Structural Water Content in Pigment-Grade TiO2 Particles Coated with Al2O3 and SiO2, and Their Effect on Polypropylene Photodegradation
Abstract
1. Introduction
2. Materials and Methods
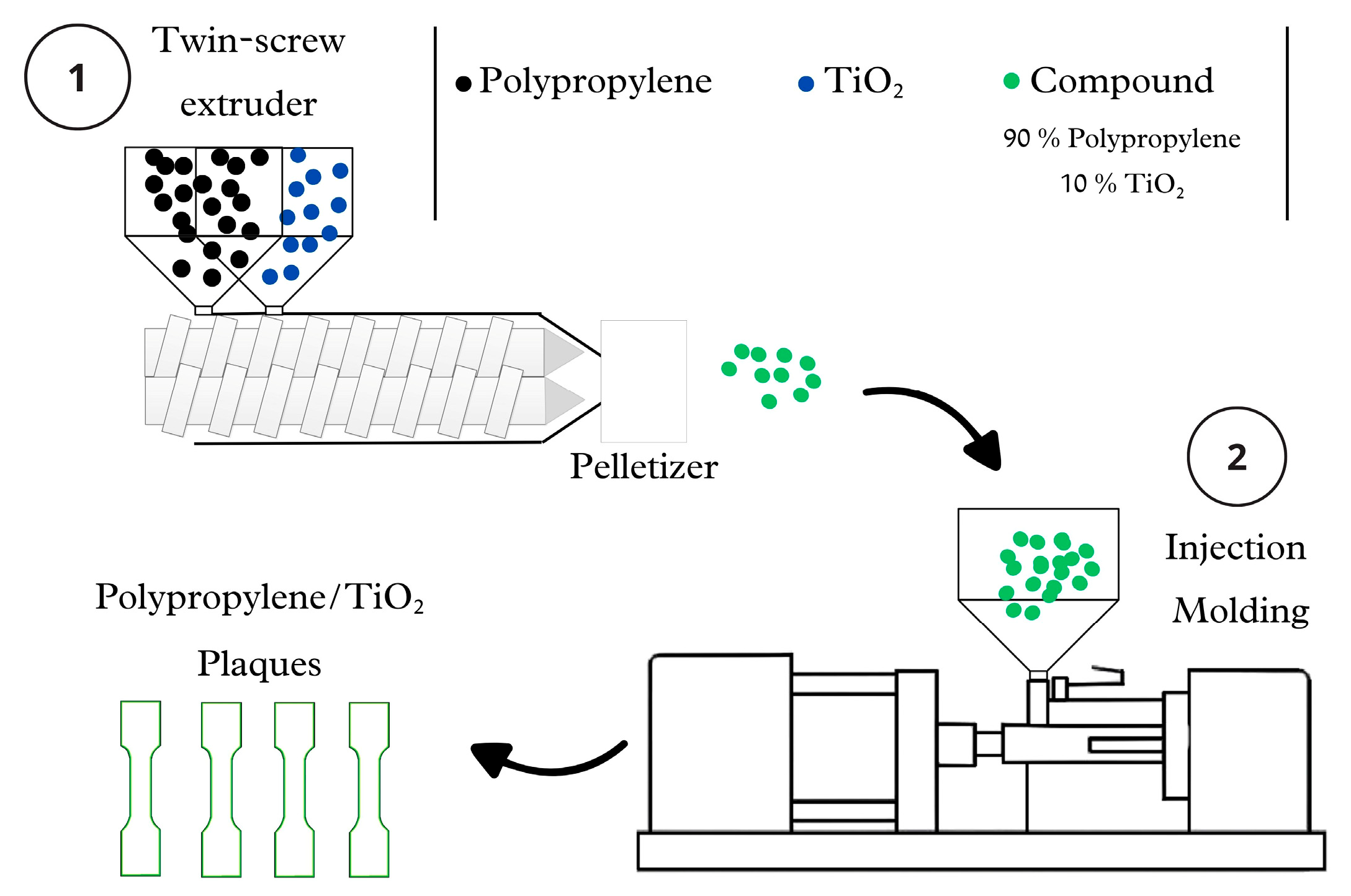
2.1. Polypropylene/TiO2 Plaques Fabrication, Degradation, and Mechanical Tests
2.2. Characterization of TiO2 Pigment
2.3. ANOVA Statistical Analysis and Mathematical Modeling
3. Results and Discussion
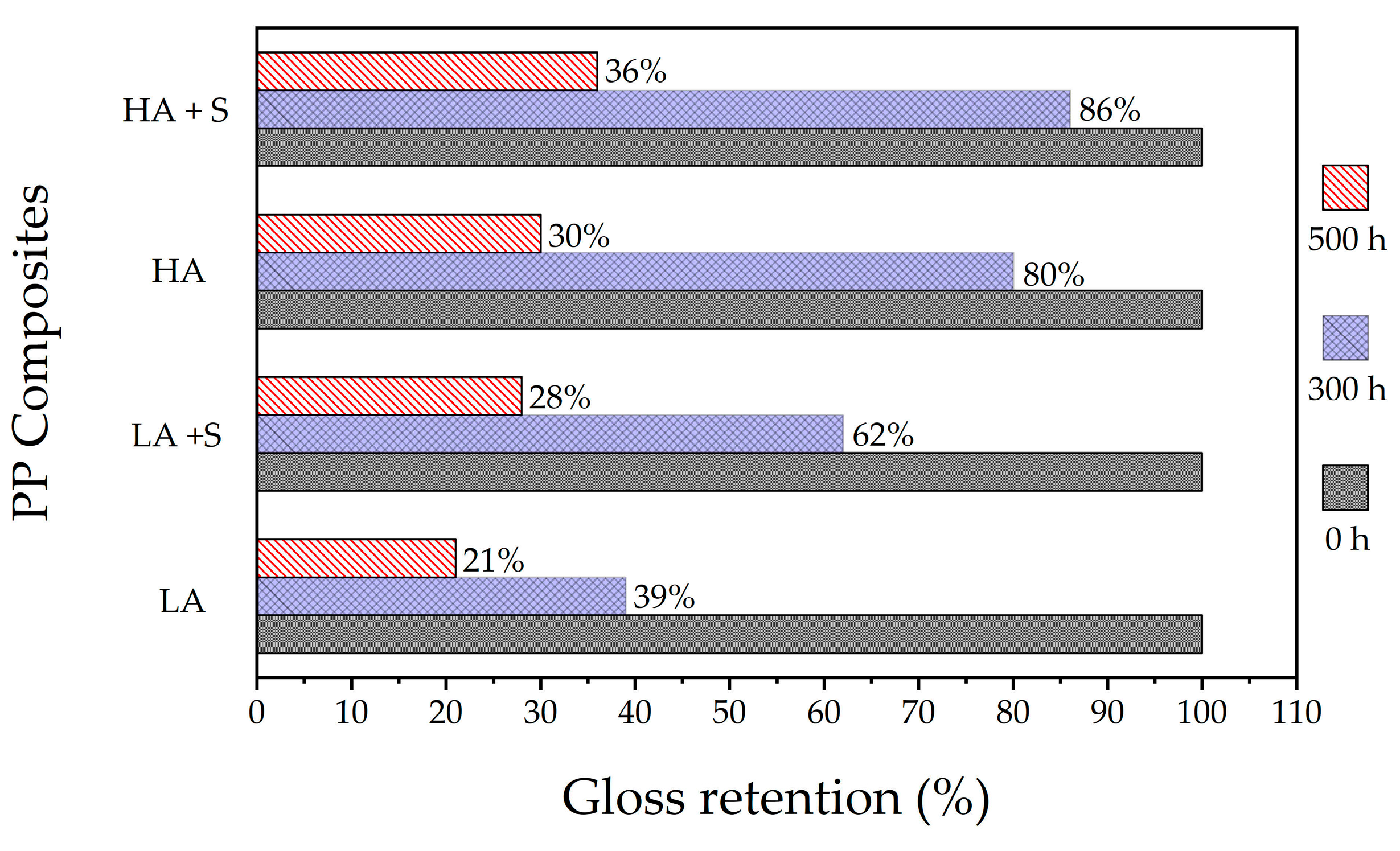
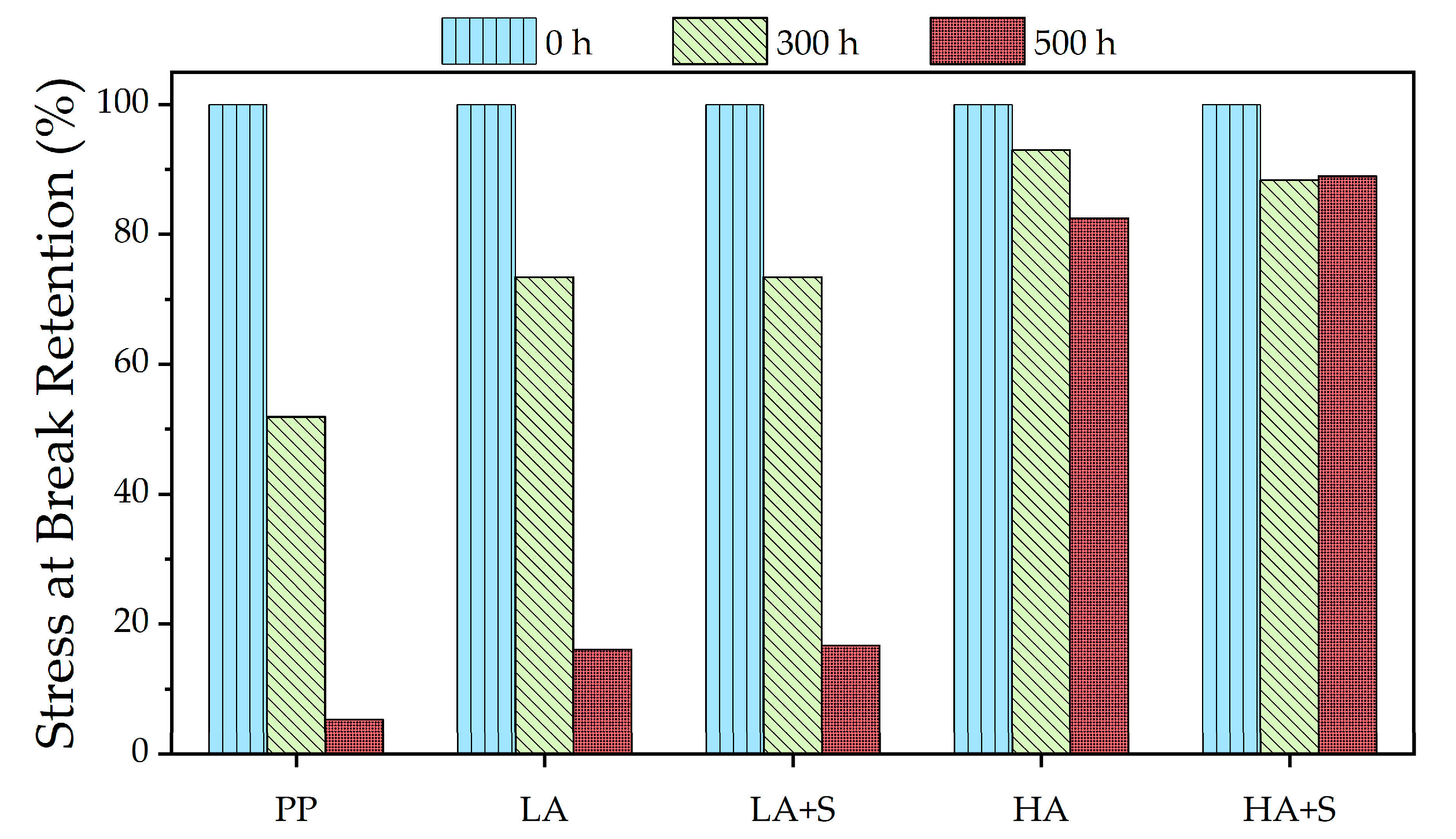
3.1. Effect of Inorganic Coatings on Photodegradation of Polypropylene/TiO2 Plaques
3.2. Characterization of TiO2 Pigment and Statistical Analysis
3.2.1. Structural Analysis by X-Ray Diffraction
