Preparation of Tung Oil Microcapsules Coated with Chitosan Sodium Tripolyphosphate and Their Effects on Coating Film Properties
Abstract
1. Introduction
2. Test Materials and Methods
2.1. Materials
2.2. Preparation of CST-T Microcapsules
2.3. Preparation of UV Coating Film with Addition of CST-T Microcapsules
2.4. Testing and Characterization
2.4.1. Sample Characterization of Microcapsules
2.4.2. Coating Film Performance Tests
3. Results and Discussion
3.1. Yield and Encapsulation Rate of CST-T Microcapsules
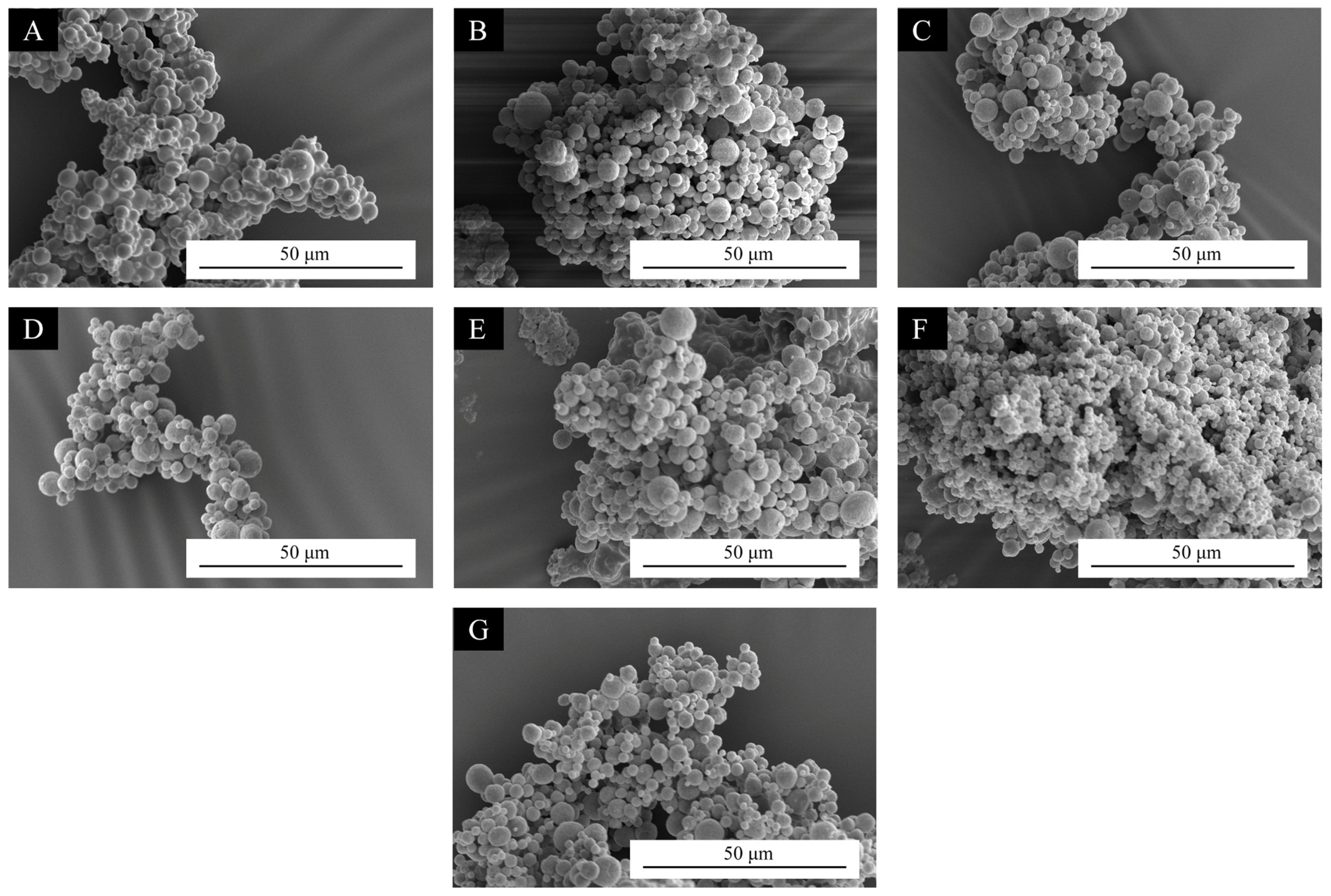
3.2. Morphology and Chemical Composition of CST-T Microcapsules
3.2.1. Morphology of Microcapsules
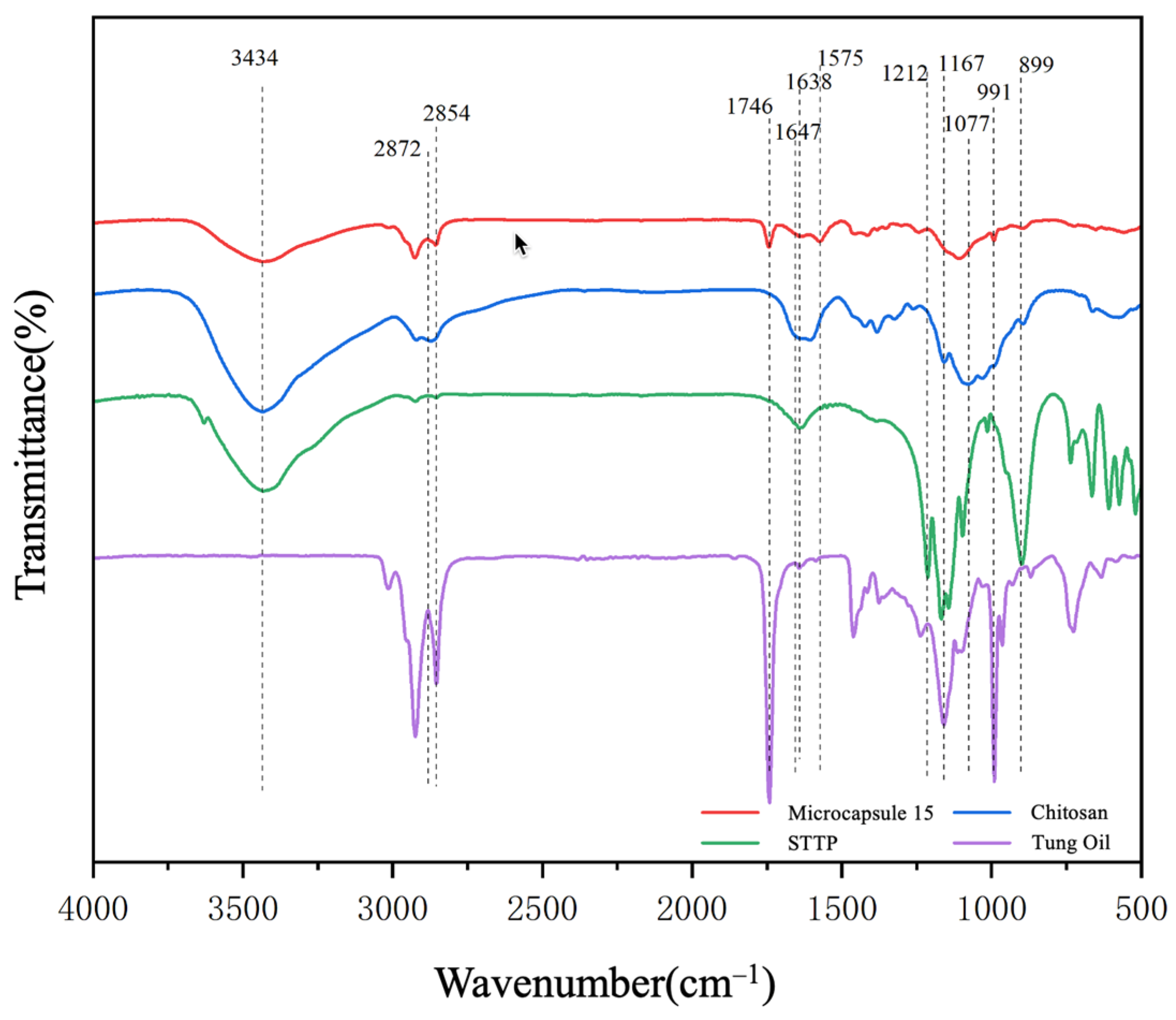
3.2.2. Chemical Composition Analysis of CST-T Microcapsules
3.3. Morphology and Chemical Composition of UV Coating Films with Added CST-T Microcapsules
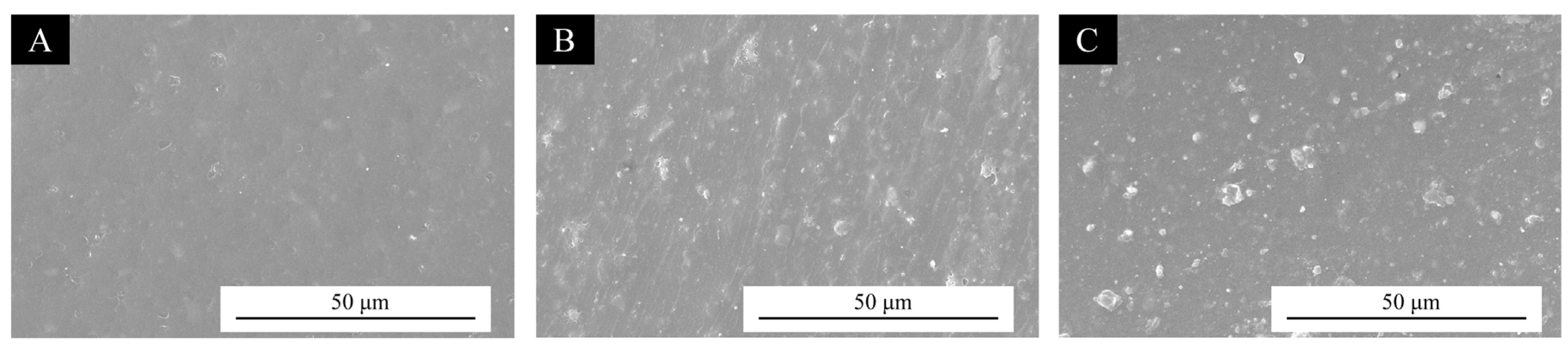
3.3.1. Morphology of UV Coating Film
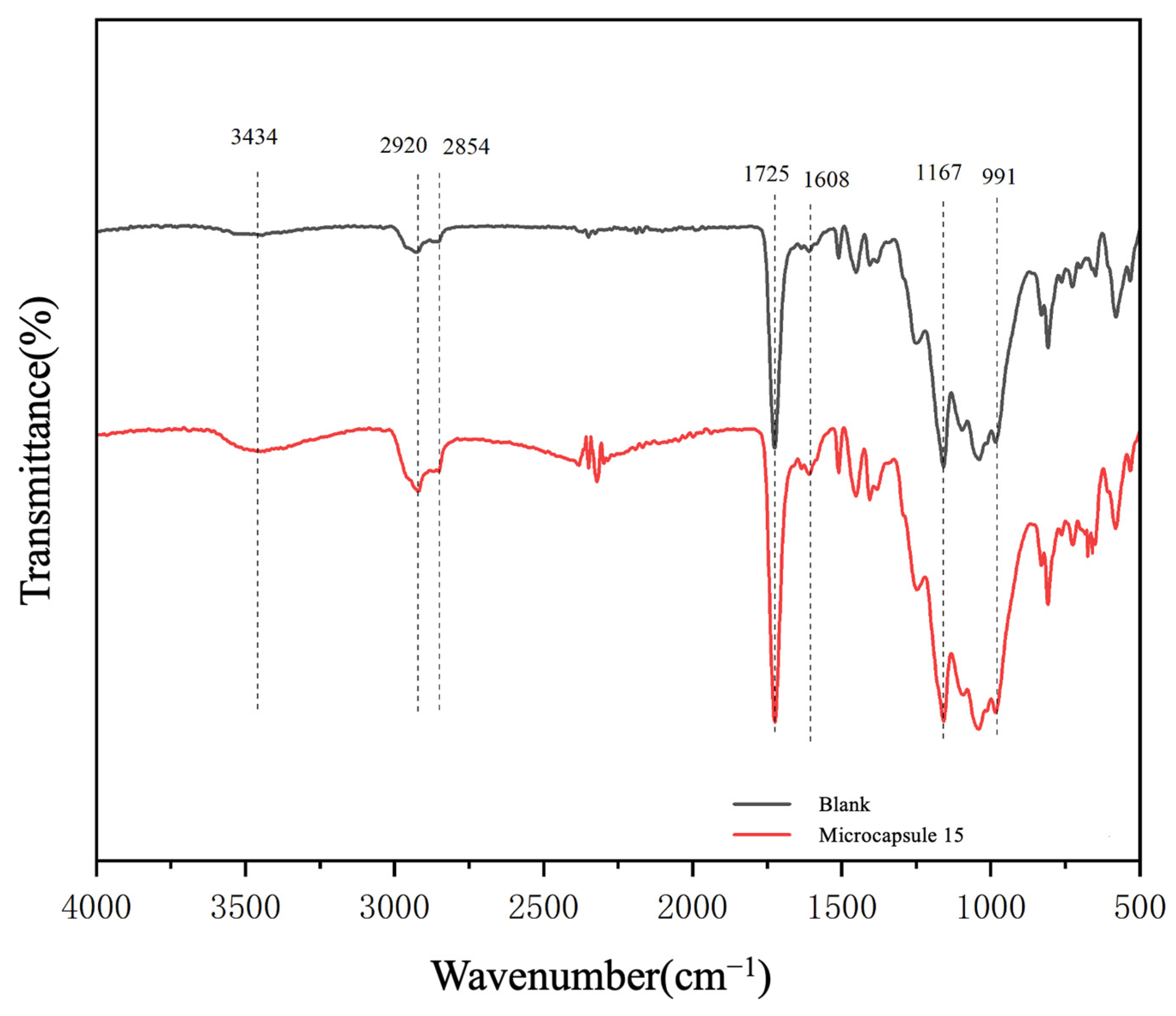
3.3.2. Chemical Composition Analysis of UV Coating Film
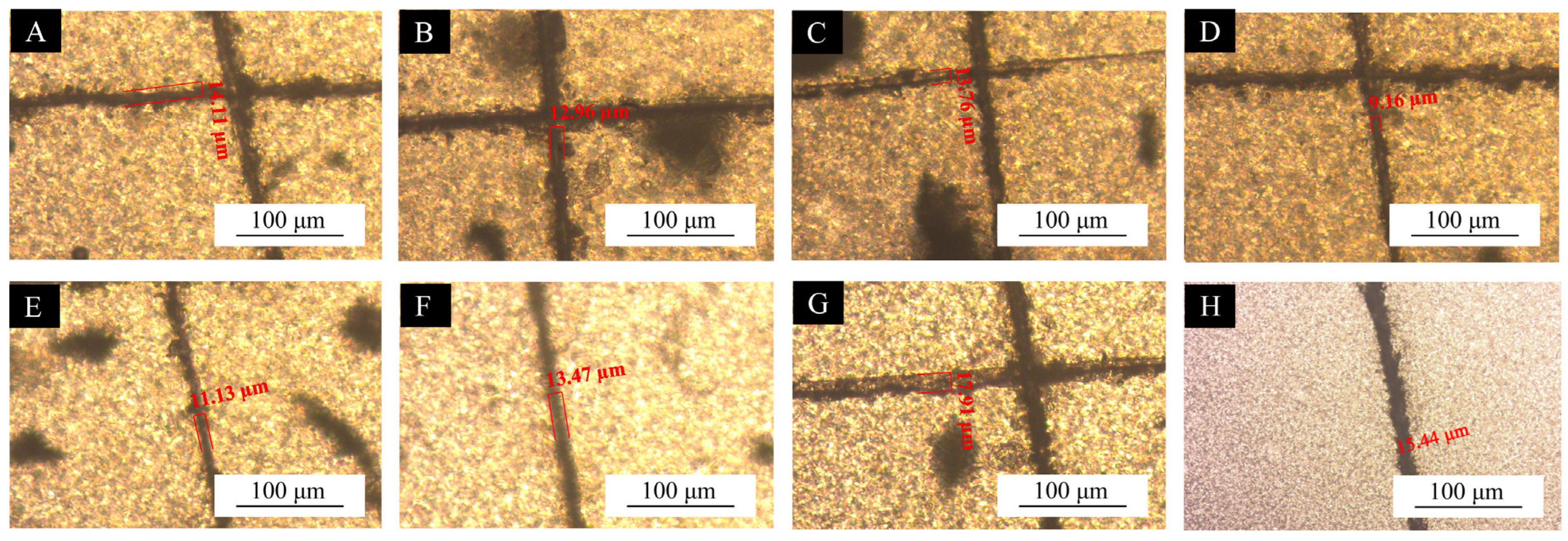
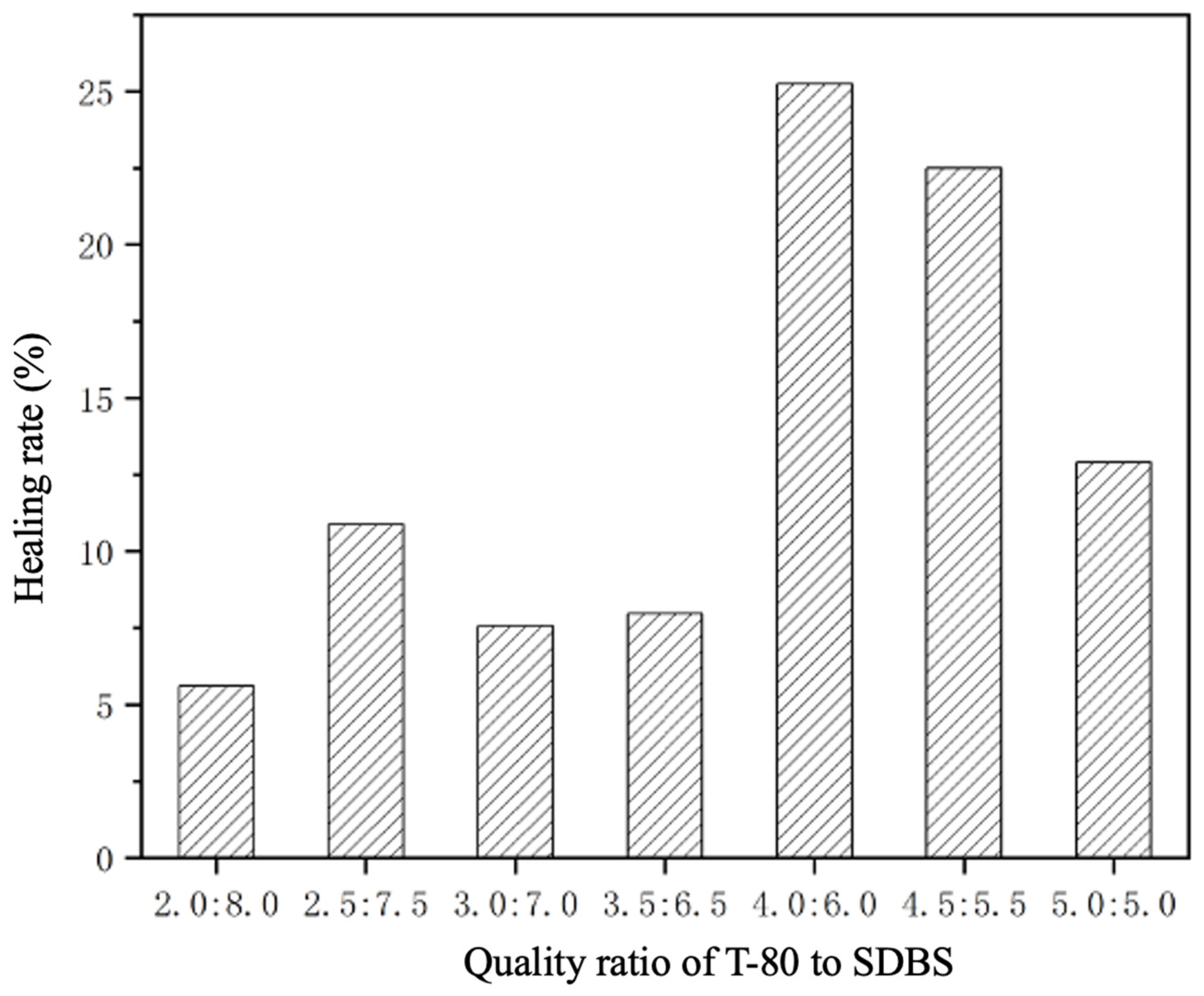
3.4. Self-Healing Properties of UV Coating Films with the Addition of CST-T Microcapsules
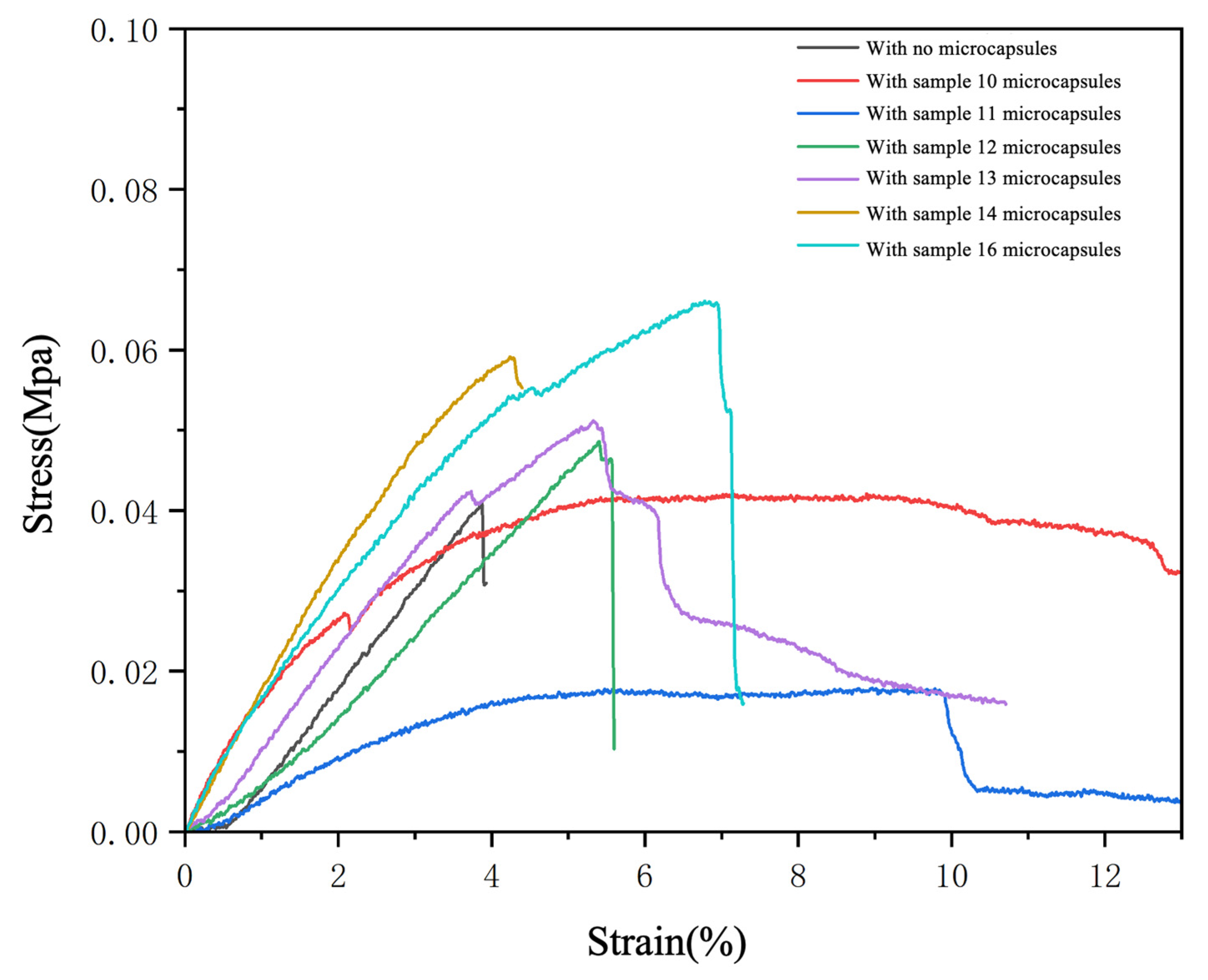
3.5. Mechanical Properties of UV Coating Films with Added CST-T Microcapsules
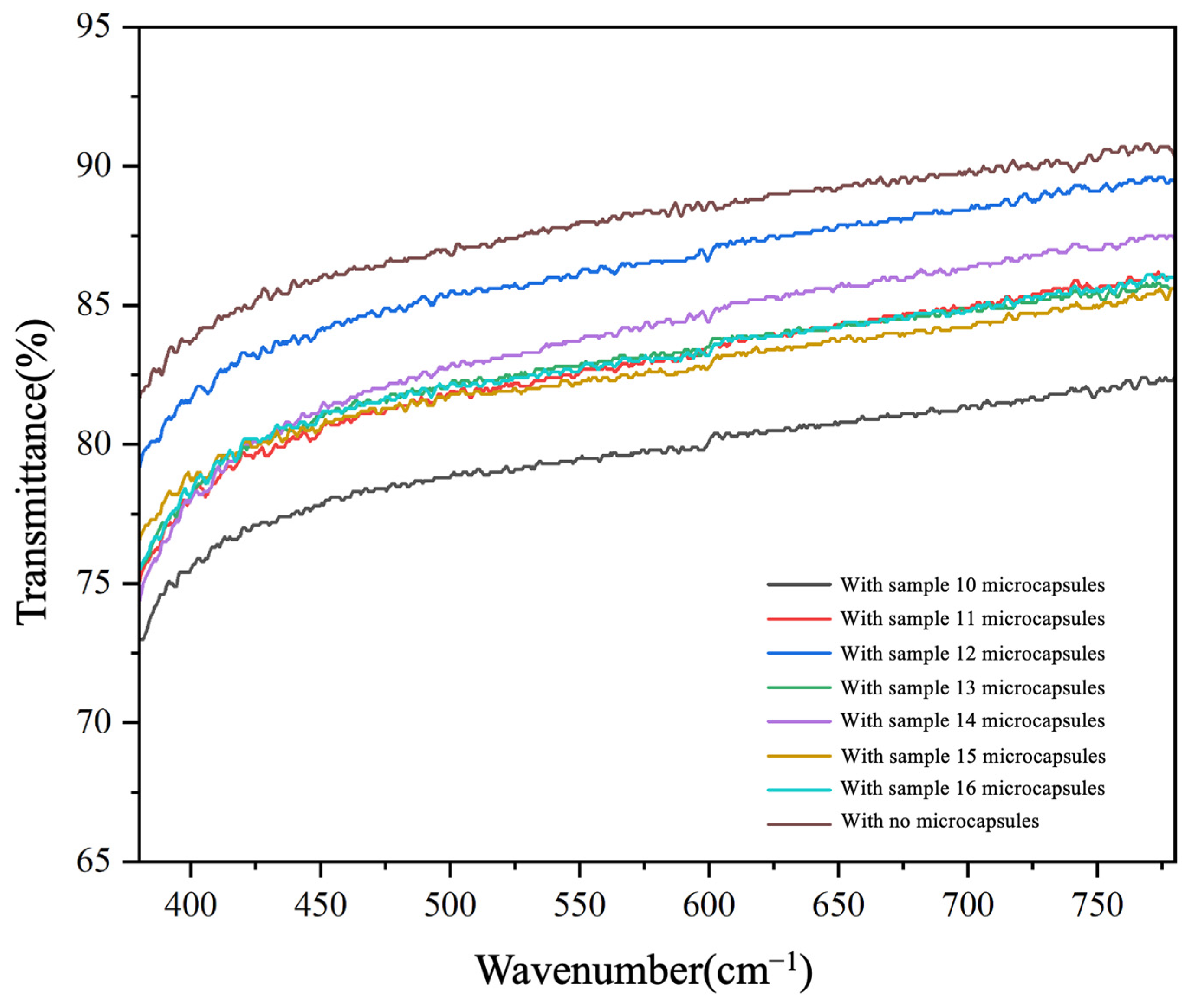
3.6. Optical Properties of UV Coating Films with Added CST-T Microcapsules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, J.; Liu, Y.; Xu, W. Influence of Cell Characteristics on the Construction of Structural Color Layers on Wood Surfaces. Forests 2024, 15, 676. [Google Scholar] [CrossRef]
- Brito, A.F.; Calonego, F.W.; Bond, B.H.; Severo, E. Color Changes, EMC and Biological Resistance of Thermally Modified Yellow Poplar. Wood Fiber Sci. 2018, 50, 439–446. [Google Scholar] [CrossRef]
- Syed, M.R.; Aati, S.; Flematti, G.; Matinlinna, J.P.; Fawzy, A. Development and Characterization of 3D-printed Denture Base Resin Composites Having Self-Healing Potential. Dent. Mater. 2025, 41, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yang, Z.; Shi, N.; Yu, X. Experimental study on effects of the selected load parameters on fatigue life of the mortise-and-tenon furniture joint. Wood Mater. Sci. Eng. 2025, 1–7. [Google Scholar] [CrossRef]
- Hu, W.; Yang, P.; Wang, Y.; Zhang, J. Damage zone analysis and its effect on the elastic constants of beech blocks with different grain orientations under compression. Wood Sci. Technol. 2025, 59, 62. [Google Scholar] [CrossRef]
- Hu, W.-G.; Zhao, Y.; Xu, W.; Liu, Y. Study on withdrawal force capacity of insert nut in wood-based materials used for case furniture. Wood Mater. Sci. Eng. 2024, 1–10. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, X.; Wu, Z. Application and prospect of self-healing microcapsules in surface coating of wood. Colloid Interface Sci. Commun. 2023, 56, 100736. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Nevejans, S.; Reck, B.; Irusta, L.; Sardon, H.; Asua, J.M.; Ballard, N. Healable and self-healing polyu-rethanes using dynamic chemistry. Prog. Polym. Sci. 2021, 114, 101362. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, Y.; Xu, W.; Liu, Y. The Influences of Selected Factors on Bending Moment Capacity of Case Furniture Joints. Appl. Sci. 2024, 14, 10044. [Google Scholar] [CrossRef]
