Flexibility-Engineered Nb2O5 Carbon Nanofiber Film Anodes: Concentration-Dependent Optimization for Mechanically Robust and Stable Sodium Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
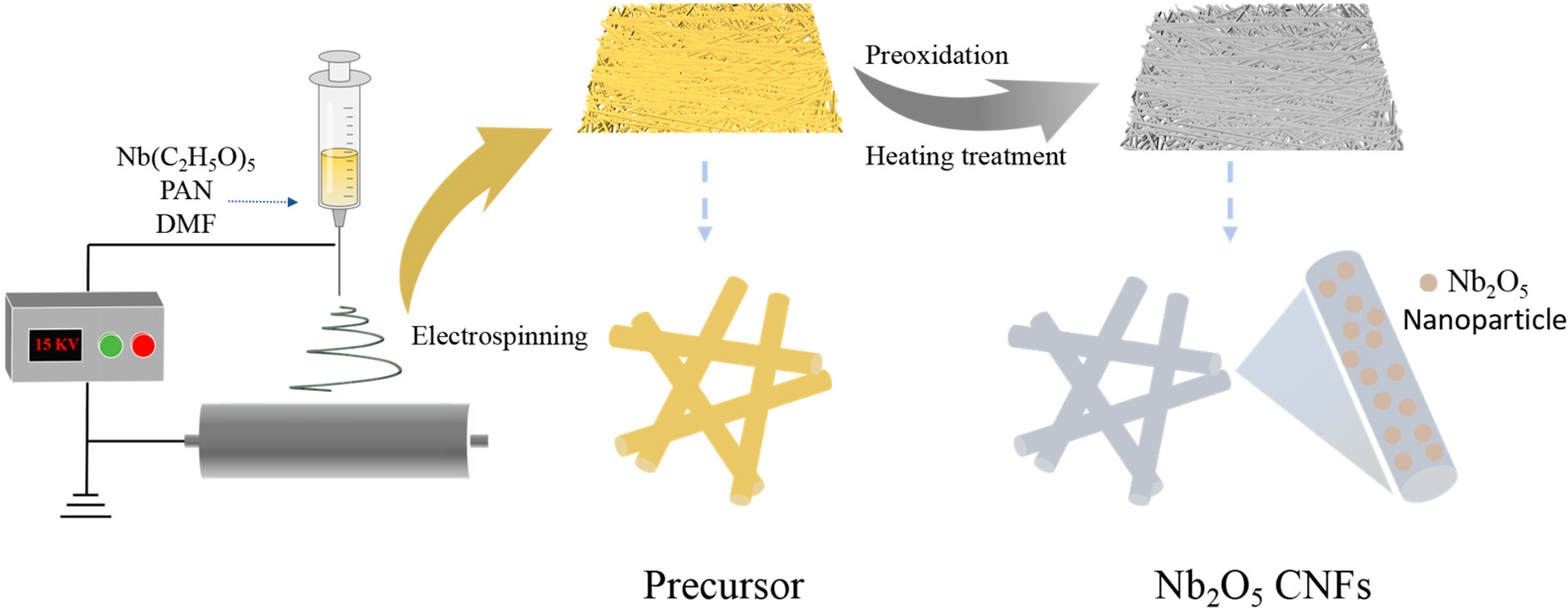
2.2. Methods
2.3. Characterizations
2.4. Electrochemical Test0073
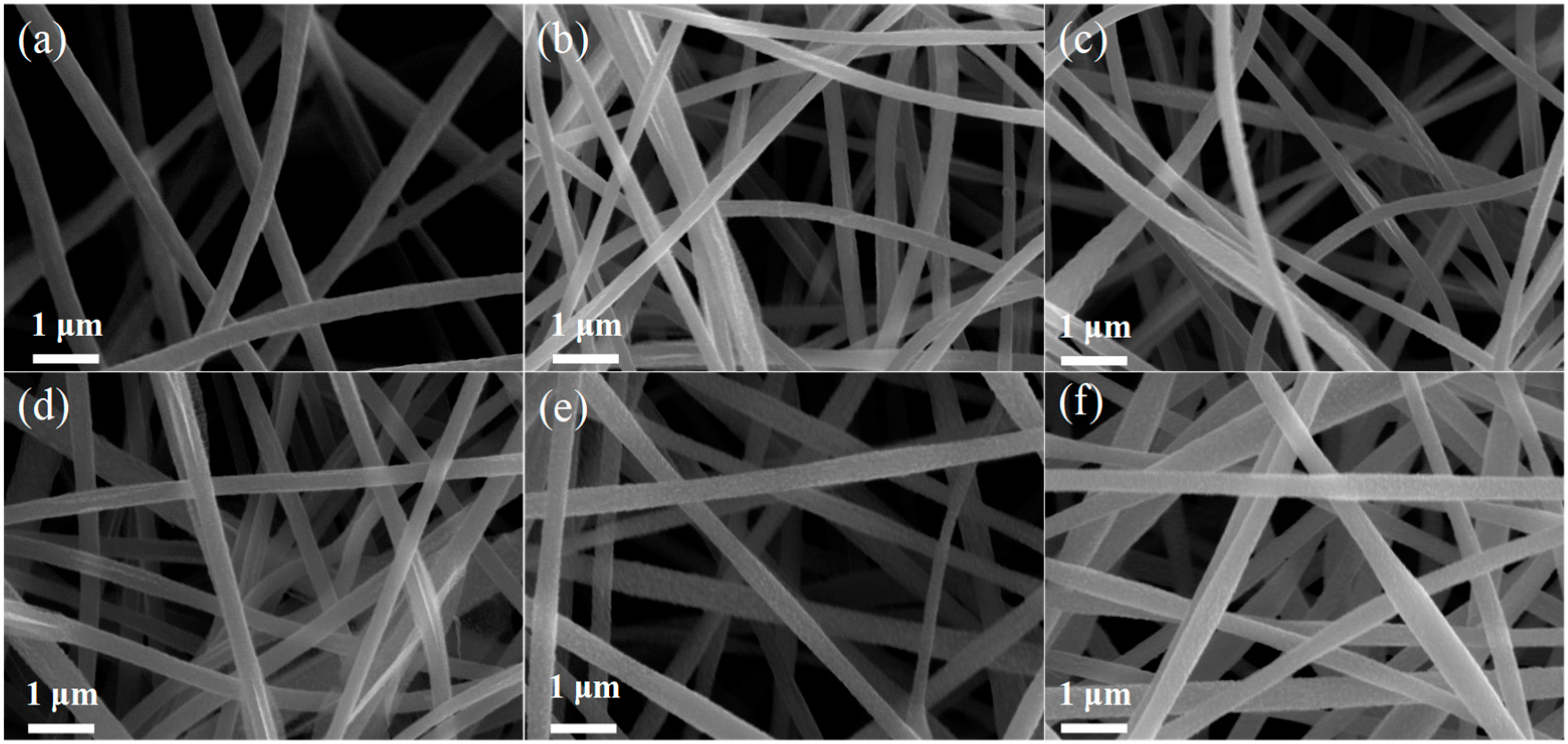
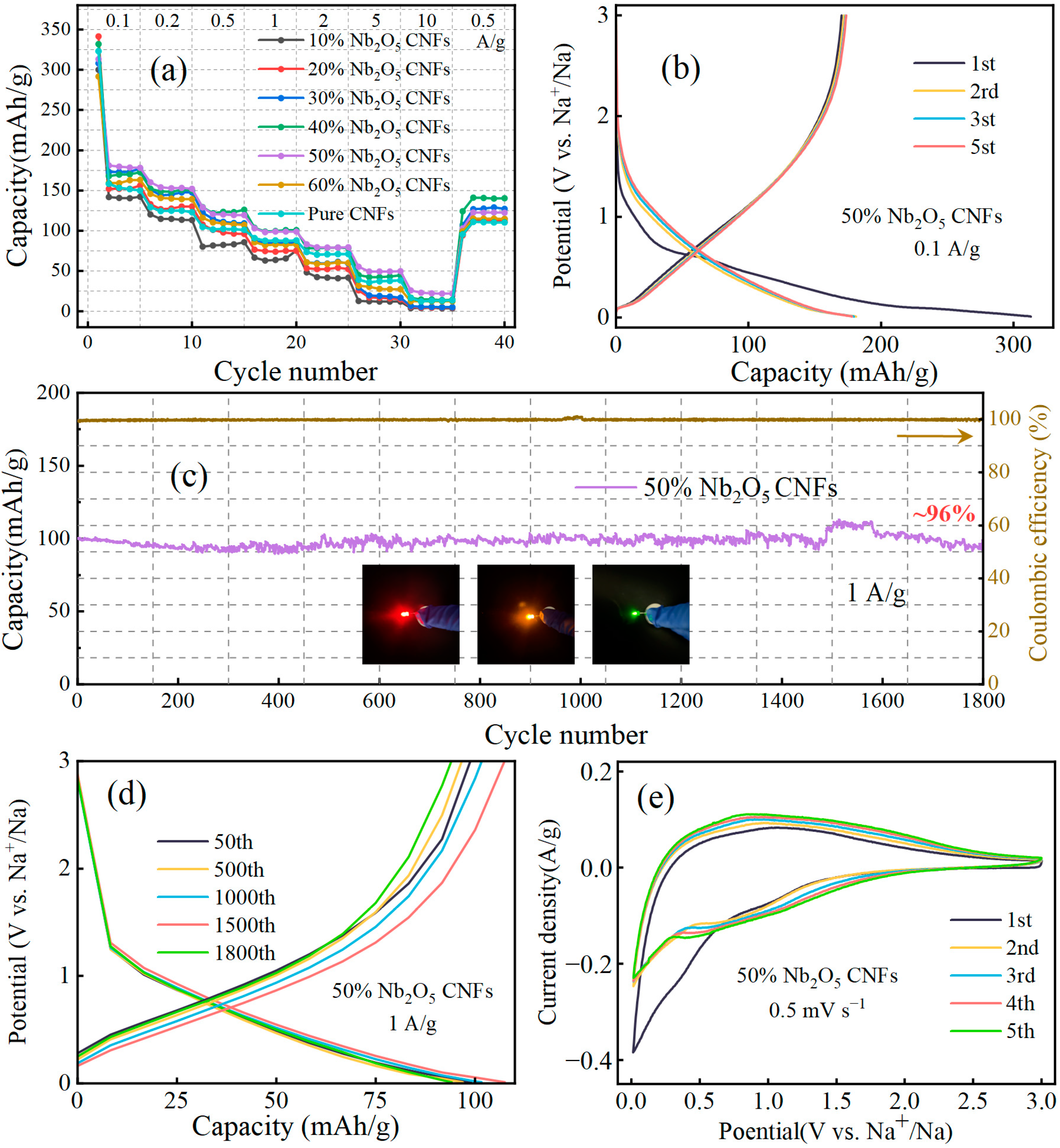
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, H.; Gao, S.; Ren, M.; Huang, Y.; Cheng, F.; Chen, J.; Li, F. Dual-Function Presodiation with Sodium Diphenyl Ketone towards Ultra-Stable Hard Carbon Anodes for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202214717. [Google Scholar] [CrossRef]
- Sun, N.; Qiu, J.; Xu, B. Understanding of Sodium Storage Mechanism in Hard Carbons: Ongoing Development under Debate. Adv. Energy Mater. 2022, 12, 2200715. [Google Scholar] [CrossRef]
- El Moctar, I.; Ni, Q.; Bai, Y.; Wu, F.; Wu, C. Hard Carbon Anode Materials for Sodium-Ion Batteries. Funct. Mater. Lett. 2018, 11, 1830003. [Google Scholar] [CrossRef]
- Kim, H.; Hyun, J.C.; Kim, D.-H.; Kwak, J.H.; Lee, J.B.; Moon, J.H.; Choi, J.; Lim, H.-D.; Yang, S.J.; Jin, H.M.; et al. Revisiting Lithium- and Sodium-Ion Storage in Hard Carbon Anodes. Adv. Mater. 2023, 35, 2209128. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, J.; Zhang, Y.; Li, Q.; Jia, Y.; Dong, X.; Xiao, J.; Tao, Y.; Yang, Q.-H. Reconfiguring Hard Carbons with Emerging Sodium-Ion Batteries: A Perspective. Adv. Mater. 2023, 35, 2212186. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, N.; Zhao, X.; Liu, N.; Qin, B.; Qiu, M.; Wang, L. A Novel PbSe@CNTs Anode Material Based on Dual Conversion-Alloying Mechanism for Sodium-Ion Batteries. Sci. China Mater. 2023, 66, 61–68. [Google Scholar] [CrossRef]
