Investigation into the Preparation and Electrochemical Energy Storage Performance of Nickel Cobalt Oxide-Based Composite Anode Materials
Abstract
1. Introduction
2. Experiment
2.1. Preparation of SSFF/GO/NiCo2O4 Electrode Materials
2.2. MFC Construction and Operation
2.3. Characterizations and Measurements
2.4. Microbial Characterization Technique
3. Results and Discussion
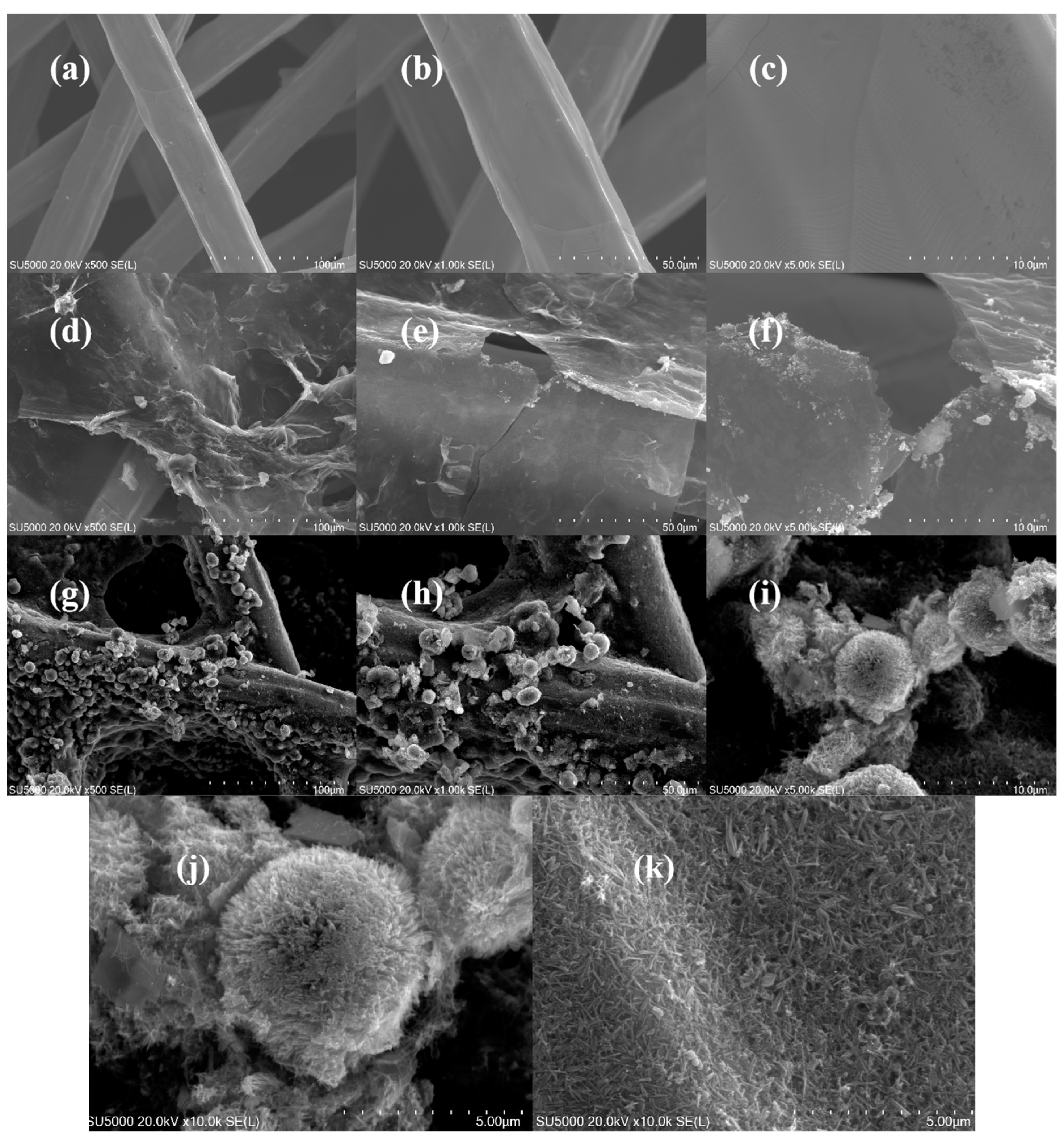