- Yu, R.; Liu, Y.; Konukcu, A.C.; Hu, W. A METHOD OF SIMULATING SEAT LOAD FOR NUMERICAL ANALYSIS OF WOOD CHAIR STRUCTURE. Wood Res. 2024, 69, 432–444. [Google Scholar] [CrossRef]
- Hu, W.-G.; Yu, R.-Z.; Yang, P. Characterizing Roughness of Wooden Mortise and Tenon Considering Effects of Measured Position and Assembly Condition. Forests 2024, 15, 1584. [Google Scholar] [CrossRef]
- Zhang, A.; Li, J.; Fan, H.; Xiang, J.; Wang, L.; Yan, J. Effect of mechanical properties on the self-healing behavior of waterborne polyurethane coatings. J. Appl. Polym. Sci. 2022, 139, 52364. [Google Scholar] [CrossRef]
- Nesterova, T.; Dam-Johansen, K.; Kiil, S. Synthesis of durable microcapsules for self-healing anticorrosive coatings: A comparison of selected methods. Prog. Org. Coatings 2011, 70, 342–352. [Google Scholar] [CrossRef]
- Beach, M.; Davey, T.; Subramanian, P.; Such, G. Self-healing organic coatings–Fundamental chemistry to commercial ap-plication. Prog. Org. Coat. 2023, 183, 107759. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, H.; Liu, H.; Liu, C.; Fang, B.; Li, Y.; Jiang, Z.; Liu, J. Self-healing performance and anti-corrosion mechanism of microcapsule-containing epoxy coatings under deep-sea environment. Prog. Org. Coatings 2025, 203, 109176. [Google Scholar] [CrossRef]
- Yabuki, A.; Nishikawa, C.; Fathona, I.W. Synergistic effect by release of corrosion inhibitors via cellulose nanofibers in self-healing polymer coatings to prevent corrosion of carbon steel. J. Ind. Eng. Chem. 2024, 145, 764–772. [Google Scholar] [CrossRef]
- Wan, J.; Cao, Y.; Li, Y.; He, Y.; Wang, S.; Wang, Y.; Song, D.; Zhang, T.; Liu, J. Robust pH-responsive double-layered self-healing coating with synergistic effects of tannic acid and Ce ions applied on Mg alloy via the phytic acid pretreatment. Prog. Org. Coatings 2025, 203, 109152. [Google Scholar] [CrossRef]
- Zhao, D.; Du, Z.; Liu, S.; Wu, Y.; Guan, T.; Sun, Q.; Sun, N.; Ren, B. UV Light Curable Self-Healing Superamphiphobic Coatings by Photopromoted Disulfide Exchange Reaction. ACS Appl. Polym. Mater. 2019, 1, 2951–2960. [Google Scholar] [CrossRef]
- Xia, Y.; Yan, X. Preparation of UV Topcoat Microcapsules and Their Effect on the Properties of UV Topcoat Paint Film. Polymers 2024, 16, 1410. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.-Z.; Zhang, M.-Q. Effect of molecular weight of PEG soft segments on photo-stimulated self-healing performance of coumarin functionalized polyurethanes. Chin. J. Polym. Sci. 2014, 32, 1286–1297. [Google Scholar] [CrossRef]
- Hu, W.-G.; Luo, M.; Yu, R.; Zhao, Y. Effects of the selected factors on cyclic load performance of T-shaped mortise-and-tenon furniture joints. Wood Mater. Sci. Eng. 2024, 1–10. [Google Scholar] [CrossRef]
- Hu, W.; Yu, R. Study on the strength mechanism of the wooden round-end mortise-and-tenon joint using the digital image correlation method. Holzforschung 2024, 78, 519–530. [Google Scholar] [CrossRef]
- Hu, W.; Fu, W.; Zhao, Y. Optimal Design of the Traditional Chinese Wood Furniture Joint Based on Experimental and Numerical Methods. Wood Res. 2024, 69, 50–59. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Z.; Li, K.; Jia, Y.; Wang, C.; Zhuang, X. Microcapsule-enabled self-healing concrete: A bibliometric analysis. Front. Struct. Civ. Eng. 2023, 17, 1611–1629. [Google Scholar] [CrossRef]