- Deng, Q.; Yao, L. Advanced Nb2O5 Anode towards Fast Pseudocapacitive Sodium Storage. Coatings 2022, 12, 1873. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, R.; Wang, J.; Zheng, M.; Wang, J.; Yu, R.; Wang, D. Delicate Co-Control of Shell Structure and Sulfur Vacancies in Interlayer-Expanded Tungsten Disulfide Hollow Sphere for Fast and Stable Sodium Storage. Adv. Mater. 2023, 35, 2209354. [Google Scholar] [CrossRef]
- Suzuki, T.; Cheng, J.; Qiao, L.; Xing, Y.; Zhang, M.F.; Nishijima, H.; Yano, T.; Pan, W. Preparation of SnO2 Nanotubes via a Template-Free Electrospinning Process. RSC Adv. 2020, 10, 22113–22119. [Google Scholar] [CrossRef]
- Wu, X.; Lan, X.; Hu, R.; Yao, Y.; Yu, Y.; Zhu, M. Tin-Based Anode Materials for Stable Sodium Storage: Progress and Perspective. Adv. Mater. 2022, 34, 2106895. [Google Scholar] [CrossRef]
- Xia, J.; Jiang, K.; Xie, J.; Guo, S.; Liu, L.; Zhang, Y.; Nie, S.; Yuan, Y.; Yan, H.; Wang, X. Tin Disulfide Embedded in N-, S-Doped Carbon Nanofibers as Anode Material for Sodium-Ion Batteries. Chem. Eng. J. 2019, 359, 1244–1251. [Google Scholar] [CrossRef]
- Zheng, Y.; He, L.; Kong, X.; Song, Y.; Zhao, Y. Three-Dimensional Porous N-Doped Graphite Carbon with Embedded CoS2 Nanoparticles as Advanced Anode for Sodium-Ion Batteries. Appl. Surf. Sci. 2022, 603, 154481. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun Nanomaterials for Supercapacitor Electrodes: Designed Architectures and Electrochemical Performance. Adv. Energy Mater. 2017, 7, 1601301. [Google Scholar] [CrossRef]
