Etching and Precursor Effects on Plasma-Modified Waste Polyethylene Terephthalate (PET) to Laccase Immobilization Applied in Catechol Biodegradation for Water Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Modification
2.2. Determination of Amino Group Density on PET Films
2.3. Contact Angle (CA) Evaluation
2.4. Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5. Scanning Electron Microscopy (SEM)
2.6. Immobilization of Laccase Enzyme
2.7. Immobilized Protein
2.8. Enzymatic Activity Assay
3. Results and Discussion
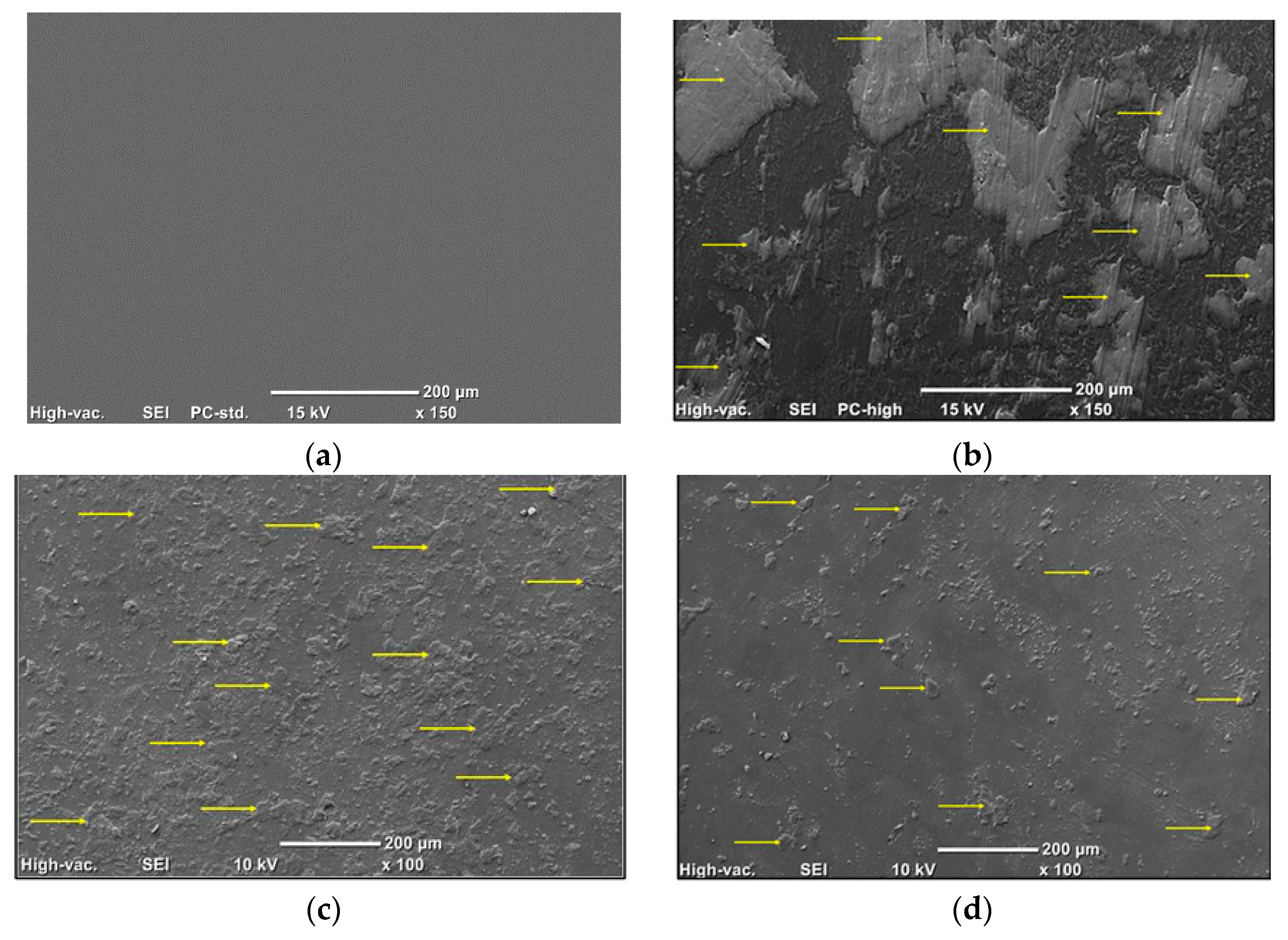
3.1. Surface Etching of PET Films with Air Plasma: Morphological Analysis
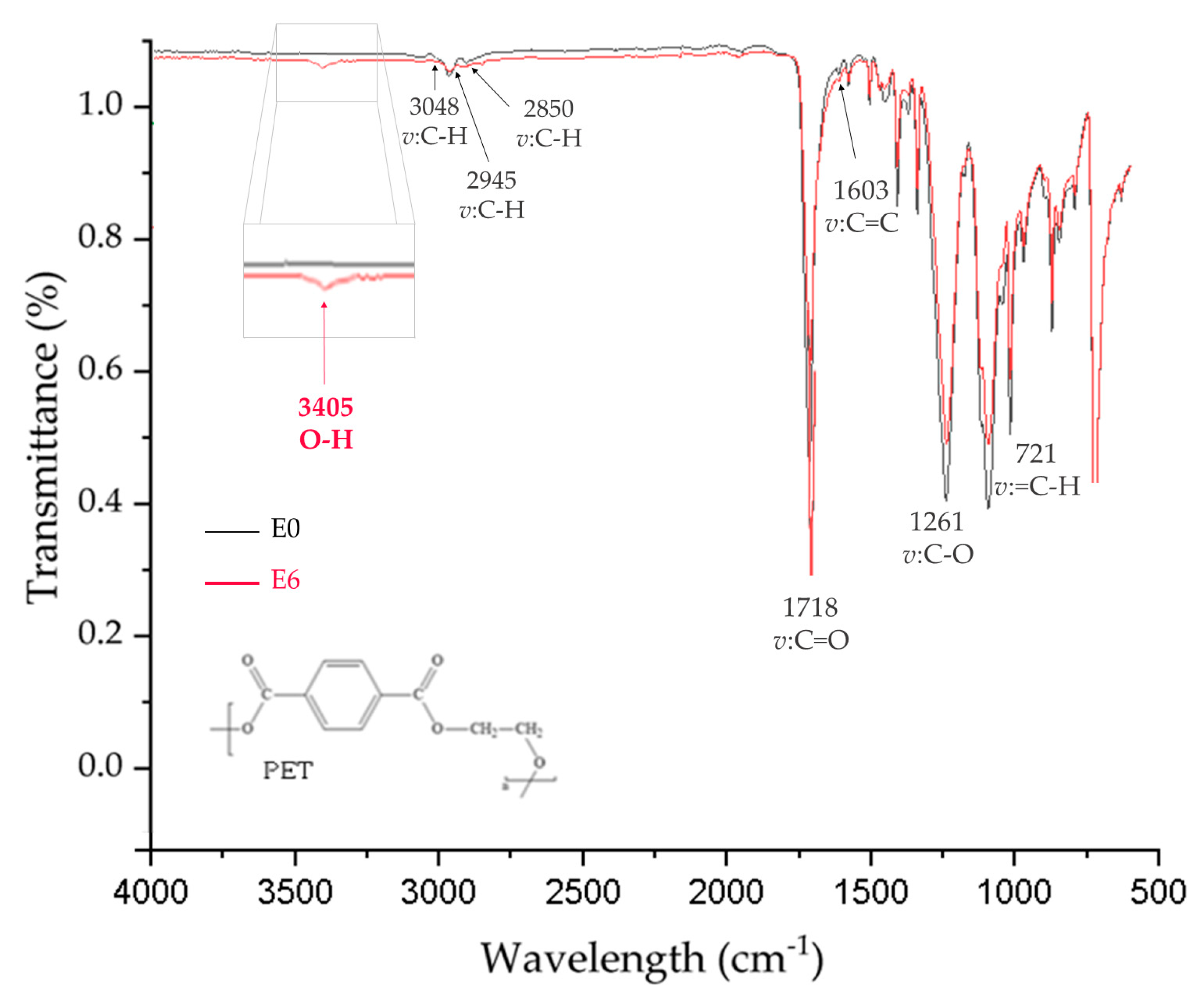
3.2. ATR-FTIR Verification of Functional Groups in Air Plasma-Treated PET Films
3.3. Surface Activation of PET Films with Ethylenediamine Plasma, Aniline, and N2/H2 Mixture
3.4. Qualitative and Quantitative Evaluation of PET Films’ Primary Amino Groups (Activation)
3.5. Contact Angle Hydrophilicity Test of PET Films
3.6. Verification of the Chemical Environment in Activated PET Films Using ATR-FTIR
3.7. SEM Analysis of Activated PET Films
3.8. Quantification of the Immobilized Protein
3.9. Quantification of Enzyme Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ángeles-Hurtado, L.A.; Rodríguez-Reséndiz, J.; Salazar-Colores, S.; Torres-Salinas, H.; Sevilla-Camacho, P.Y. Viable Disposal of Post-Consumer Polymers in Mexico: A Review. Front. Environ. Sci. 2021, 9, 749775. [Google Scholar] [CrossRef]
- Almeda, O.; Robles, E.; Pérez, I.; Martínez, J.; Noriega, S. Máquinas expendedoras inversas: El futuro para el reciclado de plásticos PET en México. In En Búsqueda de la Optimización: Herramientas y Métodos; Universidad Tecnológica de Ciudad Juárez: Chihuahua, Mexico, 2018; pp. 44–57. [Google Scholar]
- Gutiérrez-Limón, A.; Tamayo-Gómez, P.; Barajas-Aranda, S. Reciclaje de botellas de pet para manufactura aditiva. Investigación Y Ciencia Aplicada a La Ingeniería 2022, 9, 88–96. Available online: https://ojsincaing.com.mx/index.php/ediciones/article/view/96 (accessed on 25 February 2025).