- Jin, C.M.; Sinawang, G.; Osaki, M.; Zheng, Y.T.; Yamaguchi, H.; Harada, A.; Takashima, Y. Self-healing thermoplastic pol-yurethane linked via host-guest interactions. Polymers 2020, 12, 1393. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, W.; Kasal, A.; Erdil, Y.Z. The State of the Art of Biomechanics Applied in Ergonomic Furniture Design. Appl. Sci. 2023, 13, 12120. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, W. Optimizing the interface compatibility of transparent wood for green phase-change thermal storage. Wood Sci. Technol. 2025, 59, 45. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Yue, X.; Xiong, X. Design of furniture mortise-and-tenon joints: A review of mechanical properties and design recommendations. Wood Mater. Sci. Eng. 2025, 1–15. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wang, T.; Chu, Q.; Wang, X. Fused deposition 3D printing of bonsai tree guiding mold based on acrylonitrile-butadiene-styrene copolymer. BioResources 2024, 19, 5839–5846. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Zhang, Y.; Ge, Y.; Wu, R.; Song, X.; Zhang, P.; Wu, J. Mechanical behavior and self-healing mechanism of polyurea-based double-walled microcapsule/epoxy composite films. Prog. Org. Coatings 2021, 157, 106283. [Google Scholar] [CrossRef]
- Lim, Y.-J.; Song, Y.-K.; Kim, D.-M.; Chung, C.-M. Preparation of Isophorone Diisocyanate-loaded Microcapsules and Their Application to Self-healing Protective Coating. Polym. Korea 2015, 39, 56–63. [Google Scholar] [CrossRef]
- Liu, W.; Zou, S.; Pan, J.; Zhang, Z.; Chen, J.; Liu, B.; Yue, Z. Carbon nanotubes/carbon black reinforced epoxy resin microcapsule-based cement composites with combined self-healing and self-sensing capacities. J. Build. Eng. 2025, 103, 112171. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Cui, Y.X.; Zhang, M.J.; Li, M.L.; Li, H.Y. Preparation of tung oil-loaded PU/PANI microcapsules and synergetic anti-corrosion properties of self-healing epoxy coatings. Macromol. Mater. Eng. 2021, 306, 2000581. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.Y.; Zhang, C.Y. Effect of Corrected Extrusion Flow Rate on Wall Thickness Error of MEAM Thin-walled Model. Mater. Plast. 2025, 62, 78–85. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Chen, Y.; Chen, X.; Li, A.; Wang, J.; Shen, D.; Zheng, S. A review of new generation of dental restorative resin composites with antibacterial, remineralizing and self-healing capabilities. Nanoscale Res. Lett. 2024, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Wang, T.; Wang, X.; Chu, Q. Effect of Optimised Infill Parameters on the Tensile Properties of MEX Co-polyester Models. Mater. Plast. 2024, 61, 129–136. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wang, T.; Wang, X. Additive manufacturing of furniture corner guards based on thermoplastic polyurethane filament. BioResources 2025, 20, 5398–5406. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, K.; Sun, G.; Liu, R.; Luo, J. Investigation of particle size effect on the performance of micro/nano capsules and composite coatings. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 675, 132020. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, X. Preparation and Self-Repairing Properties of MF-Coated Shellac Water-Based Microcapsules. Coatings 2020, 10, 778. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, D.; Xu, W. Effect of Paint Process on the Performance of Modified Poplar Wood Antique. Coatings 2021, 11, 1174. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, D.; Xu, W. Influence of the Bottom Color Modification and Material Color Modification Process on the Performance of Modified Poplar. Coatings 2021, 11, 660. [Google Scholar] [CrossRef]