- Zhu, G.; Xia, G.; Pan, H.; Yu, X. Size-Controllable Nickel Sulfide Nanoparticles Embedded in Carbon Nanofibers as High-Rate Conversion Cathodes for Hybrid Mg-Based Battery. Adv. Sci. 2022, 9, 2106107. [Google Scholar] [CrossRef]
- Tao, B.; Yang, W.; Zhou, M.; Qiu, L.; Lu, S.; Wang, X.; Zhao, Q.; Xie, Q.; Ruan, Y. Designing a Carbon Nanofiber-Encapsulated Iron Carbide Anode and Nickel-Cobalt Sulfide-Decorated Carbon Nanofiber Cathode for High-Performance Supercapacitors. J. Colloid Interface Sci. 2022, 621, 139–148. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, L.; Shi, H.; Li, Z.; Kang, W.; Cheng, B. Three-Dimensional Porous Carbon Nanofiber Loading MoS2 Nanoflake-Flowerballs as a High-Performance Anode Material for Li-Ion Capacitor. Appl. Surf. Sci. 2019, 484, 392–402. [Google Scholar] [CrossRef]
- Xiong, Y.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Electrospun TiO2/C Nanofibers As a High-Capacity and Cycle-Stable Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 16684–16689. [Google Scholar] [CrossRef]
- Shan, C.; Feng, X.; Yang, J.; Yang, X.; Guan, H.-Y.; Argueta, M.; Wu, X.-L.; Liu, D.-S.; Austin, D.J.; Nie, P.; et al. Hierarchical Porous Carbon Pellicles: Electrospinning Synthesis and Applications as Anodes for Sodium-Ion Batteries with an Outstanding Performance. Carbon 2020, 157, 308–315. [Google Scholar] [CrossRef]
- Wang, N.; Gao, Y.; Wang, Y.; Liu, K.; Lai, W.; Hu, Y.; Zhao, Y.; Chou, S.; Jiang, L. Nanoengineering to Achieve High Sodium Storage: A Case Study of Carbon Coated Hierarchical Nanoporous TiO2 Microfibers. Adv. Sci. 2016, 3, 1600013. [Google Scholar] [CrossRef]
