Preparation of Transparent MTMS/BNNS Composite Siloxane Coatings with Anti-Biofouling Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anti-Biofouling Coatings Preparation
2.3. Characterization
2.4. Adhesion and Chemical Stability Test
2.5. Anti-Biofouling Test
2.6. Zeta Potential Measurement
3. Results and Discussion
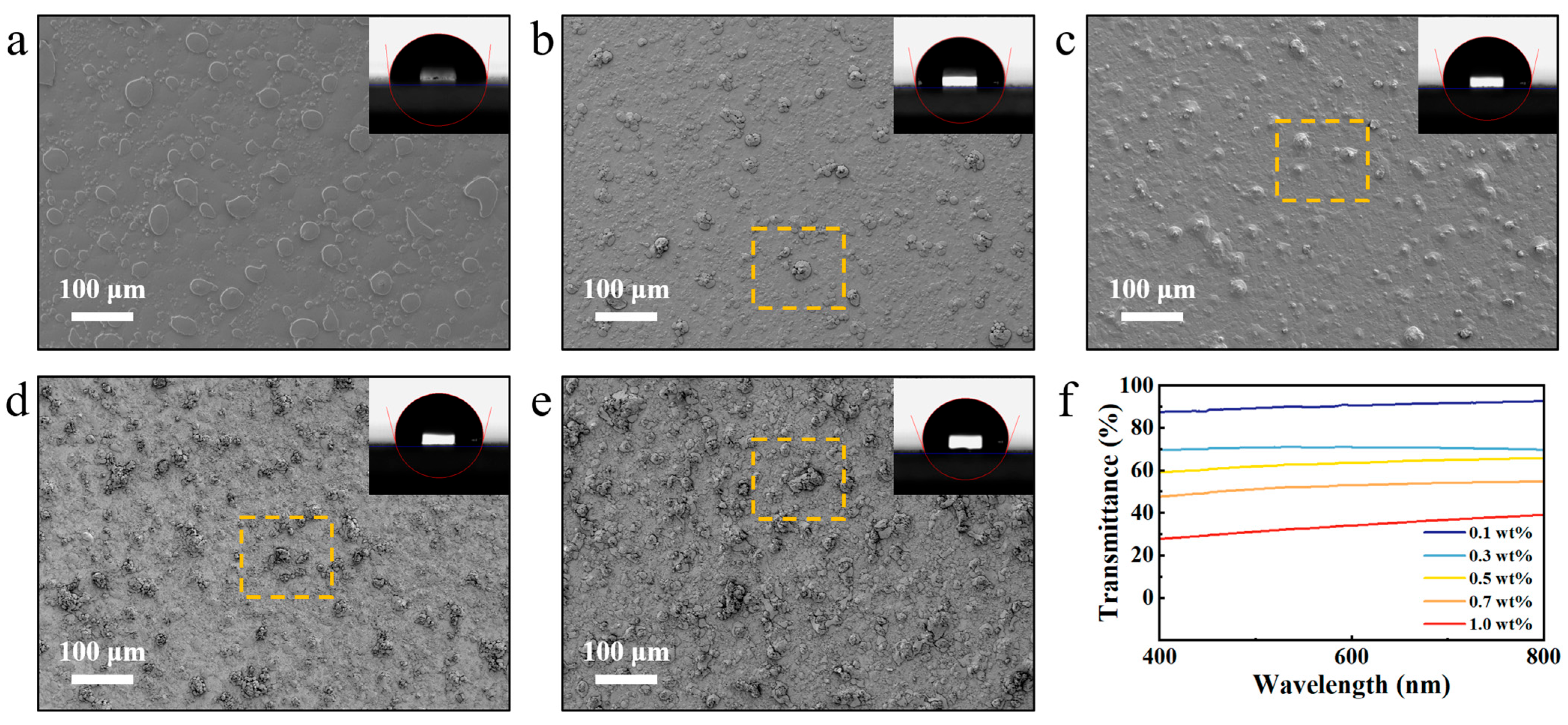
3.1. Effect of BNNS Loading on Transparency and Hydrophobicity of Coatings
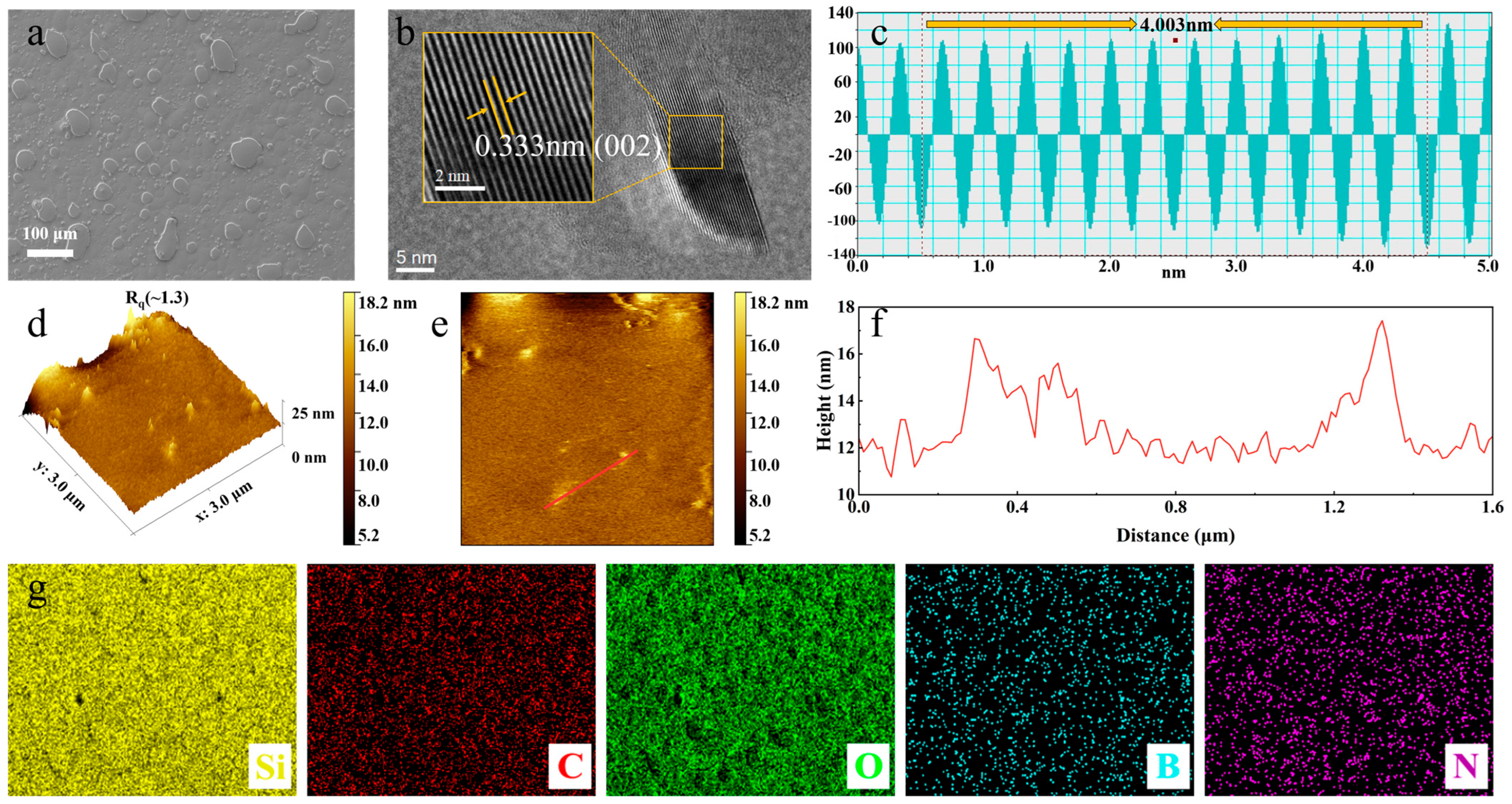
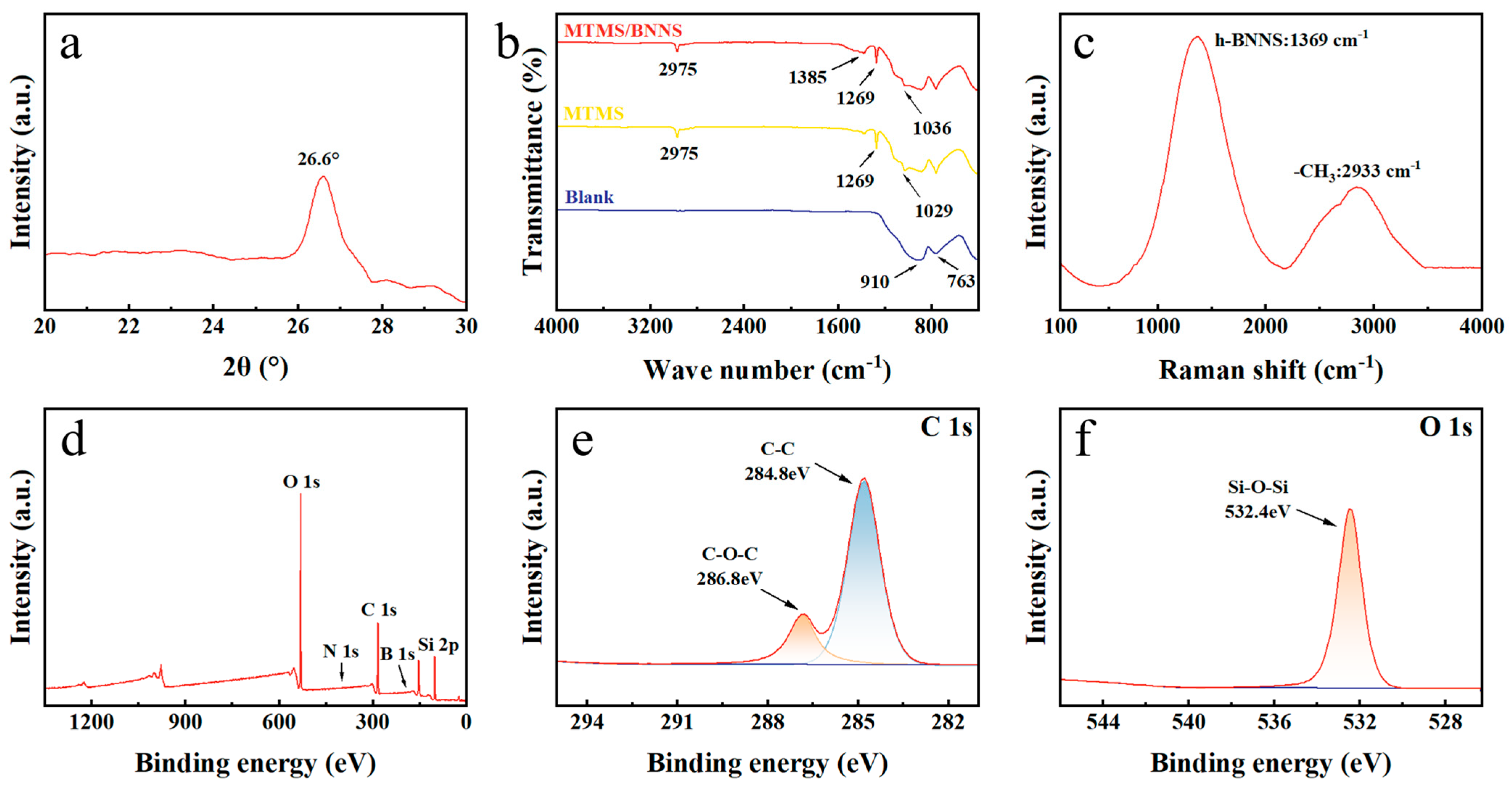
3.2. Surface Morphology and Chemical Composition
3.3. Adhesion Strength and Chemical Stability
3.4. Self-Cleaning Performance
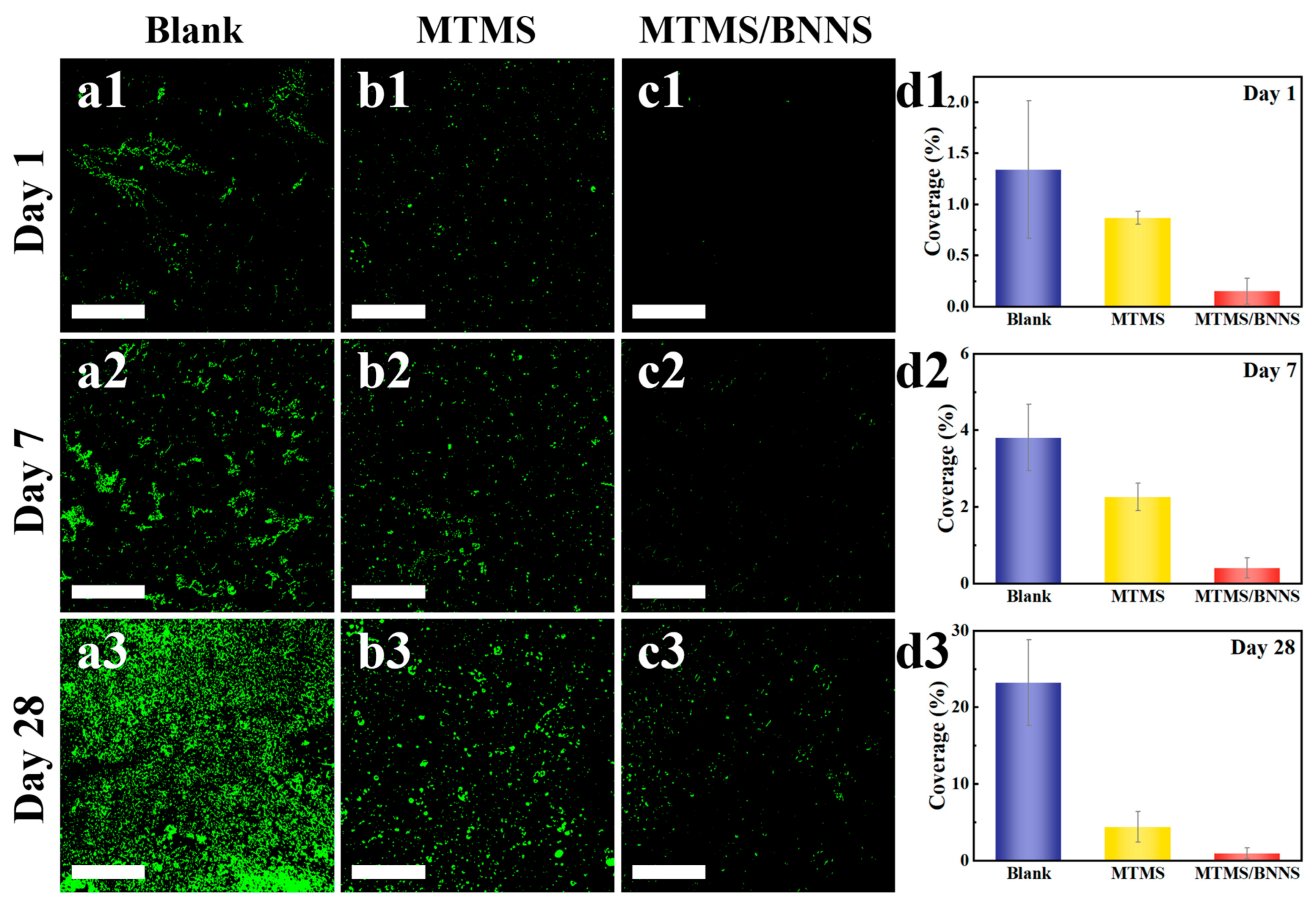
3.5. Biofouling Resistance
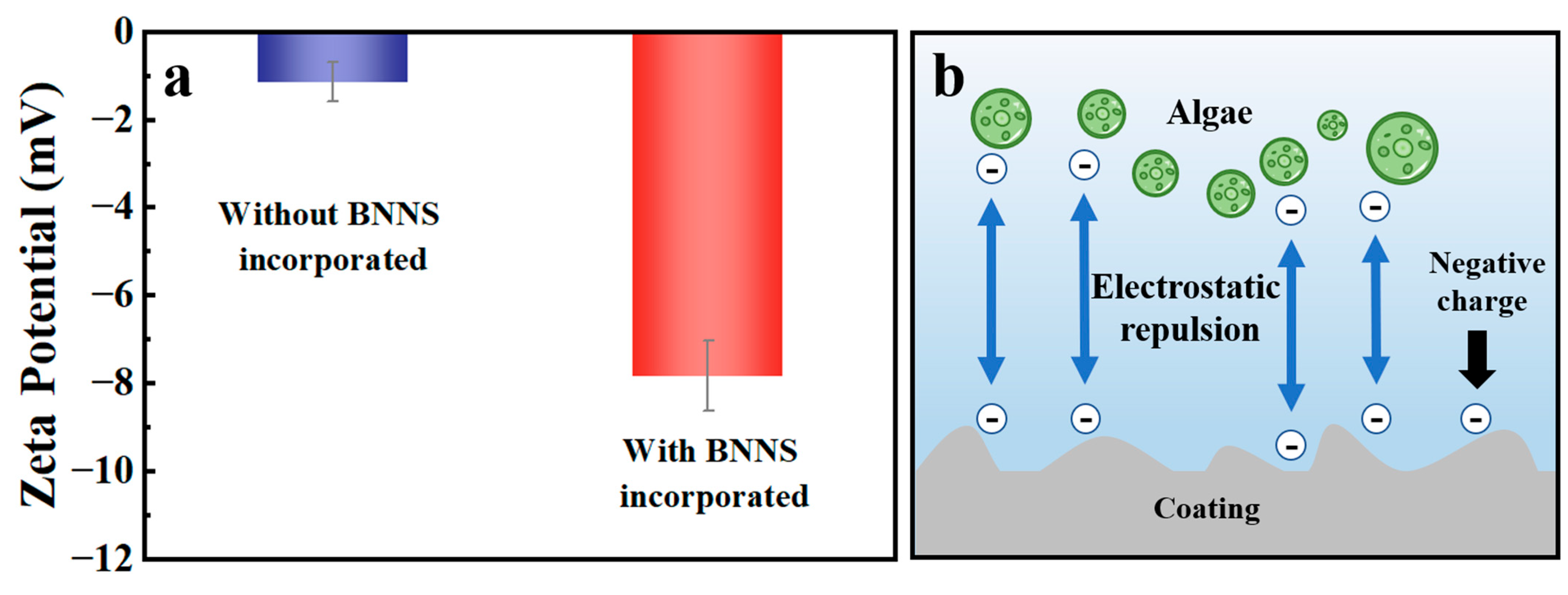
3.6. Anti-Biofouling Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cazzaniga, R.; Rosa-Clot, M. The Booming of Floating PV. Sol. Energy 2021, 219, 3–10. [Google Scholar] [CrossRef]
- Kumar, M.; Mohammed Niyaz, H.; Gupta, R. Challenges and Opportunities towards the Development of Floating Photovoltaic Systems. Sol. Energy Mater. Sol. Cells 2021, 233, 111408. [Google Scholar] [CrossRef]
- Micheli, L.; Talavera, D.L.; Marco Tina, G.; Almonacid, F.; Fernández, E.F. Techno-Economic Potential and Perspectives of Floating Photovoltaics in Europe. Sol. Energy 2022, 243, 203–214. [Google Scholar] [CrossRef]
- Wen, Y.; Lin, P. Offshore Solar Photovoltaic Potential in the Seas around China. Appl. Energy 2024, 376, 124279. [Google Scholar] [CrossRef]
