanciulli, M.; Pavia, G. Atomic layer deposition of NiO films on Si(100) using cyclopentadienyl-type compounds and ozone as precursors. J. Electrochem. Soc. 2008, 155, H807–H811. [Google Scholar] [CrossRef]
- Wang, G.Z.; Peng, X.G.; Yu, L.; Wan, G.P.; Lin, S.W.; Qin, Y. Enhanced microwave absorption of ZnO coated with Ni nanoparticles produced by atomic layer deposition. J. Mater. Chem. A 2015, 3, 2734–2740. [Google Scholar] [CrossRef]
- Gao, Z.; Dong, M.; Wang, G.Z.; Sheng, P.; Wu, Z.W.; Yang, H.M.; Zhang, B.; Wang, G.F.; Wang, J.G.; Qin, Y. Multiply confined nickel nanocatalysts produced by atomic layer deposition for hydrogenation reactions. Angew. Chem. Int. Ed. 2015, 54, 9006–9010. [Google Scholar] [CrossRef]
- Alburquenque, D.; Del Canto, M.; Arenas, C.; Tejo, F.; Pereira, A.; Escrig, J. Dewetting of Ni thin films obtained by atomic layer deposition due to the thermal reduction process: Variation of the thicknesses. Thin Solid Film. 2017, 638, 114–118. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Wei, H.H.; Si, W.J.; Ou, G.; Zhao, C.S.; Song, M.J.; Zhang, C.; Wu, H. Enhanced electrocatalytic activity for water splitting on NiO/Ni/carbon fiber paper. Materials 2017, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.K.; Chen, C.Q.; Yan, W.J.; Duan, F.F.; Zhang, B.; Gao, Z.; Qin, Y. Ni nanoparticles supported on cnts with excellent activity produced by atomic layer deposition for hydrogen generation from the hydrolysis of ammonia borane. Catal. Sci. Technol. 2016, 6, 2112–2119. [Google Scholar] [CrossRef]
- Pereira, A.; Palma, J.L.; Denardin, J.C.; Escrig, J. Temperature-dependent magnetic properties of Ni nanotubes synthesized by atomic layer deposition. Nanotechnology 2016, 27, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lamperti, A.; Spiga, S.; Lu, H.L.; Wiemer, C.; Perego, M.; Cianci, E.; Alia, M.; Fanciulli, M. Study of the interfaces in resistive switching NiO thin films deposited by both ald and e-beam coupled with different electrodes (Si, Ni, Pt, W, TiN). Microelectron. Eng. 2008, 85, 2425–2429. [Google Scholar] [CrossRef]
- Spiga, S.; Lamperti, A.; Wiemer, C.; Perego, M.; Cianci, E.; Tallarida, G.; Lu, H.L.; Alia, M.; Volpe, F.G.; Fanciulli, M. Resistance switching in amorphous and crystalline binary oxides grown by electron beam evaporation and atomic layer deposition. Microelectron. Eng. 2008, 85, 2414–2419. [Google Scholar] [CrossRef]
- Huber, R.; Berberich, P.; Rapp, T.; Schwarze, T.; Grundler, D.; Bachmann, J.; Nielsch, K. Spin wave resonances in ferromagnetic thin films prepared via atomic layer deposition. In Proceedings of the Baltic ALD/GerALD Conference 2010, Hamburg, Germany, 16–17 September 2010. [Google Scholar]
- Yu, L.; Wang, G.L.; Wan, G.P.; Wang, G.Z.; Lin, S.W.; Li, X.Y.; Wang, K.; Bai, Z.M.; Xiang, Y. Highly effective synthesis of NiO/CNT nanohybrids by atomic layer deposition for high-rate and long-life supercapacitors. Dalton Trans. 2016, 45, 13779–13786. [Google Scholar] [CrossRef] [PubMed]
- Daub, M.; Knez, M.; Goesele, U.; Nielsch, K. Ferromagnetic nanotubes by atomic layer deposition in anodic alumina membranes. J. Appl. Phys. 2007, 101, 3. [Google Scholar] [CrossRef]