- Xu, W.; Fang, X.; Han, J.; Wu, Z.; Zhang, J. Effect of Coating Thickness on Sound Absorption Property of Four Wood Species Commonly Used for Piano Soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, D.; Xu, W. Effect of Sanding Processes on the Surface Properties of Modified Poplar Coated by Primer Compared with Mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]
- Mi, T.; Mao, J.; Cai, Y.; Luo, S.; Wang, X.; Xiao, M.; Zhu, H.; Yang, K.; Ren, J. Application of Raman spectroscopy for detecting the repairing behaviour of microcapsules in self-healing cementitious system. Constr. Build. Mater. 2023, 387, 131637. [Google Scholar] [CrossRef]
- Cots, A.; Camacho, N.M.; Palma, S.D.; Alustiza, F.; Pedraza, L.; Bonino, F.; Carreño, J.; Bracamonte, C.F.; Acevedo, D.; Bozzo, A.; et al. Chitosan-alginate microcapsules: A strategy for improving stability and antibacterial potential of bovine Lactoferrin. Int. J. Biol. Macromol. 2025, 307, 141870. [Google Scholar] [CrossRef]
- Yan, X.X.; Tao, Y.; Chang, Y.J. Effect of Shellac Waterborne Coating Microcapsules on the Optical, Mechanical and Self-Healing Properties of Waterborne Primer on Tilia europaea L. Wood. Coatings 2021, 11, 785. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Shehzad, H.; Farooqi, Z.H.; Sharif, A.; Ahmed, E.; Habiba, U.; Qaisar, F.; Fatima, N.-E.; Begum, R.; et al. Innovative free radical induced synthesis of WO3-doped diethyl malonate grafted chitosan encapsulated with phosphorylated alginate matrix for UO22+ adsorption: Parameters optimisation through response surface methodology. Sep. Purif. Technol. 2024, 353, 128455. [Google Scholar] [CrossRef]
- Baiocco, D.; Preece, J.A.; Zhang, Z. Encapsulation of hexylsalicylate in an animal-free chitosan-gum Arabic shell by complex coacervation. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 625, 126861. [Google Scholar] [CrossRef]
- Qi, L.F.; Xu, Z.R.; Jiang, X.; Hu, C.H.; Zou, X.F. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Ngan, L.T.K.; Wang, S.-L.; Hiep, Đ.M.; Luong, P.M.; Vui, N.T.; Đinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Liu, J.; Chen, F.; Zhang, Q.; Xing, X.; Cui, G. Study on Preparation and Performance of Acid pH-Responsive Intelligent Self-Healing Coating. Polymers 2024, 16, 2473. [Google Scholar] [CrossRef]
- Lv, L.T.; Wang, Y.T.; Dai, J.; Yang, Z.Y.; Yang, Q.L. The study on preparation, stability and anti-allergic efficacy of phy-cocyanin microcapsules. Food Res. Int. 2025, 201, 115530. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Q.; Li, Z.; Han, Y.; Song, Y.; Yang, S.; Song, X.; Qi, T.; Möhwald, H.; Shchukin, D.; et al. Adaptive Polymeric Coatings with Self-Reporting and Self-Healing Dual Functions from Porous Core–Shell Nanostructures. Macromol. Mater. Eng. 2018, 303, 1700616. [Google Scholar] [CrossRef]