- Sahu, A.; Yadav, N.; Sudhakar, K. Floating Photovoltaic Power Plant: A Review. Renew. Sustain. Energy Rev. 2016, 66, 815–824. [Google Scholar] [CrossRef]
- Djalab, A.; Djalab, Z.; El Hammoumi, A.; Marco Tina, G.; Motahhir, S.; Laouid, A.A. A Comprehensive Review of Floating Photovoltaic Systems: Tech Advances, Marine Environmental Influences on Offshore PV Systems, and Economic Feasibility Analysis. Sol. Energy 2024, 277, 112711. [Google Scholar] [CrossRef]
- Mavraki, N.; Bos, O.G.; Vlaswinkel, B.M.; Roos, P.; de Groot, W.; van der Weide, B.; Bittner, O.; Coolen, J.W.P. Fouling Community Composition on a Pilot Floating Solar-Energy Installation in the Coastal Dutch North Sea. Front. Mar. Sci. 2023, 10, 1223766. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Xu, M.; Zhou, P.; Ren, Y. Research Strategies to Develop Environmentally Friendly Marine Antifouling Coatings. Mar. Drugs 2020, 18, 371. [Google Scholar] [CrossRef]
- Bian, W.; Li, H.; Xiong, W.; Leung, M.K.H. Photocatalytic Antifouling Coating: From Fundamentals to Applications. Photocatal. Res. Potential 2024, 1, 10008. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired Marine Antifouling Coatings: Status, Prospects, and Future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, Y.; Ju, P.; Zhang, D. Controlled Synthesis and Photocatalytic Antifouling Properties of BiVO4 with Tunable Morphologies. J. Electron. Mater. 2017, 46, 758–765. [Google Scholar] [CrossRef]