- Yang, T.S.; Cho, W.T.; Kim, M.; An, K.S.; Chung, T.M.; Kim, C.G.; Kim, Y. Atomic layer deposition of nickel oxide films using Ni(dmamp)2 and water. J. Vac. Sci. Technol. A 2005, 23, 1238–1243. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, S.Y.; Song, W.S.; Shin, Y.S.; Park, C.Y.; Kim, H.J.; Cho, W.; An, K.S. Field emission properties of ZnO nanorods coated with NiO film. J. Vac. Sci. Technol. B 2008, 26, 1021–1024. [Google Scholar] [CrossRef]
- So, B.S.; You, Y.H.; Kim, K.H.; Hwang, J.; Cho, W.; Lee, S.S.; Chung, T.M.; Lee, Y.K.; Kim, C.G.; An, K.S.; et al. Crystallization of amorphous silicon thin films using self-limiting ALD of nickel oxide. Electrochem. Solid State Lett. 2007, 10, J61–J64. [Google Scholar] [CrossRef]
- You, Y.H.; So, B.S.; Hwang, J.H.; Cho, W.; Lee, S.S.; Chung, T.M.; Kim, C.G.; An, K.S. Impedance spectroscopy characterization of resistance switching NiO thin films prepared through atomic layer deposition. Appl. Phys. Lett. 2006, 89, 3. [Google Scholar] [CrossRef]
- Lu, H.L.; Scarel, G.; Li, X.L.; Fanciulli, M. Thin MnO and NiO films grown using atomic layer deposition from ethylcyclopentadienyl type of precursors. J. Cryst. Growth 2008, 310, 5464–5468. [Google Scholar] [CrossRef]
- Pore, V.; Tois, E.; Matero, R.; Haukka, S.; Tuominen, M.; Woodruff, J.; Milligan, B.; Tang, F.; Givens, M. Nickel silicide for source-drain contacts from ALD NiO films. In Proceedings of the 2015 IEEE International Interconnect Technology Conference and 2015 IEEE Materials for Advanced Metallization Conference (IITC/MAM), Grenoble, France, 18–21 May 2015. [Google Scholar]
- Dashjav, E.; Lipinska-Chwalek, M.; Gruner, D.; Mauer, G.; Luysberg, M.; Tietz, F. Atomic layer deposition and high-resolution electron microscopy characterization of nickel nanoparticles for catalyst applications. Surf. Coat. Technol. 2016, 307, 428–435. [Google Scholar] [CrossRef]
- Kumagai, H.; Matsumoto, M.; Toyoda, K.; Obara, M. Preparation and characteristics of nickel oxide thin film by controlled growth with sequential surface chemical reactions. J. Mater. Sci. Lett. 1996, 15, 1081–1083. [Google Scholar] [CrossRef]
- Song, S.J.; Lee, S.W.; Kim, G.H.; Seok, J.Y.; Yoon, K.J.; Yoon, J.H.; Hwang, C.S.; Gatineau, J.; Ko, C. Substrate dependent growth behaviors of plasma-enhanced atomic layer deposited nickel oxide films for resistive switching application. Chem. Mater. 2012, 24, 4675–4685. [Google Scholar] [CrossRef]
- Lindahl, E.; Ottosson, M.; Carlsson, J.O. Atomic layer deposition of NiO by the Ni(thd)2/H2O precursor combination. Chem. Vap. Depos. 2009, 15, 186–191. [Google Scholar] [CrossRef]
- Lindahl, E.; Lu, J.; Ottosson, M.; Carlsson, J.O. Epitaxial NiO (100) and NiO (111) films grown by atomic layer deposition. J. Cryst. Growth 2009, 311, 4082–4088. [Google Scholar] [CrossRef]
- Lindahl, E.; Ottosson, M.; Carlsson, J.O. Growth and stability of CVD Ni3N and ALD NiO dual layers. Surf. Coat. Technol. 2010, 205, 710–716. [Google Scholar] [CrossRef]
- Hagen, D.J.; Tripathi, T.S.; Karppinen, M. Atomic layer deposition of nickel-cobalt spinel thin films. Dalton Trans. 2017, 46, 4796–4805. [Google Scholar] [CrossRef] [PubMed]
- Seim, H.; Molsa, H.; Nieminen, M.; Fjellvag, H.; Niinisto, L. Deposition of LaNiO3 thin films in an atomic layer epitaxy reactor. J. Mater. Chem. 1997, 7, 449–454. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Maximov, M.Y.; Novikov, P.A.; Popovich, A.A.; Silin, A.O.; Smirnov, V.M.; Bobrysheva, N.P.; Osmolovskaya, O.M.; Osmolovsky, M.G.; Rumyantsev, A.M. Atomic layer deposition of tin oxide using tetraethyltin to produce high-capacity Li-ion batteries. J. Vac. Sci. Technol. A 2017, 35, 11. [Google Scholar] [CrossRef]