- Michalowicz, J.; Duda, W. Phenols--Sources and Toxicity. Pol. J. Environ. Stud. 2007, 6, 347. [Google Scholar]
- Honarkar, H.; Arjmand, F.; Askari, F.; Barikani, M. Structural Study on Aminolyzed Polyethylene Terephthalate. Polym. Sci. 2022, 64, 800. [Google Scholar] [CrossRef]
- Mohan, J.; Prakash, R.; Behari, J.R. Electrochemical detection and catalytic oxidation of phenolic compounds over nickel complex modified graphite electrode. Appl. Ecol. Environ. Res. 2004, 2, 25. [Google Scholar] [CrossRef]
- Singh, T.; Bhatiya, A.K.; Mishra, P.K.; Srivastava, N. An effective approach for the degradation of phenolic waste: Phenols and cresols. In Abatement of Environmental Pollutants; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–243. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, M.A. Potential of extra cellular enzymes in remediation of polluted soils: A review. Enzyme Microb. Technol. 2004, 35, 339. [Google Scholar] [CrossRef]
- Girelli, A.M.; Quattrocchi, L.; Scuto, F.R. Design of bioreactor based on immobilized laccase on silica-chitosan support for phenol removal in continuous mode. J. Biotechnol. 2021, 337, 8. [Google Scholar] [CrossRef]
- Ameri, A.; Taghizadeh, T.; Talebian-Kiakalaieh, A.; Forootanfar, H.; Mojtabavi, S.; Jahandar, H.; Tarighi, S.; Faramarzi, M.A. Bio-removal of phenol by the immobilized laccase on the fabricated parent and hierarchical NaY and ZSM-5 zeolites. J. Taiwan Inst. Chem. Eng. 2021, 120, 300. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Exploring current tendencies in techniques and materials for immobilization of laccases—A review. Int. J. Biol. Macromol. 2021, 181, 683. [Google Scholar] [CrossRef]
- Hong, J.; Jung, D.; Park, S.; Oh, Y.; Oh, K.K.; Lee, S.H. Immobilization of laccase via cross-linked enzyme aggregates prepared using genipin as a natural cross-linker. Int. J. Biol. Macromol. 2021, 169, 541. [Google Scholar] [CrossRef]
- Chen, H.Y.; Ting, Y.; Kuo, H.C.; Hsieh, C.W.; Hsu, S.Y.; Wu, C.N.; Cheng, K.C. Enzymatic degradation of ginkgolic acids by laccase immobilized on core/shell Fe3O4/nylon composite nanoparticles using novel coaxial electrospraying process. Int. J. Biol. Macromol. 2021, 172, 270. [Google Scholar] [CrossRef]
- Latif, A.; Maqbool, A.; Zhou, R.; Arsalan, M.; Sun, K.; Si, Y. Optimized degradation of bisphenol A by immobilized laccase from Trametes versicolor using Box-Behnken design (BBD) and artificial neural network (ANN). J. Environ. Chem. Eng. 2022, 10, 107331. [Google Scholar] [CrossRef]
- Jia, J.; Xue, P.; Ma, L.; Shi, K.; Li, R. A novel approach to efficient degradation of pesticide intermediate 2,4,5-trichlorophenol by co-immobilized laccase-acetosyringone biocatalyst. Biochem. Eng. J. 2022, 187, 108607. [Google Scholar] [CrossRef]
- Angelova, G.; Stoilova, I.; Dinkov, K.; Krastanov, A. Biodegradation of phenolic mixtures at high initial concentrations by Trametes Versicolor in a “Fed-batch” process. Eur. J. Biomed. Pharm. Sci. 2018, 5, 45–50. [Google Scholar]
- Karami, C.; Taher, M. A catechol biosensor based on immobilizing laccase to Fe3O4@Au core-shell nanoparticles. Int. J. Biol. Macromol. 2019, 129, 84. [Google Scholar] [CrossRef]
- Liu, L.; Anwar, S.; Ding, H.; Xu, M.; Yin, Q.; Xiao, Y.; Yang, X.; Yan, M.; Bi, H. Electrochemical sensor based on F,N-doped carbon dots decorated laccase for detection of catechol. J. Electroanal. Chem. 2019, 840, 84. [Google Scholar] [CrossRef]
- Li, D.; Cheng, Y.; Zuo, H.; Pan, G.; Fu, Y.; Wei, Q. Dual-functional biocatalytic membrane containing laccase-embedded metal-organic frameworks for detection and degradation of phenolic pollutant. J. Colloid Interface Sci. 2021, 603, 771. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, N.; Anupama, S.; Navya, K.; Shalini, H.; Idris, M.; Hampannavar, U. Biological removal of phenol from wastewaters: A mini review. Appl. Water Sci. 2015, 5, 105. [Google Scholar] [CrossRef]