- Hu, H.; Chen, M.; Cao, M. TiO2 Antifouling Coating Based on Epoxy-Modified Tung Oil Waterborne Resin. Polym. Polym. Compos. 2021, 29, S521–S529. [Google Scholar] [CrossRef]
- Valenzuela, L.; Iglesias, A.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial Surfaces with Self-Cleaning Properties Functionalized by Photocatalytic ZnO Electrosprayed Coatings. J. Hazard. Mater. 2019, 369, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Kumar, A.M.; Kumar Sadasivuni, K.; Xing, R.; Liu, S. Self-Cleaning Superhydrophobic Coatings: Potential Industrial Applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Sakai, M.; Fujishima, A. Development of Sol–Gel Processed Semi-Transparent and Self-Cleaning Superhydrophobic Coatings. J. Mater. Chem. A 2014, 2, 5548–5553. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, B.; Zhang, X.; Tang, L.; Lin, X.; Hu, C.; Ren, K. Enhanced Corrosion Resistance and Self-Cleaning Properties of Superhydrophobic Nickel Coating Fabricated by One-Step Electrodeposition. Ceram. Int. 2023, 49, 13109–13118. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Guo, Z.; Wang, P.; Wang, A. Preparation of Microcapsules Coating and the Study of Their Bionic Anti-Fouling Performance. Materials 2020, 13, 1669. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, R.; Sun, G.; Liu, Q.; Liu, J.; Yu, J.; Lin, C.; Duan, J.; Wang, J. The Mussel-Inspired Micro-Nano Structure for Antifouling:A Flowering Tree. J. Colloid Interface Sci. 2021, 603, 307–318. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, S.; Tong, Z.; Wang, Y.; Gao, F.; Hu, J.; Hou, Y.; Lu, J.; Cong, W.; Sun, Y.; et al. A Bioinspired Antifouling Coating Based on “Host–Guest Interaction” Strategy: Durable Slipperiness and Tunable Transparency. Small 2025, 21, 2409771. [Google Scholar] [CrossRef]
- Hu, P.; Xie, Q.; Ma, C.; Zhang, G. Silicone-Based Fouling-Release Coatings for Marine Antifouling. Langmuir 2020, 36, 2170–2183. [Google Scholar] [CrossRef]
- Sun, J.; Duan, J.; Liu, X.; Dong, X.; Zhang, Y.; Liu, C.; Hou, B. Environmentally Benign Smart Self-Healing Silicone-Based Coating with Dual Antifouling and Anti-Corrosion Properties. Appl. Mater. Today 2022, 28, 101551. [Google Scholar] [CrossRef]
- An, X.; Cheng, J.; Li, Q.; Tang, J.; Li, Z.; Liu, L.; Yang, H.; Wei, C. Development of a Mechanically Robust Silicon-Based Cross-Linking Polymer for the Sustainable Marine Antifouling Coatings. Sustain. Mater. Technol. 2024, 41, e01015. [Google Scholar] [CrossRef]
- Lu, G.; Tian, S.; Li, J.; Xu, Y.; Liu, S.; Pu, J. Fabrication of Bio-Based Amphiphilic Hydrogel Coating with Excellent Antifouling and Mechanical Properties. Chem. Eng. J. 2021, 409, 128134. [Google Scholar] [CrossRef]
- Yu, R.; Yuan, X. Rising of Boron Nitride: A Review on Boron Nitride Nanosheets Enhanced Anti-Corrosion Coatings. Prog. Org. Coat. 2024, 186, 107990. [Google Scholar] [CrossRef]