- Bai, H.; Zhu, X.; Ao, H.; He, G.; Xiao, H.; Chen, Y. Advances in Sodium-Ion Batteries at Low-Temperature: Challenges and Strategies. J. Energy Chem. 2024, 90, 518–539. [Google Scholar] [CrossRef]
- Lígia da Silva Lima, L.; Wu, J.; Cadena, E.; Groombridge, A.S.; Dewulf, J. Towards Environmentally Sustainable Battery Anode Materials: Life Cycle Assessment of Mixed Niobium Oxide (XNOTM) and Lithium-titanium-Oxide (LTO). Sustain. Mater. Technol. 2023, 37, e00654. [Google Scholar] [CrossRef]
- Mu, Q.; Liu, R.; Kimura, H.; Li, J.; Jiang, H.; Zhang, X.; Yu, Z.; Sun, X.; Algadi, H.; Guo, Z.; et al. Supramolecular Self-Assembly Synthesis of Hemoglobin-like Amorphous CoP@N, P-Doped Carbon Composites Enable Ultralong Stable Cycling under High-Current Density for Lithium-Ion Battery Anodes. Adv. Compos. Hybrid Mater. 2023, 6, 23. [Google Scholar] [CrossRef]
- Etesami, M.; Nguyen, M.T.; Yonezawa, T.; Tuantranont, A.; Somwangthanaroj, A.; Kheawhom, S. 3D Carbon Nanotubes-Graphene Hybrids for Energy Conversion and Storage Applications. Chem. Eng. J. 2022, 446, 137190. [Google Scholar] [CrossRef]
- Xie, F.; Xu, J.; Liao, Q.; Zhang, Q.; Liu, B.; Shao, L.; Cai, J.; Shi, X.; Sun, Z.; Wong, C.-P. Progress in Niobium-Based Oxides as Anode for Fast-Charging Li-Ion Batteries. Energy Rev. 2023, 2, 100027. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, T.; Li, W.; Li, T.; Zhang, L.; Zhang, X.; Wang, Z. Engineering of Sodium-Ion Batteries: Opportunities and Challenges. Engineering 2023, 24, 172–183. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, L.; Deng, Q.; Mai, G.; Mei, Z.; Ji, H.; Yao, L. Flexible Nb2O5/MoS2/Carbon Nanofiber Paper Anodes for Sodium-Ion Batteries. ACS Appl. Nano Mater. 2025, 8, 200–209. [Google Scholar] [CrossRef]