3.2.2. Coating of TiO2 Surface
3.2.3. Particle Size
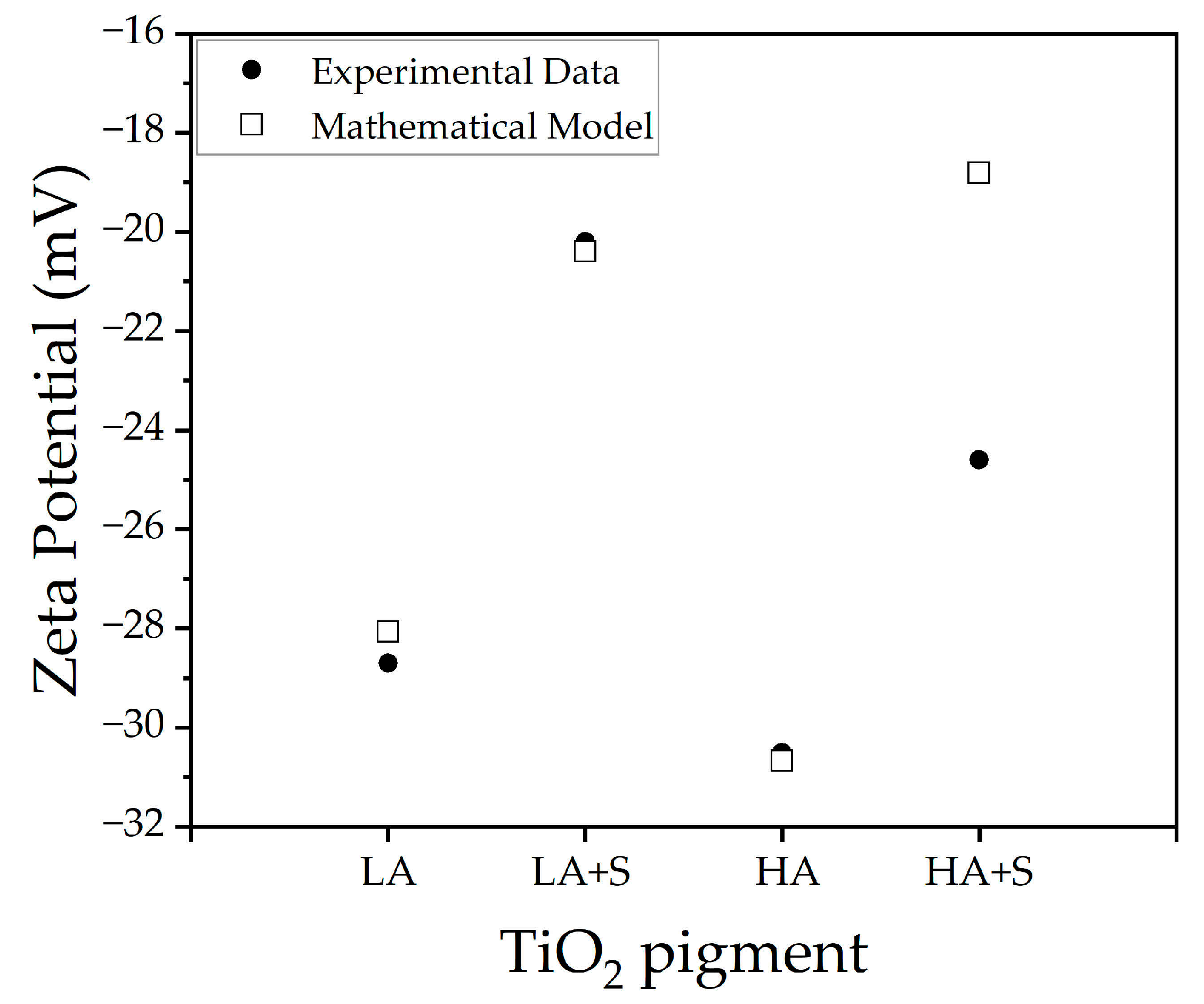
3.2.4. Zeta Potential (ζ)
3.2.5. Water Content
3.3. Water Stabilization Mechanism by Silica on TiO2 Surfaces
- Hydrogen Bond Formation: The tetrahedral structure of silica, with its surface silanol (Si-OH) groups, offers numerous sites for hydrogen bond formation with water molecules [16]. These bonds are stronger than water-TiO2 interactions, leading to more stable adsorption.
- Physical Barrier for Reactive Species Migration: The silica layer functions as a physical barrier, preventing the migration of photogenerated electron–hole pairs to the surface [19]. This reduction in migration significantly decreases the likelihood of these pairs interacting with water molecules to form reactive oxygen species (ROS).
3.4. Practical Implications for Polymer Applications
- Coating optimization for outdoor applications: For polypropylene products designed for outdoor applications, including garden furniture, exterior automotive components, and construction materials, the use of TiO2 pigments with combined alumina and silica coatings (like the HA+S sample) is recommended to enhance gloss retention and durability [10,27].
- Processing considerations: The presence of silica coatings on TiO2 particles may influence the rheological properties of the molten polymer during processing [7]. To account for these effects, manufacturers should consider adjusting processing parameters, including melting temperature and extrusion speed.
- Cost–benefit balance: Although TiO2 pigments with alumina and silica coatings may have a higher initial cost, their significant improvement in durability and gloss retention can lead to lower long-term costs due to extended product life [4].
- Specific Applications: For applications where gloss retention is essential, such as consumer packaging or decorative pieces, selecting TiO2 pigments with an optimal balance of alumina and silica coatings can significantly enhance product performance [6].