- Zuo, X.; Chang, K.; Zhao, J.; Xie, Z.; Tang, H.; Li, B.; Chang, Z. Bubble-Template-Assisted Synthesis of Hollow Fullerene-like MoS2 Nanocages as a Lithium Ion Battery Anode Material. J. Mater. Chem. A 2016, 4, 51–58. [Google Scholar] [CrossRef]
- Jiang, H.; Cai, Q.; Mateti, S.; Yu, Y.; Zhi, C.; Chen, Y. Boron Nitride Nanosheet Dispersion at High Concentrations. ACS Appl. Mater. Interfaces 2021, 13, 44751–44759. [Google Scholar] [CrossRef]
- Hao, W.; Yanpeng, L.; Zhou, S.; Xiangying, R.; Wenjun, Z.; Jun, L. Surface Characteristics of Microalgae and Their Effects on Harvesting Performance by Air Flotation. Int. J. Agric. Biol. Eng. 2017, 10, 125–133. [Google Scholar] [CrossRef]
- Li, N.; Wang, P.; Wang, S.; Wang, C.; Zhou, H.; Kapur, S.; Zhang, J.; Song, Y. Electrostatic Charges on Microalgae Surface: Mechanism and Applications. J. Environ. Chem. Eng. 2022, 10, 107516. [Google Scholar] [CrossRef]
- ASTM D1141-98(2021); Standard Practice for Preparation of Substitute Ocean Water. ASTM: West Conshohocken, PA, USA, 2021.
- ISO 2409:2020; Paints and Varnishes—Cross-Cut Test. ISO: Geneva, Switzerland, 2020.
- Zhang, Y.; Wang, T.; Lv, Y. Durable Biomimetic Two-Tier Structured Superhydrophobic Surface with Ultralow Adhesion and Effective Antipollution Property. Langmuir 2023, 39, 2548–2557. [Google Scholar] [CrossRef]
- Yue, D.; Jiang, X.; Yu, H.; Sun, D. In-Situ Fabricated Hierarchical Nanostructure on Titanium Alloy as Highly Stable and Durable Super-Lubricated Surface for Anti-Biofouling in Marine Engineering. Chem. Eng. J. 2023, 463, 142389. [Google Scholar] [CrossRef]