- Li, H.; Cui, Y.; Li, Z.; Zhu, Y.; Wang, H. Fabrication of microcapsules containing dual-functional tung oil and properties suitable for self-healing and self-lubricating coatings. Prog. Org. Coatings 2018, 115, 164–171. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending Viability of Bifidobacterium longum in Chitosan-Coated Alginate Microcapsules Using Emulsification and Internal Gelation Encapsulation Technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef]
- Varankovich, N.; Martinez, M.F.; Nickerson, M.T.; Korber, D.R. Survival of probiotics in pea protein-alginate microcapsules with or without chitosan coating during storage and in a simulated gastrointestinal environment. Food Sci. Biotechnol. 2017, 26, 189–194. [Google Scholar] [CrossRef]
- GB/T 4893.6-2013; Physical and Chemical Property Test of Furniture Surface Paint Film Part 6 Gloss Determination Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Paolini, N.A.; Neto, A.G.C.; Pellanda, A.C.; Jorge, A.R.d.C.; Soares, B.d.B.; Floriano, J.B.; Berton, M.A.C.; P, P.V.; Thomas, S.; Li, Z. Evaluation of Corrosion Protection of Self-Healing Coatings Containing Tung and Copaiba Oil Microcapsules. Int. J. Polym. Sci. 2021, 2021, 6650499. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, H.-S.; Kim, J.-W.; Lee, E.-Y.; Rhee, M.; You, Y.-H.; Khang, G.; Park, C.-G.; Yoon, K.-H. Suppression of Fibrotic Reactions of Chitosan-Alginate Microcapsules Containing Porcine Islets by Dexamethasone Surface Coating. Endocrinol. Metab. 2021, 36, 146–156. [Google Scholar] [CrossRef]
- Li, P.H.; Lu, Z.; Ma, K.X.; Zou, G.F.; Chang, L.; Guo, W.C.; Tian, K.S.; Li, X.A.; Wang, H.Y. UV-triggered self-healing SiO2/PDA hybrid microcapsules with both enhanced UV-shielding ability and improved compatibility for epoxy resin coating. Prog. Org. Coat. 2022, 163, 106636. [Google Scholar] [CrossRef]
- Cong, Y.; Chen, K.; Zhou, S.; Wu, L. Synthesis of pH and UV dual-responsive microcapsules with high loading capacity and their application in self-healing hydrophobic coatings. J. Mater. Chem. A 2015, 3, 19093–19099. [Google Scholar] [CrossRef]


| Name | Molecular Formula | CAS No. | Manufacturer |
|---|---|---|---|
| Chitosan | (C6H11NO4)n | 9012-76-4 | Sinopharm Chemical Reagent Co., Ltd., Beijing, China |
| Gum Arabic Powder | C12H36 | 9000-01-5 | Tianjin Zhonglian Chemical Reagent Co., Ltd., Tianjin, China |
| Acetic Acid (CH3COOH) | C2H4O2 | 64-19-7 | Sinopharm Chemical Reagent Co., Ltd. Beijing, China |
| Tung Oil | C65H100O14 | - | Shanghai Shenmeng Home Furnishing Co., Ltd., Shanghai, China |
| Tannic Acid | C76H52O46 | 1401-55-4 | Tianjin Zhonglian Chemical Reagent Co., Ltd., Tianjin, China |
| Polyoxyethylene Sorbitan Monooleate | C24H44O6 | 9005-65-6 | Tianjin Beichen Fangzheng Reagent Co., Ltd., Tianjin, China |
| Sodium Dodecylbenzene Sulfonate | CH3(CH2)11C6H4SO3Na | 25155-30-0 | Tianjin Zhiyuan Chemical Reagent Co., Ltd., Tianjin, China |
| Sodium Tripolyphosphate (SDPP) | Na5P3O10 | 7758-29-4 | Sinopharm Chemical Reagent Co., Ltd., Beijing, China |
| Name | Model Number | Manufacturer |
|---|---|---|
| Collector-type constant-temperature heating magnetic stirrer | DF-101Z | Shanghai Yixin Scientific Instrument Co., Ltd., Shanghai, China |
| Ultrasonic emulsifying disperser | BILONG-500 | Shanghai Biron Instrument Co., Ltd., Shanghai, China |
| Small spray-dryer | JA-PWGZ100 | Shenyang Jing’ao Instrument Technology Co., Ltd., Shenyang, China |