3.5. Comparison with Previous Studies on TiO2 Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and Application of Titanium Dioxide Photocatalysis for Energy, Decontamination and Viral Disinfection: A Review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Umar, M.; Maryam, R.; Nawaz, I.; Razzaq, H.; Malik, T.; Liu, X. Polymer Nanocomposites Based on TiO2 as a Reinforcing Agent: An Overview. Adv. Eng. Mater. 2022, 24, 2200844. [Google Scholar] [CrossRef]
- Samoylov, A.M.; Popov, V.N. Titanium Dioxide (TiO₂) and Its Applications; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128199602. [Google Scholar]
- Yang, C.; Liu, C.; Xu, L.; Nie, M.; Wang, Q. Flow-Induced Alignment of Titanium Dioxide Nanorods in Polyolefin Tubes for Use in Catheters. Mater. Des. 2023, 233, 112277. [Google Scholar] [CrossRef]
- Wu, X. Applications of Titanium Dioxide Materials. In Titanium Dioxide—Advances and Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A Review on the Synthesis of the Various Types of Anatase TiO2 Facets and Their Applications for Photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Zapata-Tello, D.L.; Escobar-Barrios, V.; Gonzalez-Calderon, J.A.; Pérez, E. Chemical Modification of Titanium Dioxide Nanoparticles with Dicarboxylic Acids to Mediate the UV Degradation in Polyethylene Films. Polym. Bull. 2020, 77, 6409–6431. [Google Scholar] [CrossRef]
- Parvate, S.; Singh, J.; Dixit, P.; Vennapusa, J.R.; Maiti, T.K.; Chattopadhyay, S. Titanium Dioxide Nanoparticle-Decorated Polymer Microcapsules Enclosing Phase Change Material for Thermal Energy Storage and Photocatalysis. ACS Appl. Polym. Mater. 2021, 3, 1866–1879. [Google Scholar] [CrossRef]
- Andrady, A.L.; Barnes, P.W.; Bornman, J.F.; Gouin, T.; Madronich, S.; White, C.C.; Zepp, R.G.; Jansen, M.A.K. Oxidation and Fragmentation of Plastics in a Changing Environment; from UV-Radiation to Biological Degradation. Sci. Total Environ. 2022, 851, 158022. [Google Scholar] [CrossRef]
- Bourgogne, D.; Therias, S.; Gardette, J.-L. Wavelength Effect on Polymer Photooxidation under LED Weathering Conditions. Polym. Degrad. Stab. 2022, 202, 110021. [Google Scholar] [CrossRef]
- Alavian Petroody, S.S.; Hashemi, S.H.; Škrlep, L.; Mušič, B.; van Gestel, C.A.M.; Sever Škapin, A. UV Light Causes Structural Changes in Microplastics Exposed in Bio-Solids. Polymers 2023, 15, 4322. [Google Scholar] [CrossRef]
- Vital-Grappin, A.D.; Ariza-Tarazona, M.C.; Luna-Hernández, V.M.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; Siligardi, C.; Cedillo-González, E.I. The Role of the Reactive Species Involved in the Photocatalytic Degradation of HDPE Microplastics Using C,N-TiO2 Powders. Polymers 2021, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Sharara, A.; Samy, M.; Mossad, M.; Gar Alalm, M. Photodegradation of Polyethylene Debris in Water by Sulfur-Doped TiO2: System Optimization, Degradation Mechanism, and Reusability. Environ. Sci. Pollut. Res. 2023, 31, 3951–3963. [Google Scholar] [CrossRef]
- Bussiere, P.-O.; Gardette, J.-L.; Rapp, G.; Masson, C.; Therias, S. New Insights into the Mechanism of Photodegradation of Chitosan. Carbohydr. Polym. 2021, 259, 117715. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Tang, F.; Wang, F.; Livingstone, R.A.; Schlegel, S.J.; Ohto, T.; Bonn, M.; Nagata, Y.; Backus, E.H.G. Chemisorbed and Physisorbed Water at the TiO2/Water Interface. J. Phys. Chem. Lett. 2017, 8, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Litke, A.; Su, Y.; Tranca, I.; Weber, T.; Hensen, E.J.M.; Hofmann, J.P. Role of Adsorbed Water on Charge Carrier Dynamics in Photoexcited TiO2. J. Phys. Chem. C 2017, 121, 7514–7524. [Google Scholar] [CrossRef]
- Chapa-Rodríguez, R.; Avila-de la Rosa, G.; Pérez, E. Thermal Stability and Ageing Properties of PP–PE Film Modulated by Nano-Silica Particles: Comparison between Dry and Moist Particles. Polym. Bull. 2021, 78, 3071–3088. [Google Scholar] [CrossRef]
- Burak, D.; Han, J.H.; Han, J.S.; Kim, I.S.; Rahman, M.A.; Yang, J.K.W.; Cho, S.-H. Controlling TiO2 Photocatalytic Behaviour via Perhydropolysilazane-Derived SiO 2 Ultrathin Shell. Nanoscale 2024, 16, 21960–21969. [Google Scholar] [CrossRef]
- Guo, J.; Van Bui, H.; Valdesueiro, D.; Yuan, S.; Liang, B.; Van Ommen, J. Suppressing the Photocatalytic Activity of TiO2 Nanoparticles by Extremely Thin Al2O3 Films Grown by Gas-Phase Deposition at Ambient Conditions. Nanomaterials 2018, 8, 61. [Google Scholar] [CrossRef]
- Ti-PureTM TiO2 by Chemours Titanium Dioxide Solutions for Plastics. Available online: https://www.tipure.com/en/applications/plastics (accessed on 22 May 2025).