- Lesniewska, N.; Duval, J.F.L.; Caillet, C.; Razafitianamaharavo, A.; Pinheiro, J.P.; Bihannic, I.; Gley, R.; Cordier, H.L.; Vyas, V.; Pagnout, C.; et al. Physicochemical Surface Properties of Chlorella Vulgaris: A Multiscale Assessment, from Electrokinetic and Proton Uptake Descriptors to Intermolecular Adhesion Forces. Nanoscale 2024, 16, 5149–5163. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Yang, J.; Wang, F.; Liu, L.; Liu, H.; Yan, C.; Guo, Z. Sol-Gel Synthesized Hexagonal Boron Nitride/Titania Nanocomposites with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2019, 465, 154–163. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Yuan, X.; Wan, J.; Wang, L.; Pan, H.; Shen, Y. One-Pot Preparation of Robust, Ultraviolet-Proof Superhydrophobic Cotton Fabrics for Self-Cleaning and Oil/Water Separation. Cellulose 2020, 27, 9005–9026. [Google Scholar] [CrossRef]
- Siuda, J.; Perdoch, W.; Mazela, B.; Zborowska, M. Catalyzed Reaction of Cellulose and Lignin with Methyltrimethoxysilane—FT-IR, 13C NMR and 29Si NMR Studies. Materials 2019, 12, 2006. [Google Scholar] [CrossRef]
- Sato, Y.; Hayami, R.; Gunji, T. Characterization of NMR, IR, and Raman Spectra for Siloxanes and Silsesquioxanes: A Mini Review. J. Sol-Gel Sci. Technol. 2022, 104, 36–52. [Google Scholar] [CrossRef]
- Song, J.; Dai, Z.; Li, J.; Tong, X.; Zhao, H. Polydopamine-Decorated Boron Nitride as Nano-Reinforcing Fillers for Epoxy Resin with Enhanced Thermomechanical and Tribological Properties. Mater. Res. Express 2018, 5, 075029. [Google Scholar] [CrossRef]