| Circulating-water vacuum pumps | SHZ-D | Shanghai Sematic Instrument Co., Ltd., Shanghai, China |
| Air-drying oven | DHG-9240A | Shanghai Aojin Instrument Manufacturing Co., Ltd., Shanghai, China |
| UV-curing machine | 620# | Huzhou Tongxu Machinery Equipment Co., Ltd., Huzhou, China |
| Optical microscope | AX-10 | Carl Zeiss Co., Ltd., Baden-Württemberg, Germany |
| Fourier infrared spectrometer | VERTEX 80V | Bruker Technology Co., Ltd., Hamburg, Germany |
| Scanning electron microscope | QUANTA-200 | Thermo Fisher Scientific Co., Ltd., Waltham, MA, USA |
| Roughness tester | J8-4C | Shanghai Tai Ming Optical Instrument Co., Ltd., Shanghai, China |
| Gloss meter | HG268 | Shenzhen SUNSHI Technology Co., Ltd., Shenzhen, China |
| Spectrocolorimeter | CR7 | Shenzhen SUNSHI Technology Co., Ltd., Shenzhen, China |
| Universal mechanical testing machine | AGS-X | Shimadzu Manufacturing, Kyoto, Japan |
| Ultraviolet spectrophotometer | U-3900 | Hitachi Instrument Co., Ltd., Suzhou, China |
| Level | Factor A Core-to-Wall Ratio | Factor B Emulsifier Compounding Ratio (T-80:SDBS) | Factor C Concentration of Crosslinker STPP (%) | Factor D Spray-Drying Temperature (°C) |
|---|---|---|---|---|
| 1 | 1.0:1.0 | 0.5:9.5 | 2 | 125 |
| 2 | 2.0:1.0 | 2.0:8.0 | 3 | 130 |
| 3 | 3.0:1.0 | 3.5:6.5 | 4 | 135 |
| Samples | Core-to-Wall Ratio | Emulsifier Compounding Ratio (T-80:SDBS) | Concentration of Crosslinking Agent STPP (%) | Spray-Drying Temperature (°C) |
|---|---|---|---|---|
| 1 | 1.0:1.0 | 0.5:9.5 | 2 | 125 |
| 2 | 1.0:1.0 | 2.0:8.0 | 3 | 130 |
| 3 | 1.0:1.0 | 3.5:6.5 | 4 | 135 |
| 4 | 2.0:1.0 | 0.5:9.5 | 3 | 135 |
| 5 | 2.0:1.0 | 2.0:8.0 | 4 | 125 |
| 6 | 2.0:1.0 | 3.5:6.5 | 2 | 130 |
| 7 | 3.0:1.0 | 0.5:9.5 | 4 | 130 |
| 8 | 3.0:1.0 | 2.0:8.0 | 2 | 135 |
| 9 | 3.0:1.0 | 3.5:6.5 | 3 | 125 |
| Type of Test | Samples | Chitosan (g) | 1% Acetic Acid (g) | Tung Oil (g) | T-80 (g) | SDBS (g) | Pure Water for Emulsification (g) | STPP (g) | Pure Water for STPP (g) |
|---|---|---|---|---|---|---|---|---|---|
| Orthogonal test | 1 | 0.800 | 79.200 | 0.800 | 0.119 | 2.261 | 76.820 | 0.200 | 9.800 |
| 2 | 0.800 | 79.200 | 0.800 | 0.476 | 1.904 | 76.820 | 0.300 | 9.700 | |
| 3 | 0.800 | 79.200 | 0.800 | 0.833 | 1.547 | 76.820 | 0.400 | 9.600 | |
| 4 | 0.800 | 79.200 | 1.600 | 0.117 | 2.233 | 76.050 | 0.300 | 9.700 | |
| 5 | 0.800 | 79.200 | 1.600 | 0.470 | 1.880 | 76.050 | 0.400 | 9.600 | |
| 6 | 0.800 | 79.200 | 1.600 | 0.823 | 1.528 | 76.050 | 0.200 | 9.800 | |
| 7 | 0.800 | 79.200 | 2.400 | 0.116 | 2.214 | 75.270 | 0.400 | 9.600 | |
| 8 | 0.800 | 79.200 | 2.400 | 0.466 | 1.864 | 75.270 | 0.200 | 9.800 | |
| 9 | 0.800 | 79.200 | 2.400 | 0.816 | 1.516 | 75.270 | 0.300 | 9.700 | |
| One-way test | 10 | 0.800 | 79.200 | 1.600 | 0.470 | 1.880 | 76.050 | 0.400 | 9.600 |
| 11 | 0.800 | 79.200 | 1.600 | 0.587 | 1.763 | 76.050 | 0.400 | 9.600 | |
| 12 | 0.800 | 79.200 | 1.600 | 0.705 | 1.645 | 76.050 | 0.400 | 9.600 | |
| 13 | 0.800 | 79.200 | 1.600 | 0.823 | 1.528 | 76.050 | 0.400 | 9.600 | |
| 14 | 0.800 | 79.200 | 1.600 | 0.940 | 1.410 | 76.050 | 0.400 | 9.600 | |
| 15 | 0.800 | 79.200 | 1.600 | 1.058 | 1.292 | 76.050 | 0.400 | 9.600 | |
| 16 | 0.800 | 79.200 | 1.600 | 1.175 | 1.175 | 76.050 | 0.400 | 9.600 |
| Samples | Microencapsulated Mass (g) | UV Topcoat Mass (g) |
|---|---|---|
| 10 | 0.05 | 0.95 |
| 11 | 0.05 | 0.95 |
| 12 | 0.05 | 0.95 |