- Nagendran, V.; Goveas, L.; Vinayagam, R.; Varadavenkatesan, T.; Selvaraj, R. Nanozymes in environmental remediation: A bibliometric and comprehensive review of their oxidoreductase-mimicking capabilities. Microchem. J. 2024, 207, 111748. [Google Scholar] [CrossRef]
- Patil, P.; Salokhe, S.; Karvekar, A.; Suryavanshi, P.; Phirke, A.; Tiwari, M.; Nadar, S. Microfluidic based continuous enzyme immobilization: A comprehensive review. Int. J. Biol. Macromol. 2023, 253, 127358. [Google Scholar] [CrossRef]
- Borham, A.; Bkhit, M.; Wang, J.; Qian, X. Immobilization of fungal laccase onto red seaweed biomass as a novel support for efficient dye decolorization. Environ. Technol. Innov. 2025, 38, 104143. [Google Scholar] [CrossRef]
- Prabhakar, T.; Giaretta, J.; Zulli, R.; Rath, R.; Farajikhah, S.; Talebian, S.; Dehghani, F. Covalent immobilization: A review from an enzyme perspective. Chem. Eng. J. 2025, 503, 158054. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. Enzym. Immobil. Technol. Ind. Appl. 2023, 6, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Daronch, N.; Kelbert, M.; Senna, C.; Hermes, P.; Oliveira, D. Elucidating the choice for a precise matrix for laccase immobilization: A review. Chem. Eng. J. 2020, 397, 125506. [Google Scholar] [CrossRef]
- Oromiehie, A.; Mamizadeh, A. Recycling PET beverage bottles and improving propert. Polym. Int. 2004, 53, 728. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Rosmaninho, M.G.; Jardim, E.; Ferreira, G.L.; Araújo, M.H.; Lago, R.M. Hidrólise parcial da superfície do polyethylene terephthalate (PET): Transformando um rejeito em um material de troca catiônica para aplicação ambiental. Quim. Nova 2009, 32, 1673. [Google Scholar] [CrossRef]
- Rajesh, S.; Murty, Z.V.P. Ultrafiltration membranes from waste polyethylene terephthalate and additives: Synthesis and characterization. Quim. Nova 2014, 37, 653. [Google Scholar] [CrossRef]
- Nasser Rabab, M.; Siddiq Maid, A.; Jamil Fakhra, J. Water purification using cellulosic fibers extracted from agriculture wastes and their modified copolymers. Kuwait J. Sci. 2024, 51, 1. [Google Scholar] [CrossRef]
- Young, J.; Sadeghi, K.; Seo, J. Chain-Extending Modification for Value-Added Recycled PET: A Review. Polym. Rev. 2022, 62, 860–889. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 1, 2912–2938. [Google Scholar] [CrossRef]
- Bech, L.; Meylheuc, T.; Lepoittevin, B.; Roger, P. Chemical surface modification of poly(ethylene terephthalate) fibers by aminolysis and grafting of carbohydrates. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2172–2183. [Google Scholar] [CrossRef]
- Noel, S.; Liberelle, B.; Robitaille, L.; De Crescenzo, G. Quantification of primary amine groups available for subsequent biofunctionalization of polymer surfaces. Bioconjugate Chem. 2011, 22, 1690. [Google Scholar] [CrossRef]
- Chen, C.; Liang, B.; Lu, D.; Ogino, A.; Wang, X.; Nagatsu, M. Amino group introduction onto multiwall carbon nanotubes by NH3/Ar plasma treatment. Carbon 2010, 48, 939–948. [Google Scholar] [CrossRef]
- Purkait, M.; Sinha, M.; Mondal, P.; Singh, R. Chapter 3—Temperature-Responsive Membranes. Interface Sci. Technol. 2018, 25, 67–113. [Google Scholar] [CrossRef]
- Casimiro, J.; Lepoittevin, B.; Boisse-Laporte, C.; Barthés-Labrousse, M.G.; Jegou, P.; Brisset, F. Introduction of Primary Amino Groups on Poly(ethylene terephthalate) Surfaces by Ammonia and a Mix of Nitrogen and Hydrogen Plasma. Plasma Chem. Plasma Process. 2012, 32, 305–323. [Google Scholar] [CrossRef]
- Delcorte, A.; Cristaudo, V.; Zarshenas, M.; Merche, D.; Reniers, F.; Bertrand, P. Chemical Analysis of Plasma-treated Organic Surfaces and Plasma Polymers by Secondary Ion Mass Spectrometry. Plasma Process. Polym. 2015, 12, 905–918. [Google Scholar] [CrossRef]
- Pal, D.; Neogi, S.; De, S. Surface modification of polyacrylonitrile co-polymer membranes using pulsed direct current nitrogen plasma. Thin Solid Films 2015, 597, 171–182. [Google Scholar] [CrossRef]