- Chaturbedy, P.; Ahamed, M.; Eswaramoorthy, M. Oxidative Dehydrogenation of Propane over a High Surface Area Boron Nitride Catalyst: Exceptional Selectivity for Olefins at High Conversion. ACS Omega 2018, 3, 369–374. [Google Scholar] [CrossRef]
- Ma, J.W.; Lee, W.J.; Bae, J.M.; Jeong, K.S.; Oh, S.H.; Kim, J.H.; Kim, S.-H.; Seo, J.-H.; Ahn, J.-P.; Kim, H.; et al. Carrier Mobility Enhancement of Tensile Strained Si and SiGe Nanowires via Surface Defect Engineering. Nano Lett. 2015, 15, 7204–7210. [Google Scholar] [CrossRef]
- Rashid, M.; Yousif, M.; Rashid, Z.; Muhammad, A.; Altaf, M.; Mustafa, A. Effect of Dust Accumulation on the Performance of Photovoltaic Modules for Different Climate Regions. Heliyon 2023, 9, e23069. [Google Scholar] [CrossRef]
- Li, Y.; Ning, C. Latest Research Progress of Marine Microbiological Corrosion and Bio-Fouling, and New Approaches of Marine Anti-Corrosion and Anti-Fouling. Bioact. Mater. 2019, 4, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Hashizume, M.; Ariga, K.; Terashima, T.; Kikuchi, J. Preparation and Characterization of a Novel Organic–Inorganic Nanohybrid “Cerasome” Formed with a Liposomal Membrane and Silicate Surface. Chem.—Eur. J. 2007, 13, 5272–5281. [Google Scholar] [CrossRef] [PubMed]