- Titanium Dioxide—Venator. Available online: https://www.venatorcorp.com/products-and-applications/products/titanium-dioxide (accessed on 22 May 2025).
- Ti-PureTM Solutions for Coatings: Applications. Available online: https://www.tipure.com/en/applications/coatings (accessed on 22 May 2025).
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, X.; Lu, Y.; Dao, X.; Qiu, L. Accelerated Laboratory Weathering of Polypropylene/Poly (Lactic Acid) Blends. Polymers 2022, 15, 17. [Google Scholar] [CrossRef]
- Wang, L.; Xie, G.; Mi, X.; Zhang, B.; Du, Y.; Zhu, Q.; Yu, Z. Surface-Modified TiO2 @SiO2 Nanocomposites for Enhanced Dispersibility and Optical Performance to Apply in the Printing Process as a Pigment. ACS Omega 2023, 8, 20116–20124. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, Y.; Ma, M.; Chen, S.; He, H.; Zhu, Y.; Wang, X. LLDPE/TiO2 Composites with High UV Aging Resistance and Mechanical Properties by Controlling the Alumina Coating on TiO2. Polym. Compos. 2025, 46, 1383–1399. [Google Scholar] [CrossRef]
- Al-Fahham, A.A. Development of New LSD Formula When Numbers of Observations Are Unequal. Open J. Stat. 2018, 8, 258–263. [Google Scholar] [CrossRef]
- Mui, J.; Ngo, J.; Kim, B. Aggregation and Colloidal Stability of Commercially Available Al2O3 Nanoparticles in Aqueous Environments. Nanomaterials 2016, 6, 90. [Google Scholar] [CrossRef]
- Ceballos-Chuc, M.C.; Ramos-Castillo, C.M.; Rodríguez-Pérez, M.; Ruiz-Gómez, M.Á.; Rodríguez-Gattorno, G.; Villanueva-Cab, J. Synergistic Correlation in the Colloidal Properties of TiO2 Nanoparticles and Its Impact on the Photocatalytic Activity. Inorganics 2022, 10, 125. [Google Scholar] [CrossRef]
- Kang, X.; Chatzitakis, A.; Aarholt, T.; Sun, X.; Negri, C.; Norby, T. Facet-Engineered TiO2 Nanomaterials Reveal the Role of Water–Oxide Interactions in Surface Protonic Conduction. J. Mater. Chem. A Mater. 2022, 10, 218–227. [Google Scholar] [CrossRef]
- Kunc, F.; Gallerneault, M.; Kodra, O.; Brinkmann, A.; Lopinski, G.P.; Johnston, L.J. Surface Chemistry of Metal Oxide Nanoparticles: NMR and TGA Quantification. Anal. Bioanal. Chem. 2022, 414, 4409–4425. [Google Scholar] [CrossRef]
- Li, W.; Franco, D.C.; Yang, M.S.; Zhu, X.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Comparative Study of the Performances of Al(OH)3 and BaSO4 in Ultrafine Powder Coatings. Processes 2019, 7, 316. [Google Scholar] [CrossRef]