- Neira, M.; Borjas, J.; Hernández, E.; Hernández, C.; Narro, R.I.; Hernández, J. Nanocomposites Prepared with High Density Polyethylene and Carbon Nanofibers Modified by Ethylene Plasma. Plasma Process. Polym. 2015, 12, 477–485. [Google Scholar] [CrossRef]
- Thiry, D.; Konstantinidis, S.; Cornil, J.; Snyders, R. Plasma diagnostics for the low-pressure plasma polymerization process: A critical review. Thin Solid Films 2016, 606, 19–44. [Google Scholar] [CrossRef]
- Gotoh, K.; Shohbuke, E.; Kobayashi, Y.; Yamada, H. Wettability control of PET surface by plasma-induced polymer film deposition and plasma/UV oxidation in ambient air. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 1. [Google Scholar] [CrossRef]
- Narimis, M.; Onyshchenko, Y.; Morent, R.; Geyter, N. Improvement of PET surface modification using an atmospheric pressure plasma jet with different shielding gases. Polymer 2021, 215, 123421. [Google Scholar] [CrossRef]
- Elammari, F.A.; Daniels, S. Polymer Surface Modification Using Atmospheric Pressure Plasma. Encycl. Mater. Plast. Polym. 2022, 3, 575. [Google Scholar] [CrossRef]
- Vo, T.S.; Vo, T. Surface characterization of polyimide and polyethylene terephthalate membranes toward plasma and UV treatments. Prog. Nat. Sci. Mater. Int. 2022, 32, 314. [Google Scholar] [CrossRef]
- Peñarrieta, J.; Tejeda, L.; Mollinedo, P.; Vila, J.; Bravo, J. Phenolic Compounds in Food. Rev. Boliv. Quim. 2014, 3, 68. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Mozetic, M.; Primc, G. Surface modification of PS polymer by oxygen-atom treatment from remote plasma: Initial kinetics of functional groups formation. Appl. Surf. Sci. 2021, 561, 150058. [Google Scholar] [CrossRef]
- Burmeister, N.; Vollstedt, C.; Kröger, C.; Friedrich, T.; Scharnagl, N.; Rohnke, M.; Zorn, E.; Wicha, S.; Streit, W.; Maison, W. Zwitterionic surface modification of polyethylene via atmospheric plasma-induced polymerization of (vinylbenzyl-)sulfobetaine and evaluation of antifouling properties. Colloids Surf. B Biointerfaces 2023, 224, 113195. [Google Scholar] [CrossRef]
- Chytrosz-Wrobel, P.; Golda-Cepa, M.; Stodolak-Zych, E.; Rysz, J.; Kotarba, A. Effect of oxygen plasma-treatment on surface functional groups, wettability, and nanotopography features of medically relevant polymers with various crystallinities. Appl. Surf. Sci. Adv. 2023, 18, 100497. [Google Scholar] [CrossRef]
- Nakulan, A.; Sumithra, K.; Sheethal, Z.; Yuvaraj, S.; Peranantham, A.P.; Jeyachandran, Y. Surface modification and patterning of polymer thin films by plasma and adsorption behavior of proteins. Surf. Interfaces 2024, 55, 105342. [Google Scholar] [CrossRef]
- Prasad, D.; Prakash, R.; Man, U. Surface modification of polymers by 50 Hz dielectric barrier discharge (DBD) plasma produced in air at 40 Torr. Fundam. Plasma Phys. 2024, 10, 100058. [Google Scholar] [CrossRef]
- Sánchez, J.; Martínez, J.; López, R.; Segura, E.; Saade, H.; Ramos, R.; Ilyna, A. Waste Biomass Valorization; Springer Nature: Berlin, Germany, 2018; Volume 9, p. 223. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248. [Google Scholar] [CrossRef]
- Wang, F.; Chen, G.; Liang-rong, Y.; Chun-Zhao, L. Magnetic mesoporous silica nanoparticles: Fabrication and their laccase immobilization performance. Bioresour. Technol. 2010, 101, 8931. [Google Scholar]
- Burkersroda, F.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4121. [Google Scholar] [CrossRef] [PubMed]
- Chernik, V.N.; Paskhalov, A.A.; Gaidar, A.I. Polymer surface erosion under an oxygen plasma stream. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2009, 3, 215–217. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Valera-Zaragoza, M.; Aparicio-Saguilán, A.; Conde-Acevedo, J. Comportamiento térmico de películas de almidón de platano con polietileno tereftalato degradado. Rev. Mex. Ing. Quim. 2015, 14, 513–521. Available online: https://www.redalyc.org/articulo.oa?id=62041194027 (accessed on 25 February 2025).