- Pavan, C.; Sydor, M.J.; Bellomo, C.; Leinardi, R.; Cananà, S.; Kendall, R.L.; Rebba, E.; Corno, M.; Ugliengo, P.; Mino, L.; et al. Molecular Recognition between Membrane Epitopes and Nearly Free Surface Silanols Explains Silica Membranolytic Activity. Colloids Surf. B Biointerfaces 2022, 217, 112625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Su, J.; Su, F.; Zhao, T.; Li, J.; Yang, P.; Zhang, Q. Synthesis of Robust Superhydrophobic EP/F-CNTs/SiO2 Composite Coating with Excellent Anti-Corrosion and Wear-Resistant Property. J. Coat. Technol. Res. 2025. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Ding, Z.; Chen, Q.; Ji, Y.; Xiong, Y.; Gao, Y.; Huo, Z. Preparation of Transparent MTMS/BNNS Composite Siloxane Coatings with Anti-Biofouling Properties. Coatings 2025, 15, 769. https://doi.org/10.3390/coatings15070769
Cao L, Ding Z, Chen Q, Ji Y, Xiong Y, Gao Y, Huo Z. Preparation of Transparent MTMS/BNNS Composite Siloxane Coatings with Anti-Biofouling Properties. Coatings. 2025; 15(7):769. https://doi.org/10.3390/coatings15070769
Chicago/Turabian StyleCao, Lu, Zhutao Ding, Qi Chen, Yefeng Ji, Ying Xiong, Yun Gao, and Zhongyan Huo. 2025. "Preparation of Transparent MTMS/BNNS Composite Siloxane Coatings with Anti-Biofouling Properties" Coatings 15, no. 7: 769. https://doi.org/10.3390/coatings15070769
APA StyleCao, L., Ding, Z., Chen, Q., Ji, Y., Xiong, Y., Gao, Y., & Huo, Z. (2025). Preparation of Transparent MTMS/BNNS Composite Siloxane Coatings with Anti-Biofouling Properties. Coatings, 15(7), 769. https://doi.org/10.3390/coatings15070769