- Deng, Q.; Chen, F.; Liu, S.; Bayaguud, A.; Feng, Y.; Zhang, Z.; Fu, Y.; Yu, Y.; Zhu, C. Advantageous Functional Integration of Adsorption-Intercalation-Conversion Hybrid Mechanisms in 3D Flexible Nb2O5 @Hard Carbon@MoS2 @Soft Carbon Fiber Paper Anodes for Ultrafast and Super-Stable Sodium Storage. Adv. Funct. Mater. 2020, 30, 1908665. [Google Scholar] [CrossRef]
- Deng, Q.; Fu, Y.; Zhu, C.; Yu, Y. Niobium-Based Oxides Toward Advanced Electrochemical Energy Storage: Recent Advances and Challenges. Small 2019, 15, 1804884. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, J.; Wu, Z.; Zhao, J.; Zeng, Y.; Li, H.; Meng, M.; He, Y.; Jiao, P.; Pan, H.; et al. Surface Gradient Desodiation Chemistry in Layered Oxide Cathode Materials. Angew. Chem. Int. Ed. 2024, 63, e202410080. [Google Scholar] [CrossRef]
- Nakayama, H.; Nose, M.; Nakanishi, S.; Iba, H. Electrochemical Reactions of Niobium Based Oxide Materials as Anodes for Sodium Ion Batteries. Meet. Abstr. 2014, MA2014-02, 98. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Li, Y.; Feng, X.; Zheng, L.; Liu, M.; Li, S.; Qian, J.; Wang, Z.; Ren, H.; et al. Multilevel Gradient-Ordered Silicon Anode with Unprecedented Sodium Storage. Adv. Mater. 2024, 36, 2310270. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Krumeich, F.; Ono, L.K.; Guo, T.; Morimoto, R.; Ding, C.; Xu, Z.; Liu, M.; Qi, Y. Exploring Niobium Oxide-Based Materials for Fast-Charging Lithium-Ion Anodes: Insights from Structure to Property. Mater. Sci. Eng. R Rep. 2025, 162, 100887. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wang, J.; Wu, C.; Wang, W.; Chen, Y.; Hu, C.; Mo, K.; Gao, T.; He, Y.-S.; et al. Gradient-Porous-Structured Ni-Rich Layered Oxide Cathodes with High Specific Energy and Cycle Stability for Lithium-Ion Batteries. Nat. Commun. 2024, 15, 10216. [Google Scholar] [CrossRef]