- Liu, X. DRIFTS Study of Surface of γ-Alumina and Its Dehydroxylation. J. Phys. Chem. C 2008, 112, 5066–5073. [Google Scholar] [CrossRef]
- Milligan, L.H. The Mechanism of the Dehydration of Crystalline Aluminum Hydroxide and of the Adsorption of Water by the Resulting Alumina. J. Phys. Chem. 1922, 26, 247–255. [Google Scholar] [CrossRef][Green Version]
- Vasile, B.S.; Dobra, G.; Iliev, S.; Cotet, L.; Neacsu, I.A.; Surdu, V.A.; Nicoara, A.I.; Boiangiu, A.; Filipescu, L. Thermally Activated Al(OH)3 Part II—Effect of Different Thermal Treatments. Ceramics 2021, 4, 564–575. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, F.; Ma, A.; Tian, S. Experimental Study on Microwave Drying Aluminum Hydroxide. Coatings 2024, 14, 687. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Eckhoff, R.K. Kinetics Study of Hydration Reaction between Aluminum Powder and Water Based on an Improved Multi-Stage Shrinking Core Model. Int. J. Hydrogen Energy 2021, 46, 33635–33655. [Google Scholar] [CrossRef]
- Egerton, T. The Influence of Surface Alumina and Silica on the Photocatalytic Degradation of Organic Pollutants. Catalysts 2013, 3, 338–362. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, Y.; Wang, W.; Wang, D.; Qi, T. Hydrous Alumina/Silica Double-Layer Surface Coating of TiO2 Pigment. Colloids Surf. A Physicochem. Eng. Asp. 2012, 407, 77–84. [Google Scholar] [CrossRef]
- Oguma, J.; Kakuma, Y.; Murayama, S.; Nosaka, Y. Effects of Silica Coating on Photocatalytic Reactions of Anatase Titanium Dioxide Studied by Quantitative Detection of Reactive Oxygen Species. Appl. Catal. B 2013, 129, 282–286. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Abbasi, S.; Li, D.; Mojiri, A.; Zhou, J.L. Investigation of Advanced Oxidation Process in the Presence of TiO2 Semiconductor as Photocatalyst: Property, Principle, Kinetic Analysis, and Photocatalytic Activity. Catalysts 2023, 13, 232. [Google Scholar] [CrossRef]


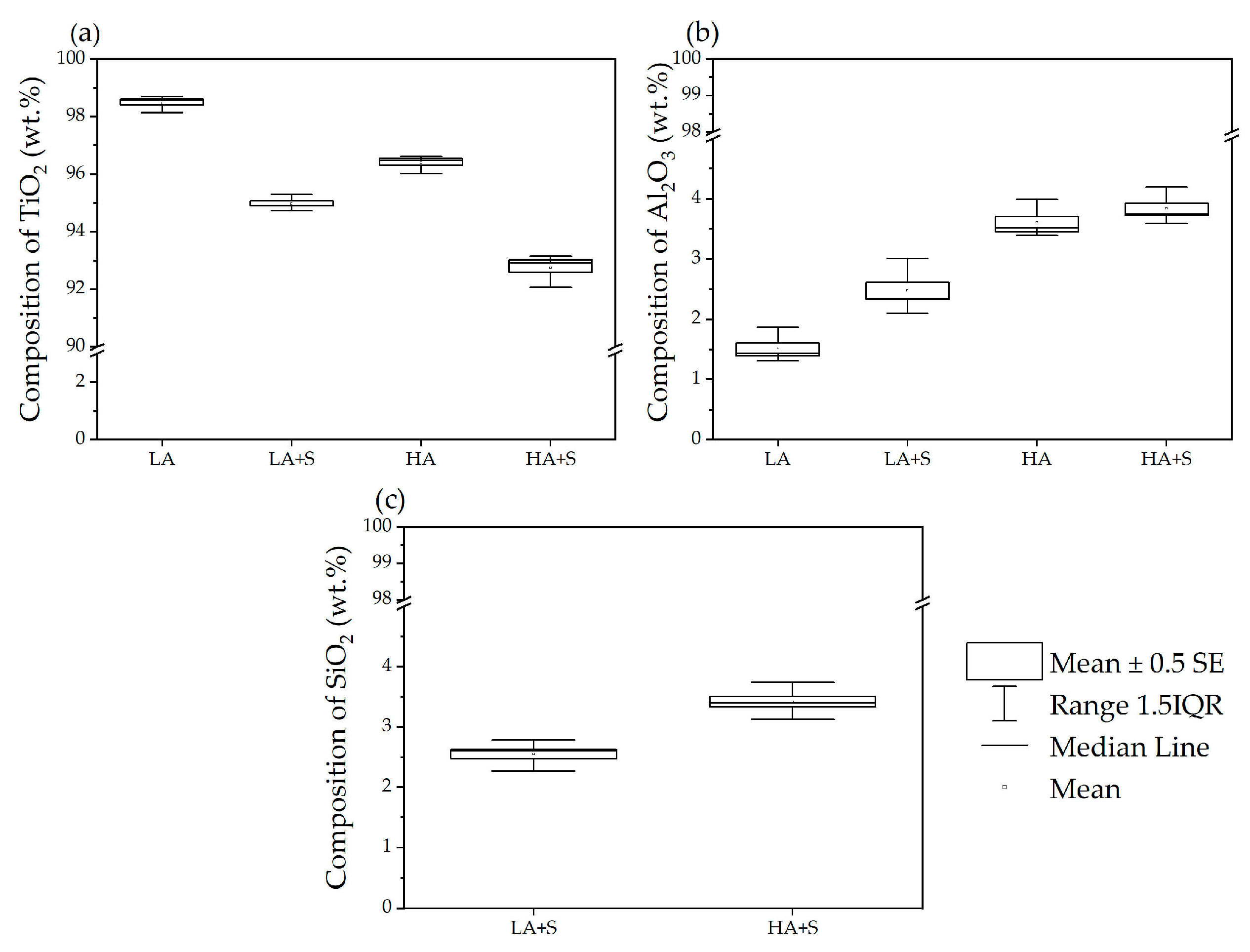






| Sample ID | Inorganic Coatings Content | ||
|---|---|---|---|
| Experimental (wt.%) | |||
| TiO2 | Al2O3 | SiO2 | |
| LA | 98.5 ± 0.3 | 1.5 ± 0.3 | / |
| LA+S | 95.0 ± 0.3 | 2.5 ± 0.5 | 2.5 ± 0.3 |
| HA | 96.4 ± 0.3 | 3.6 ± 0.3 | / |
| HA+S | 92.7 ± 0.6 | 3.8 ± 0.3 | 3.4 ± 0.3 |