| 13 | 0.05 | 0.95 |
| 14 | 0.05 | 0.95 |
| 15 | 0.05 | 0.95 |
| 16 | 0.05 | 0.95 |
| Samples | Factor A Core-to-Wall Ratio | Factor B Emulsifier Compounding Ratio (T-80:SDBS) | Factor C STPP Concentration (%) | Factor D Spray-Drying Temperature (°C) | Coating Rate (%) |
|---|---|---|---|---|---|
| 1 | 1.0:1.0 | 0.5:9.5 | 2 | 125 | 42.00 |
| 2 | 1.0:1.0 | 2.0:8.0 | 3 | 130 | 50.00 |
| 3 | 1.0:1.0 | 3.5:6.5 | 4 | 135 | 52.00 |
| 4 | 2.0:1.0 | 0.5:9.5 | 3 | 135 | 50.00 |
| 5 | 2.0:1.0 | 2.0:8.0 | 4 | 125 | 68.00 |
| 6 | 2.0:1.0 | 3.5:6.5 | 2 | 130 | 62.00 |
| 7 | 3.0:1.0 | 0.5:9.5 | 4 | 130 | 48.00 |
| 8 | 3.0:1.0 | 2.0:8.0 | 2 | 135 | 58.00 |
| 9 | 3.0:1.0 | 3.5:6.5 | 3 | 125 | 68.00 |
| Mean value 1 | 48.00 | 46.67 | 54.00 | 59.33 | |
| Average value 2 | 60.00 | 58.67 | 56.00 | 53.33 | |
| Average value 3 | 58.00 | 60.67 | 56.00 | 53.33 | |
| range | 12.00 | 14.00 | 2.00 | 6.00 | |
| Factor priority level | B > A > D > C | ||||
| Optimal preparation program | A2 B3 C3 D1 | ||||
| Considerations | Square Sum (e.g., Equation of Squares) | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Factor A Core-to-wall ratio | 496.000 | 2 | 248.000 | 11.160 | 0.004 ** |
| Factor B Emulsifier compounding ratio | 688.000 | 2 | 344.000 | 15.480 | 0.001 ** |
| Factor C Crosslinker concentration | 16.000 | 2 | 8.000 | 0.360 | 0.707 |
| Factor D Drying Temperature | 144.000 | 2 | 72.000 | 3.240 | 0.087 |
| Inaccuracies | 200.000 | 9 | 22.222 |
| Samples | Factor A Core-to-Wall Ratio | Factor B Emulsifier Compounding Ratio (T-80:SDBS) | Factor C Concentration of Crosslinker STPP (%) | Factor D Spray-Drying Temperature (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 1.0:1.0 | 0.5:9.5 | 2 | 125 | 37.32 |
| 2 | 1.0:1.0 | 2.0:8.0 | 3 | 130 | 32.48 |
| 3 | 1.0:1.0 | 3.5:6.5 | 4 | 135 | 41.94 |
| 4 | 2.0:1.0 | 0.5:9.5 | 3 | 135 | 32.87 |
| 5 | 2.0:1.0 | 2.0:8.0 | 4 | 125 | 50.68 |
| 6 | 2.0:1.0 | 3.5:6.5 | 2 | 130 | 37.98 |
| 7 | 3.0:1.0 | 0.5:9.5 | 4 | 130 | 27.49 |
| 8 | 3.0:1.0 | 2.0:8.0 | 2 | 135 | 35.08 |
| 9 | 3.0:1.0 | 3.5:6.5 | 3 | 125 | 25.09 |
| k1 | 37.25 | 32.56 | 36.79 | 37.70 | |
| k2 | 40.51 | 39.41 | 30.15 | 32.65 | |
| k3 | 29.22 | 35.00 | 40.04 | 36.63 | |
| range | 11.29 | 6.85 | 9.89 | 5.05 | |
| Factor priority level | A > C > B > D | ||||
| Optimal preparation program | A2 B2 C3 D1 | ||||
| Considerations | Square Sum (e.g., Equation of Squares) | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Factor A: Core-to-wall ratio | 405.082 | 2 | 202.541 | 18.323 | 0.001 ** |
| Factor B: Emulsifier compounding ratio | 144.772 | 2 | 72.386 | 6.548 | 0.018 * |
| Factor C: Crosslinker STPP concentration | 305.019 | 2 | 152.509 | 13.797 | 0.002 ** |
| Factor D: Drying temperature | 84.894 | 2 | 42.447 | 3.840 | 0.062 |
| inaccuracies | 99.486 | 9 | 11.054 |
| Samples | T-80-to-SDBS Ratio | HLB Value of Emulsifiers | Yield (%) | Coating Rate (%) |
|---|---|---|---|---|
| 10 | 2.0:8.0 | 11.510 | 40.39 | 49.50 |
| 11 | 2.5:7.5 | 11.728 | 40.97 | 60.00 |
| 12 | 3.0:7.0 | 11.947 | 44.08 | 65.00 |
| 13 | 3.5:6.5 | 12.170 | 40.77 | 55.00 |
| 14 | 4.0:6.0 | 12.383 | 42.91 | 68.50 |
| 15 | 4.5:5.5 | 12.601 | 35.73 | 69.25 |
| 16 | 5.0:5.0 | 12.819 | 34.95 | 63.50 |
| Samples | T-80-to-SDBS Ratio | Scratch Width Before Repair (μm) | Scratch Width After Repair (μm) | Healing Rate (%) |
|---|---|---|---|---|