- Savoji, H.; Lerouge, S.; Ajji, A.; Wertheimer, M. Plasma-Etching for Controlled Modification of Structural and Mechanical Properties of Electrospun PET Scaffolds. Plasma Process. Polym. 2015, 12, 314. [Google Scholar] [CrossRef]
- Mu, H.; Wang, X.; Li, Z.; Xie, Y.; Gao, Y.; Liu, H. Preparation and atomic oxygen erosion resistance of SiOx coating formed on polyimide film by plasma polymer deposition. Vacuum 2019, 165, 7–11. [Google Scholar] [CrossRef]
- Ma, J.; Fan, H.; Li, Z.; Jia, Y.; Yadav, A.; Dong, G.; Wang, W.; Dong, W.; Wang, S. Multi-walled carbon nanotubes/polyaniline on the ethylenediamine modified polyethylene terephthalate fibers for a flexible room temperature ammonia gas sensor with high responses. Sens. Actuators B Chem. 2021, 334, 129677. [Google Scholar] [CrossRef]
- Stetsiv, Y.; Yatsyshyn, M.; Nykypanchuk, D.; Korniy, S.; Saldan, I.; Reshetnyak, O.; Bednarchuk, T. Characterization of polyaniline thin films prepared on polyethylene terephthalate substrate. Polym. Bull. 2021, 78, 6251. [Google Scholar] [CrossRef]
- Brading, H.; Morton, P.; Bell, T.; Earwaker, L. Plasma Nitriding with Nitrogen, Hydrogen, and Argon Gas Mixtures: Structure and Composition of Coatings on Titanium. Surf. Eng. 1992, 8, 206. [Google Scholar] [CrossRef]
- Mora-Cortés, L.; Rivas-Muñoz, A.; Neira-Velázquez, M.; Contreras-Esquive, J.; Roger, P.; Mora-Cura, Y.; Soria-Arguello, G.; Bolaina-Lorenzo, E.; Reyna-Martínez, R.; Zugasti-Cruz, A.; et al. Biocompatible enhancement of poly(ethylene terephthalate) (PET) waste films by cold plasma aminolysis. J. Chem. Technol. Biotechnol. 2022, 97, 3001. [Google Scholar] [CrossRef]
- Wang, M.; Chang, Y.; Poncin-Epaillard, F. Effects of the Addition of Hydrogen in the Nitrogen Cold Plasma: The Surface Modification of Polystyrene. Langmuir 2003, 19, 8325–8330. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, T.; Yang, Z. Flexible polyethylene terephthalate/polyaniline composite paper with bending durability and effective electromagnetic shielding performance. Chem. Eng. J. 2020, 389, 124433. [Google Scholar] [CrossRef]
- Anbarasan, R.; Vasudevan, T.; Gopalan, A. Chemical grafting of poly(aniline) and poly(o-toluidine) onto PET fibre—A comparative study. Eur. Polym. J. 2000, 36, 1725. [Google Scholar] [CrossRef]