- Blasco, N.; Girard, J.M. Recent development of ligand chemistries for next generation conformal peald/ald of metal & oxides. In Proceedings of the ALD Workshop on Atomic Layer Processing @ Semicon Europa, Grenoble, France, 7–10 October 2014. [Google Scholar]
- Vieyra-Eusebio, M.T.; Rojas, A. Vapor pressures and sublimation enthalpies of nickelocene and cobaltocene measured by thermogravimetry. J. Chem. Eng. Data 2011, 56, 5008–5018. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kada, T.; Machida, H.; Ohshita, Y.; Ogura, A. Ni precursor for chemical vapor deposition of NiSi. Jpn. J. Appl. Phys. 2004, 43, 1833–1836. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Gong, M.; Cao, K.; Zhang, Y.H.; Li, J.L.; Chen, R. Atomic layer deposition of Ni on Cu nanoparticles for methanol synthesis from CO2 hydrogenation. ChemCatChem 2017, 9, 3772–3778. [Google Scholar] [CrossRef]
- Huber, R. Control of spin waves on the nanoscale in one-dimensional magnonic crystals and atomic layer deposition of metallic ferromagnets for second generation of nanomaterials. Ph.D. Thesis, Technische Universitat Munchen, München, Germany, 2013. [Google Scholar]
- Bachmann, J.; Zolotaryov, A.; Albrecht, O.; Goetze, S.; Berger, A.; Hesse, D.; Novikov, D.; Nielsch, K. Stoichiometry of nickel oxide films prepared by ALD. Chem. Vap. Depos. 2011, 17, 177–180. [Google Scholar] [CrossRef]
- Kang, J.K.; Rhee, S.W. Metalorganic chemical vapor deposition of nickel films from Ni(C5H5)2/H2. J. Mater. Res. 2000, 15, 1828–1833. [Google Scholar] [CrossRef]
- Siddiqui, R.A. Experimental investigations of thermodynamic properties of organometallic compounds. Universität Duisburg-Essen. Ph.D. Thesis, Universität Duisburg-Essen, Duisburg, Germany, 2009. [Google Scholar]
- Ylilammi, M. Monolayer thickness in atomic layer deposition. Thin Solid Film. 1996, 279, 124–130. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 52. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 79th ed.; CRC Press Inc.: Boca Raton, FL, USA, 1998. [Google Scholar]
- Kraytsberg, A.; Drezner, H.; Auinat, M.; Shapira, A.; Solomatin, N.; Axmann, P.; Wohlfahrt-Mehrens, M.; Ein-Eli, Y. Atomic layer deposition of a particularized protective MgF2 film on a Li-ion battery LiMn1.5Ni0.5O4 cathode powder material. ChemNanoMat 2015, 1, 577–585. [Google Scholar]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Mansour, A.N. Characterization of NiO by XPS. Surf. Sci. Spectra 1994, 3, 232–238. [Google Scholar] [CrossRef]
- Mansour, A.N. Characterization of β-Ni(OH)2 by XPS. Surf. Sci. Spectra 1994, 3, 239–246. [Google Scholar] [CrossRef]
- Hu, H.L.; Zhu, J.G.; Chen, M.S.; Guo, T.L.; Li, F.S. Inkjet-printed p-type nickel oxide thin-film transistor. Appl. Surf. Sci. 2018, 441, 295–302. [Google Scholar] [CrossRef]
- Furlan, A.; Lu, J.; Hultman, L.; Jansson, U.; Magnuson, M. Crystallization characteristics and chemical bonding properties of nickel carbide thin film nanocomposites. J. Phys.-Condes. Matter 2014, 26, 11. [Google Scholar] [CrossRef]
- Gutierrez, J.; Mondragon, I.; Tercjak, A. Quantitative nanoelectrical and nanomechanical properties of nanostructured hybrid composites by peakforce tunneling atomic force microscopy. J. Phys. Chem. C 2014, 118, 1206–1212. [Google Scholar] [CrossRef]