| Source | Sum of Squares | d.f. a | Mean Squares | F b | P c | |
|---|---|---|---|---|---|---|
| TiO2 | Between | 52.30 | 3 | 17.43 | 110.98 | 0.0000 |
| Within (error) | 1.26 | 8 | 0.16 | |||
| Total | 53.55 | 11 | ||||
| Al2O3 | Between | 10.35 | 3 | 3.45 | 26.58 | 0.0000 |
| Within (error) | 1.04 | 8 | 0.13 | |||
| Total | 11.39 | 11 | ||||
| SiO2 | Between | 1.13 | 1 | 1.13 | 14.05 | 0.0199 |
| Within (error) | 0.32 | 4 | 0.08 | |||
| Total | 1.46 | 5 |
| Source | Sum of Squares | d.f. a | Mean Squares | F b | P c |
|---|---|---|---|---|---|
| Between | 9409.43 | 3 | 3136.48 | 1.67 | 0.226 |
| Within (error) | 22,579.92 | 12 | 1881.66 | ||
| Total | 31,989.35 | 15 |
| Source | Sum of Squares | d.f. a | Mean Squares | F b | P c |
|---|---|---|---|---|---|
| Between | 411.47 | 3 | 137.16 | 110.48 | 0.0000 |
| Within (error) | 29.80 | 24 | 1.24 | ||
| Total | 441.26 | 27 |
| ID | Moisture (wt.%) |
|---|---|
| HA+S | 0.54 |
| HA | 0.45 |
| LA+S | 0.19 |
| LA | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armendáriz-Alonso, E.F.; Rivera-García, N.; Moreno-Razo, J.A.; Meza-Espinoza, L.O.; Waldo-Mendoza, M.A.; Pérez, E. Structural Water Content in Pigment-Grade TiO2 Particles Coated with Al2O3 and SiO2, and Their Effect on Polypropylene Photodegradation. Coatings 2025, 15, 685. https://doi.org/10.3390/coatings15060685
Armendáriz-Alonso EF, Rivera-García N, Moreno-Razo JA, Meza-Espinoza LO, Waldo-Mendoza MA, Pérez E. Structural Water Content in Pigment-Grade TiO2 Particles Coated with Al2O3 and SiO2, and Their Effect on Polypropylene Photodegradation. Coatings. 2025; 15(6):685. https://doi.org/10.3390/coatings15060685
Chicago/Turabian StyleArmendáriz-Alonso, Edgar F., Nancy Rivera-García, J. Antonio Moreno-Razo, Luis Octavio Meza-Espinoza, Miguel A. Waldo-Mendoza, and Elías Pérez. 2025. "Structural Water Content in Pigment-Grade TiO2 Particles Coated with Al2O3 and SiO2, and Their Effect on Polypropylene Photodegradation" Coatings 15, no. 6: 685. https://doi.org/10.3390/coatings15060685
APA StyleArmendáriz-Alonso, E. F., Rivera-García, N., Moreno-Razo, J. A., Meza-Espinoza, L. O., Waldo-Mendoza, M. A., & Pérez, E. (2025). Structural Water Content in Pigment-Grade TiO2 Particles Coated with Al2O3 and SiO2, and Their Effect on Polypropylene Photodegradation. Coatings, 15(6), 685. https://doi.org/10.3390/coatings15060685