- Liu, S.; Liu, D.; Pan, Z. The Effect of Polyaniline (PANI) Coating via Dielectric-Barrier Discharge (DBD) Plasma on Conductivity and Air Drag of Polyethylene Terephthalate (PET) Yarn. Polymers 2018, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alba, E.; Dionisio, E.; Pérez-Calixto, M.; Huerta, L.; García, L.; Hautefeuille, M.; Vázquez, G.; Burnillo, G. Surface modification of polyethylenterephthalate film with primary amines using gamma radiation and aminolysis reaction for cell adhesion studies. Radiat. Phys. Chem. 2020, 176, 109070. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Tian, D.; Liu, X.; Wu, Z. Amino-functionalized MOF immobilized laccase for enhancing enzyme activity stability and degrading Congo red. J. Taiwan Inst. Chem. Eng. 2023, 143, 104647. [Google Scholar] [CrossRef]
- Rodrigues, R.; Berenguer-Murcia, A.; Carballares, D.; Morellon-Sterling, R.; Fernández-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotech. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, J.; Martínez-Hernández, J.L.; López-Campos, R.G.; Segura-Ceniceros, E.P.; Saade, H.; Ramos-González, R.; Neira-Velázquez, M.G.; Medina-Morales, M.A.; Aguilar, C.; Ilyina, A. Laccase Validation as Pretreatment of Agave Waste Prior to Saccharification: Free and Immobilized in Superparamagnetic Nanoparticles Enzyme Preparations. Waste Biomass Valor 2018, 9, 223–224. [Google Scholar] [CrossRef]
- Rangel-Rodríguez, A.; Conxita, S.; Susana, V.; Flores-Gallardo, S.; Contreras-Esquivel, J.; Licea Jiménez, L. Immobilization of Pectinesterase in Genipin-Crosslinked Chitosan Membrane for Low Methoxyl Pectin Production. Appl. Biochem. Biotechnol. 2014, 174, 2941–2950. [Google Scholar] [CrossRef]

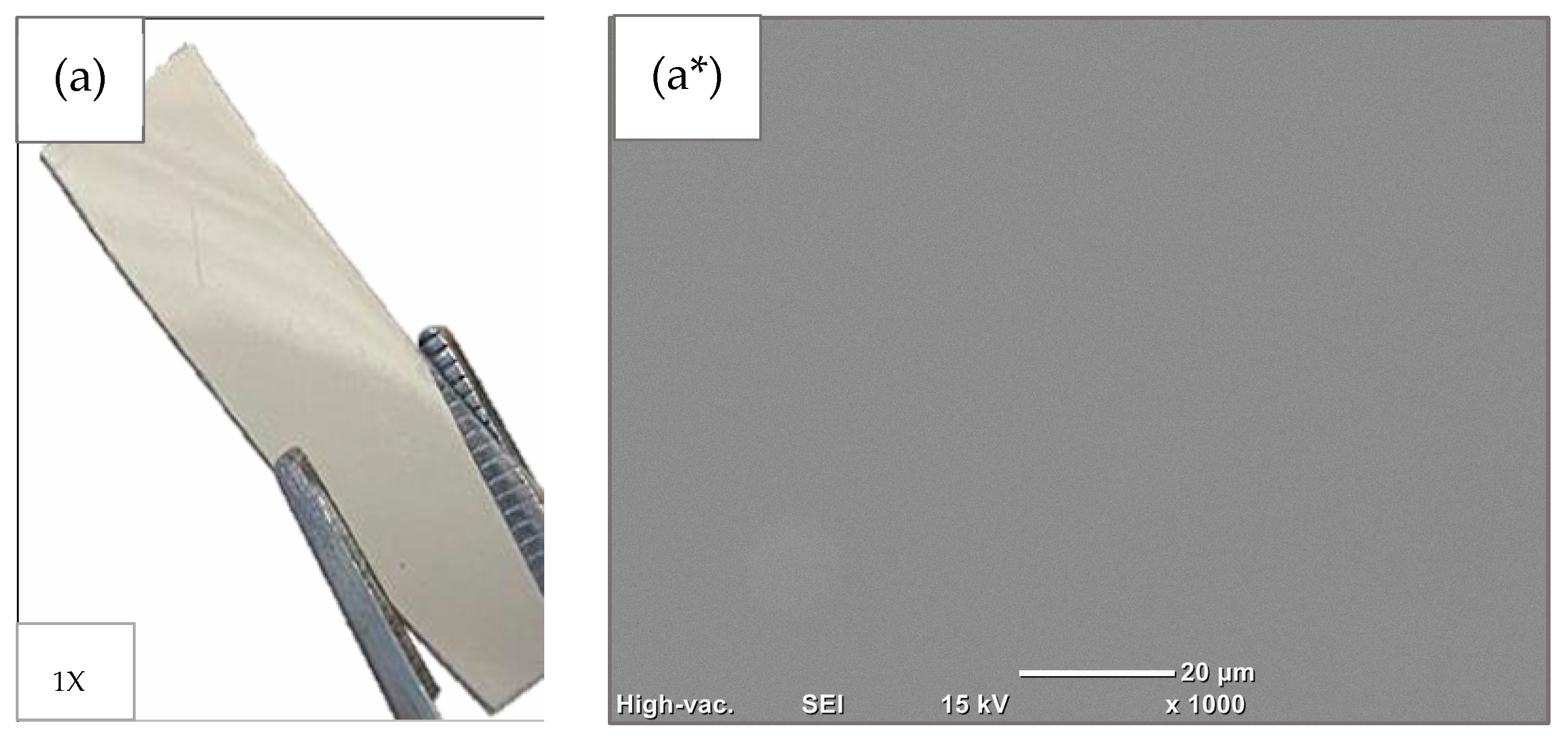


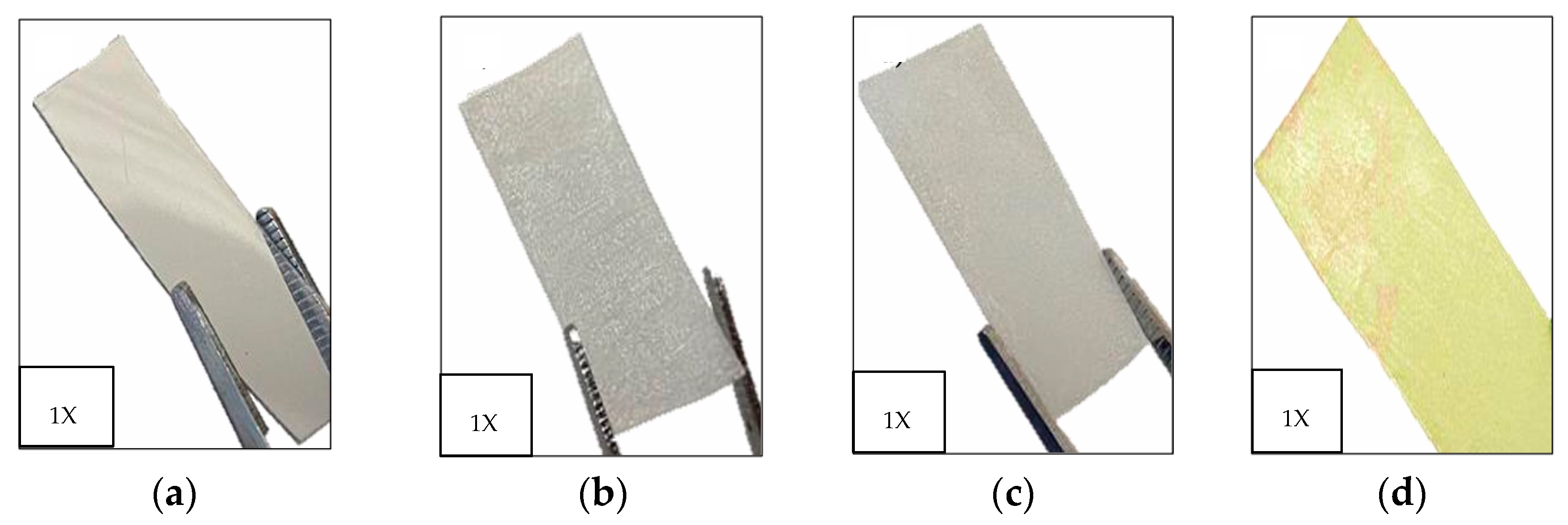


| RF Plasma-Activated Films | |||||
|---|---|---|---|---|---|
| EtDA90 | N2/H290 | An90 | |||
| Density-NH2 (mg·mm−2) | Density-NH2 (mg·mm−2) | Density-NH2 (mg·mm−2) | |||
| No etching | Etching | No etching | Etching | No etching | Etching |
| 1.02 ± 0.03 | 3.98 ± 0.10 | 0.86 ± 0.04 | 1.79 ± 0.02 | 0.33 ± 0.01 | 1.53 ± 0.10 |
 |  |  |  |  |  |
| Film | Density-NH2 | Contact Angle Average | Contact Angle Illustration |
|---|---|---|---|
| E0 | - | 79.70° ± 0.11 |  image a |
| E6 | - | 50.20° ± 0.10 |  image b |
| E0-EtDA90 | 1.02 ± 0.09 | 66.40° ± 0.10 |  image c |
| E6-EtDA90 | 3.98 ± 0.10 | 51.10° ± 0.10 |  image d |
| E0-N2/H2 | 0.86 ± 0.08 | 74.20° ± 0.11 | 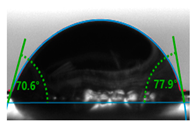 image e |
| E6-N2/H2 | 1.79 ± 0.10 | 60.60° ± 0.12 | 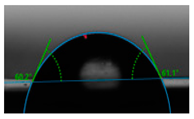 image f |
| E0-An90 | 0.33 ± 0.01 | 73.90° ± 0.12 |  image g |