- Kawamori, M.; Asai, T.; Shirai, Y.; Yagi, S.; Oishi, M.; Ichitsubo, T.; Matsubara, E. Three-dimensional nanoelectrode by metal nanowire nonwoven clothes. Nano Lett. 2014, 14, 1932–1937. [Google Scholar] [CrossRef]
- Wang, A.P.; Kadam, S.; Li, H.; Shi, S.Q.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. npj Comput. Mater. 2018, 4, 26. [Google Scholar] [CrossRef]
- Gao, X.P.; Yang, H.X. Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci. 2010, 3, 174–189. [Google Scholar] [CrossRef]
- Laruelle, S.; Grugeon, S.; Poizot, P.; Dolle, M.; Dupont, L.; Tarascon, J.M. On the origin of the extra electrochemical capacity displayed by Mo/Li cells at low potential. J. Electrochem. Soc. 2002, 149, A627–A634. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Tarascon, J.M. Rationalization of the low-potential reactivity of 3d-metal-based inorganic compounds toward Li. J. Electrochem. Soc. 2002, 149, A1212–A1217. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Zhang, C.Q.; Zhou, F. Hollow microspheres of NiO as anode materials for lithium-ion batteries. Electrochim. Acta 2010, 55, 8981–8985. [Google Scholar] [CrossRef]
- Long, H.; Shi, T.L.; Hu, H.; Jiang, S.L.; Xi, S.; Tang, Z.R. Growth of hierarchal mesoporous NiO nanosheets on carbon cloth as binder-free anodes for high-performance flexible lithium-ion batteries. Sci. Rep. 2014, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.W.; Zhang, R.L.; Zhong, S.K.; Wu, L. Electrospinning synthesis of 3D porous NiO nanorods as anode material for lithium-ion batteries. Mater. Sci. Pl. 2016, 34, 227–232. [Google Scholar]
| Reagent (CAS No.) | Counter-Reagent | Substrate Temperature (°C) | Substrate | Growth Per Cycle (nm) | Phase, Amorp, Crystal. | Preferred Orientation | Ref. |
|---|---|---|---|---|---|---|---|
| Ni(acac)2 (3264-82-2) | Air | 200–400 | Al2O3 porous | – | Cryst | – | [17] |
| Air | 200–400 | Al2O3 porous | – | Cryst | – | [18] | |
| Air | 200–400 | Al2O3 porous | – | – | – | [19] | |
| H2O | 250 | Soda lime | – | A | – | [20] | |
| O2 | 250 | Soda lime | – | – | – | [20] | |
| O3 | 200–300 (150–300) | Soda lime Si (100) | ≈0.045 | C | (100) | [21] | |
| O3 | 250 | Soda lime | 0.062 | C | (100) | [20] | |
| O3 | 280 | Soda lime | 0.067 | C | (100) | [20] | |
| O3 | 310 | Soda lime | 0.077 | C | (100) | [20] | |
| O3 + H2O | 190–310 | Soda lime | <0.072 | C | (100) | [20] | |
| Ni-Amd (940895-79-4) | H2O | 150 | Si (ITO/SiOx) | <0.06 | – | – | [22] |
| H2O | 200 | Si, glass | 0.025–0.045 | C | (111) | [23] | |
| H2O | 200 | Si | 0.01 | C | – | [24] | |
| Ni(apo)2 (not found) | N2O | 190–400 | Soda lime | – | – | – | [20] |
| O3 | 190–310 | Soda lime | 0.025–0.062 | C | (100) | [20] | |
| Ni(Cp)2 (1271-28-9) | H2O(+H2) | 165 | TiN/SiO2/Si | – | – | – | [25] |
| H2O | 250 | SiO2 | – | A | – | [26] | |
| O2 | 150 | (MIL-101) 1 | – | – | – | [27] | |
| O3 | 150 | Graphene/SiO2 | – | – | – | [28] | |
| O3 | 150 | Si (100) | 0.32 | A/C | – | [29] | |
| O3 | 150 | ZnO | 0.02 | A | – | [30] | |
| O3 | 200 | Al2O3 NT 2 | – | – | – | [31] | |
| O3 | 200 | Si (100) | 0.26 | C | – | [29] | |
| O3 | 200 | SiO2/Si (100) | 0.012 | C | –/(100) | [32] | |
| O3 | 250 | Si (100) | 0.12 | C | – | [29] | |
| O3 | 270 | Carbon paper | – | C | – | [33] | |