- Kim, Y.; Yoo, J.; Lee, K. High-Performance Binder-Free Li-Ion Batteries Using Dynamically Transformed Niobium Oxide Nanochannel Electrodes. J. Power Sources 2025, 630, 236167. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Lin, D.; Liu, S.; Li, J.; Tang, Y.; Pan, Y.; Deng, Q.; Yao, L. Flexibility-Engineered Nb2O5 Carbon Nanofiber Film Anodes: Concentration-Dependent Optimization for Mechanically Robust and Stable Sodium Storage. Coatings 2025, 15, 374. https://doi.org/10.3390/coatings15040374
Zhu X, Lin D, Liu S, Li J, Tang Y, Pan Y, Deng Q, Yao L. Flexibility-Engineered Nb2O5 Carbon Nanofiber Film Anodes: Concentration-Dependent Optimization for Mechanically Robust and Stable Sodium Storage. Coatings. 2025; 15(4):374. https://doi.org/10.3390/coatings15040374
Chicago/Turabian StyleZhu, Xuhui, Duiming Lin, Siying Liu, Jufang Li, Yi Tang, Yancheng Pan, Qinglin Deng, and Lingmin Yao. 2025. "Flexibility-Engineered Nb2O5 Carbon Nanofiber Film Anodes: Concentration-Dependent Optimization for Mechanically Robust and Stable Sodium Storage" Coatings 15, no. 4: 374. https://doi.org/10.3390/coatings15040374
APA StyleZhu, X., Lin, D., Liu, S., Li, J., Tang, Y., Pan, Y., Deng, Q., & Yao, L. (2025). Flexibility-Engineered Nb2O5 Carbon Nanofiber Film Anodes: Concentration-Dependent Optimization for Mechanically Robust and Stable Sodium Storage. Coatings, 15(4), 374. https://doi.org/10.3390/coatings15040374