| E6-An90 | 1.53 ± 0.08 | 75.30° ± 0.10 | 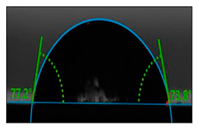 image h |
| Films | Immobilized Protein |
|---|---|
| EtDA90-G-EzL | 22.46% ± 0.12% |
| N2/H290-G-EzL | 19.73% ± 0.10% |
| An90-G-EzL | 18.55% ± 0.11% |
| E6-EtDA90-EzL | 0.00% |
| E6-N2/H290-EzL | 0.00% |
| E6-An90-EzL | 0.00% |
| E6-EtDA90-G-EzL | 97.30% ± 0.10% |
| E6-N2/H290-G-EzL | 94.30% ± 0.12% |
| E6-An90-G-EzL | 93.10% ± 0.10% |
| Films | Immobilized Protein | U (µmol·min−1) mL−1 | U % |
|---|---|---|---|
| E6-EtDA90-G-EzL | 97.30% ± 0.10% | 0.36 ± 0.08 | 86.11% ± 0.09% |
| E6-N2/H290-G-EzL | 94.30% ± 0.12% | 0.27 ± 0.07 | 77.03% ± 0.08% |
| E6-An90-G-EzL | 93.10% ± 0.10% | 0.21 ± 0.07 | 71.35% ± 0.09% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsua-Gaona, R.; Narro-Céspedes, R.I.; Ilina, A.; Mora-Cortés, L.F.; Reyes-Acosta, Y.K.; Soria-Arguello, G.; Luevano-Martínez, C.; Luévanos-Escareño, M.P.; Cuellar-Gaona, C.G. Etching and Precursor Effects on Plasma-Modified Waste Polyethylene Terephthalate (PET) to Laccase Immobilization Applied in Catechol Biodegradation for Water Treatment. Coatings 2025, 15, 421. https://doi.org/10.3390/coatings15040421
Orsua-Gaona R, Narro-Céspedes RI, Ilina A, Mora-Cortés LF, Reyes-Acosta YK, Soria-Arguello G, Luevano-Martínez C, Luévanos-Escareño MP, Cuellar-Gaona CG. Etching and Precursor Effects on Plasma-Modified Waste Polyethylene Terephthalate (PET) to Laccase Immobilization Applied in Catechol Biodegradation for Water Treatment. Coatings. 2025; 15(4):421. https://doi.org/10.3390/coatings15040421
Chicago/Turabian StyleOrsua-Gaona, Reyna, Rosa Idalia Narro-Céspedes, Anna Ilina, Luis Fernando Mora-Cortés, Yadira Karina Reyes-Acosta, Gustavo Soria-Arguello, Cynthia Luevano-Martínez, Miriam Paulina Luévanos-Escareño, and Claudia Gabriela Cuellar-Gaona. 2025. "Etching and Precursor Effects on Plasma-Modified Waste Polyethylene Terephthalate (PET) to Laccase Immobilization Applied in Catechol Biodegradation for Water Treatment" Coatings 15, no. 4: 421. https://doi.org/10.3390/coatings15040421
APA StyleOrsua-Gaona, R., Narro-Céspedes, R. I., Ilina, A., Mora-Cortés, L. F., Reyes-Acosta, Y. K., Soria-Arguello, G., Luevano-Martínez, C., Luévanos-Escareño, M. P., & Cuellar-Gaona, C. G. (2025). Etching and Precursor Effects on Plasma-Modified Waste Polyethylene Terephthalate (PET) to Laccase Immobilization Applied in Catechol Biodegradation for Water Treatment. Coatings, 15(4), 421. https://doi.org/10.3390/coatings15040421