| UV topcoats without added microcapsules | - | 15.44 | 15.44 | - |
| MC-UV10 | 2.0:8.0 | 14.11 | 13.32 | 5.60 |
| MC-UV11 | 2.5:7.5 | 12.96 | 11.55 | 10.88 |
| MC-UV12 | 3.0:7.0 | 13.76 | 12.72 | 7.56 |
| MC-UV13 | 3.5:6.5 | 9.16 | 8.43 | 7.97 |
| MC-UV14 | 4.0:6.0 | 11.13 | 8.32 | 25.25 |
| MC-UV15 | 4.5:5.5 | 13.47 | 10.44 | 22.49 |
| MC-UV16 | 5.0:5.0 | 17.91 | 15.6 | 12.90 |
| Samples | Roughness (μm) | Elongation at Break (%) |
|---|---|---|
| UV topcoats without added microcapsules | 0.31 | 4.43 |
| MC-UV10 | 0.88 | 8.89 |
| MC-UV11 | 0.76 | 9.31 |
| MC-UV12 | 0.58 | 5.40 |
| MC-UV13 | 0.54 | 5.32 |
| MC-UV14 | 0.66 | 5.17 |
| MC-UV15 | 0.54 | 6.92 |
| MC-UV16 | 0.74 | 6.78 |
| Samples | Visible Light Transmittance (%) |
|---|---|
| With no microcapsules | 87.90 |
| With sample 10 microcapsules | 79.56 |
| With sample 11 microcapsules | 82.74 |
| With sample 12 microcapsules | 86.31 |
| With sample 13 microcapsules | 82.87 |
| With sample 14 microcapsules | 83.81 |
| With sample 15 microcapsules | 82.50 |
| With sample 16 microcapsules | 82.88 |
| Samples | Glossiness (GU) | Loss of Light (%) | ||
|---|---|---|---|---|
| Angle of Incidence of Light Source: 20° | Angle of Incidence of Light Source: 60° | Angle of Incidence of Light Source: 85° | ||
| No microcapsules | 12.00 | 49.30 | 56.40 | - |
| Sample 10 microcapsules | 7.80 | 32.90 | 25.20 | 33.27 |
| Sample 11 microcapsules | 7.20 | 30.00 | 20.20 | 39.15 |
| Sample 12 microcapsules | 4.90 | 29.70 | 21.20 | 39.76 |
| Sample 13 microcapsules | 7.70 | 32.00 | 33.20 | 35.09 |
| Sample 14 microcapsules | 8.20 | 34.50 | 39.50 | 30.02 |
| Sample 15 microcapsules | 5.30 | 31.80 | 28.00 | 35.50 |
| Sample 16 microcapsules | 5.40 | 30.70 | 27.60 | 37.73 |
| Samples | Colorimetric Value | Color Difference Value ΔE | ||
|---|---|---|---|---|
| Brightness Value L | Red and Green Value a | Yellow and Blue Value b | ||
| No microcapsules | 76.93 | −2.24 | 5.08 | - |
| Sample 10 microcapsules | 74.10 | −1.62 | 6.25 | 3.12 |
| Sample 11 microcapsules | 73.32 | −2.17 | 5.65 | 3.66 |
| Sample 12 microcapsules | 75.37 | −2.00 | 5.54 | 1.64 |
| Sample 13 microcapsules | 74.76 | −2.03 | 5.70 | 2.27 |
| Sample 14 microcapsules | 74.82 | −2.21 | 5.38 | 2.14 |
| Sample 15 microcapsules | 74.90 | −2.04 | 5.77 | 2.16 |
| Sample 16 microcapsules | 73.43 | −2.17 | 5.80 | 3.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Deng, J.; Yan, X. Preparation of Tung Oil Microcapsules Coated with Chitosan Sodium Tripolyphosphate and Their Effects on Coating Film Properties. Coatings 2025, 15, 867. https://doi.org/10.3390/coatings15080867
Dong Y, Deng J, Yan X. Preparation of Tung Oil Microcapsules Coated with Chitosan Sodium Tripolyphosphate and Their Effects on Coating Film Properties. Coatings. 2025; 15(8):867. https://doi.org/10.3390/coatings15080867
Chicago/Turabian StyleDong, Yang, Jinzhe Deng, and Xiaoxing Yan. 2025. "Preparation of Tung Oil Microcapsules Coated with Chitosan Sodium Tripolyphosphate and Their Effects on Coating Film Properties" Coatings 15, no. 8: 867. https://doi.org/10.3390/coatings15080867
APA StyleDong, Y., Deng, J., & Yan, X. (2025). Preparation of Tung Oil Microcapsules Coated with Chitosan Sodium Tripolyphosphate and Their Effects on Coating Film Properties. Coatings, 15(8), 867. https://doi.org/10.3390/coatings15080867