| O3 | 275 | Si (100) | 0.063 | C | –/(100) | [1] | |
| O3 | 280 | CNT 3 | – | C | – | [34] | |
| O3 | 300 | Al2O3 | – | – | – | [35] | |
| O3 | 300 | Si (100) | 0.08 | C | – | [29] | |
| O3 | 300 | Si, Ni, Pt, W, TiN | – | Pt – C | (111) | [36] | |
| O3 | 300 | Si, Pt, W, TiN | – | Si – C Pt – C | – (111) | [37] | |
| O3 | 300 | – | – | – | – | [38] | |
| O3 + H2O | 150 | CNT 3 | 0.03 | C | – | [39] | |
| O3 + H2O | 270–330 | Al2O3 | 0.022–0.03 | – | – | [40] | |
| Ni(dmamp)2 (942311-35-5) | H2O | 100–160 (80–240) | Si (001) | 0.08 (0.07–0.16) | A | – | [41] |
| H2O | 130 | ZnO | 0.14 | A | – | [42] | |
| H2O | 140 | Amorph. Si/SiO2/glass | 0.14 | – | – | [43] | |
| H2O | 140 | Pt | – | A | – | [44] | |
| Ni(dmg)2 (13478-93-8) | O3 | 190–310 | Soda lime | 0.025–0.062 | C | (100) | [20] |
| Ni(EtCp)2 (31886-51-8) | O3 | 150 | Si (100) | 0.08 | A | – | [29,45] |
| O3 | 200 | Si (100) | 0.06 | C | – | [29,45] | |
| O3 | 250 | Si (100) | 0.04 | C | – | [29,45] | |
| O3 | 300 | Si (100) | 0.06 | C | – | [29,45] | |
| Ni(EtNacNac) (not found) | O3 | 150–175 (140–225) | Si | 0.02 | C | – | [46] |
| Ni(MeCp)2 (1293-95-4) | H2O | 200–220 | Si (111) | 0.004 | – | – | [47] |
| H2O2 | 400 | Si (100) | 0.14 | A | – | [48] | |
| O2 plasma | 250 | W | 0.084 | C | –/(100) | [49] | |
| O2 plasma | 250 | Pt | 0.058 | C | (111) | [49] | |
| O2 plasma | 250 | Ru | 0.048 | C | –/(100) | [49] | |
| Ni(thd)2 (14481-08-4) | H2O | 205 | SiO2 | 0.02 | C/A | – | [50] |
| H2O | 205–275 | Mg (100) α-Al2O3 (00l) glass | 0.017–0.035 0.02–0.029 0.023–0.039 | C C – | (100) (111) – | [51] | |
| H2O | 230 | SiO2 | 0.02 | C | 24–100 nm (100) | [50] | |
| H2O | 250 | Si (100) α-Al2O3 (00l) | – | C C | – (111) | [52] | |
| H2O | 260 | SiO2 | 0.04 | C | – | [50] | |
| H2O | 275 | SiO2 | 0.03 | C | – | [50] | |
| O3 | 200 | – | 0.02 | C | – | [53] | |
| O3 | 210–400 (150–500) | Corning 7059 glass | 0.008–0.026 | – | – | [54] |
| Layer | Material | Cell Input | Thickness, nm | Roughness, nm | Gradient | Density, g/cm3 |
|---|---|---|---|---|---|---|
| Film | NiO | density | 28.0 | 0.63 | No | 6.6 |
| Substrate | Si | density | 0 | 1.26 | No | 2.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshtyal, Y.; Nazarov, D.; Ezhov, I.; Mitrofanov, I.; Kim, A.; Rymyantsev, A.; Lyutakov, O.; Popovich, A.; Maximov, M. Atomic Layer Deposition of NiO to Produce Active Material for Thin-Film Lithium-Ion Batteries. Coatings 2019, 9, 301. https://doi.org/10.3390/coatings9050301
Koshtyal Y, Nazarov D, Ezhov I, Mitrofanov I, Kim A, Rymyantsev A, Lyutakov O, Popovich A, Maximov M. Atomic Layer Deposition of NiO to Produce Active Material for Thin-Film Lithium-Ion Batteries. Coatings. 2019; 9(5):301. https://doi.org/10.3390/coatings9050301
Chicago/Turabian StyleKoshtyal, Yury, Denis Nazarov, Ilya Ezhov, Ilya Mitrofanov, Artem Kim, Aleksander Rymyantsev, Oleksiy Lyutakov, Anatoly Popovich, and Maxim Maximov. 2019. "Atomic Layer Deposition of NiO to Produce Active Material for Thin-Film Lithium-Ion Batteries" Coatings 9, no. 5: 301. https://doi.org/10.3390/coatings9050301
APA StyleKoshtyal, Y., Nazarov, D., Ezhov, I., Mitrofanov, I., Kim, A., Rymyantsev, A., Lyutakov, O., Popovich, A., & Maximov, M. (2019). Atomic Layer Deposition of NiO to Produce Active Material for Thin-Film Lithium-Ion Batteries. Coatings, 9(5), 301. https://doi.org/10.3390/coatings9050301