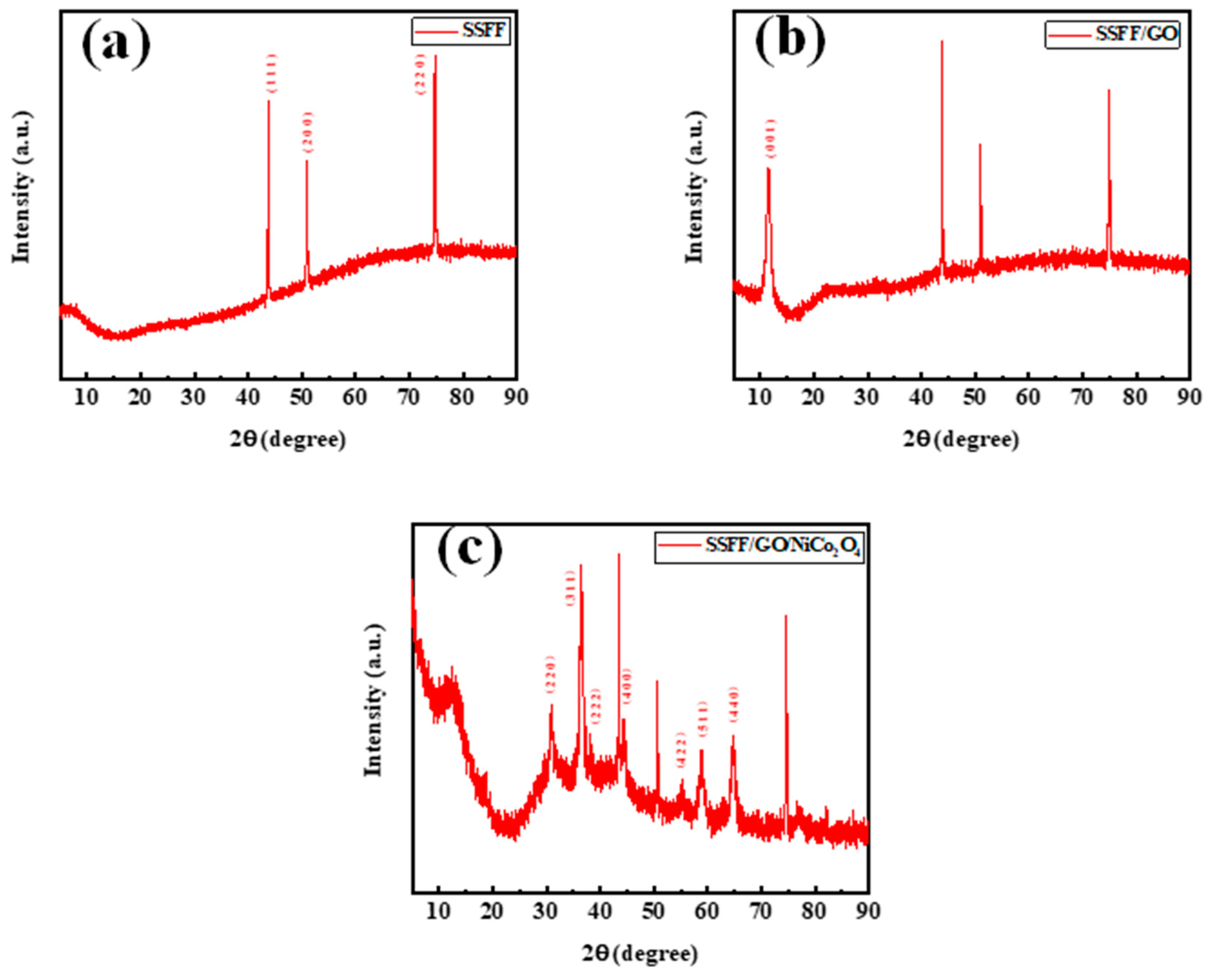
3.1. Preparation and Morphology Characteristics of SSFF/GO/NiCo2O4 Anode
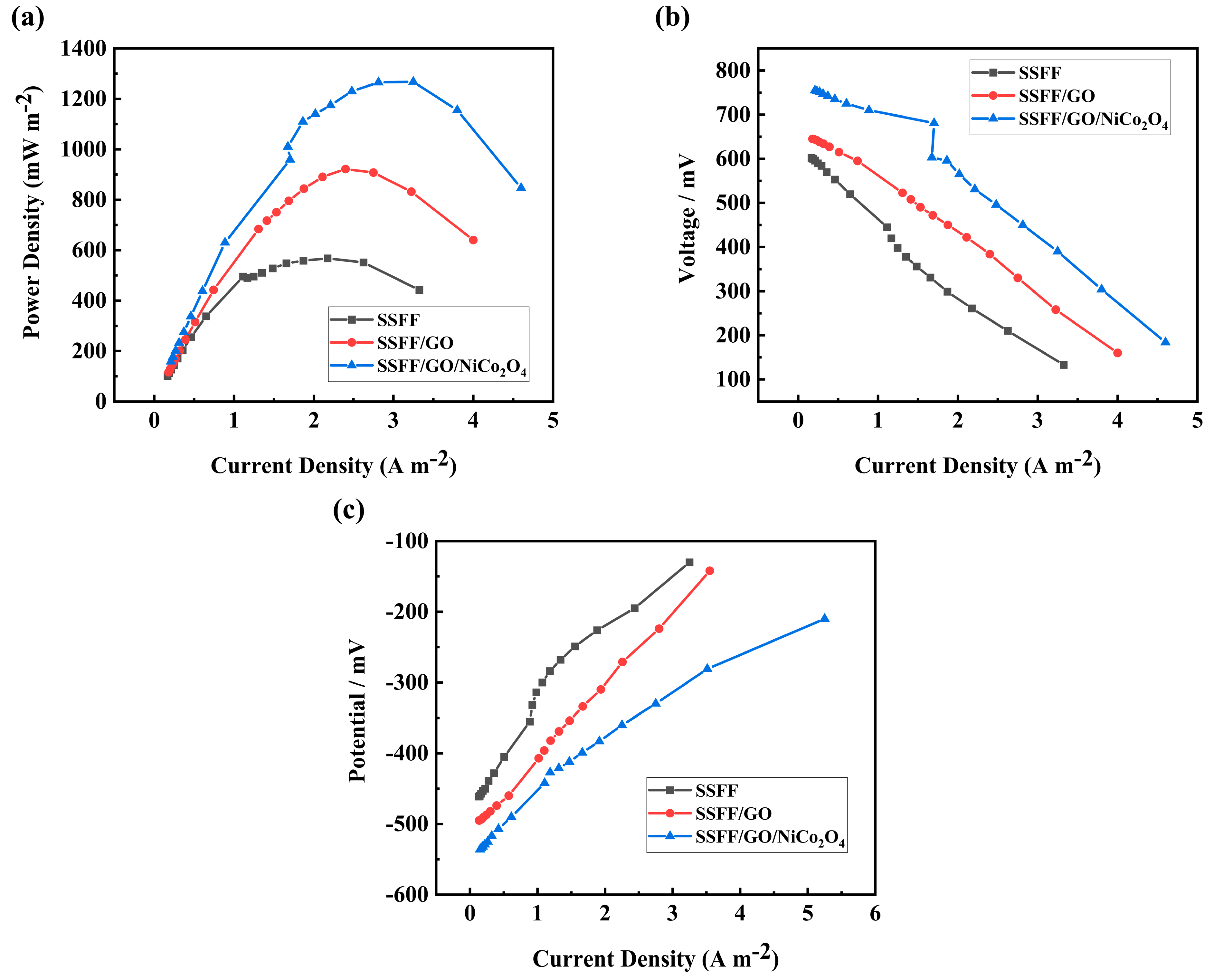
3.2. The Output of MFC
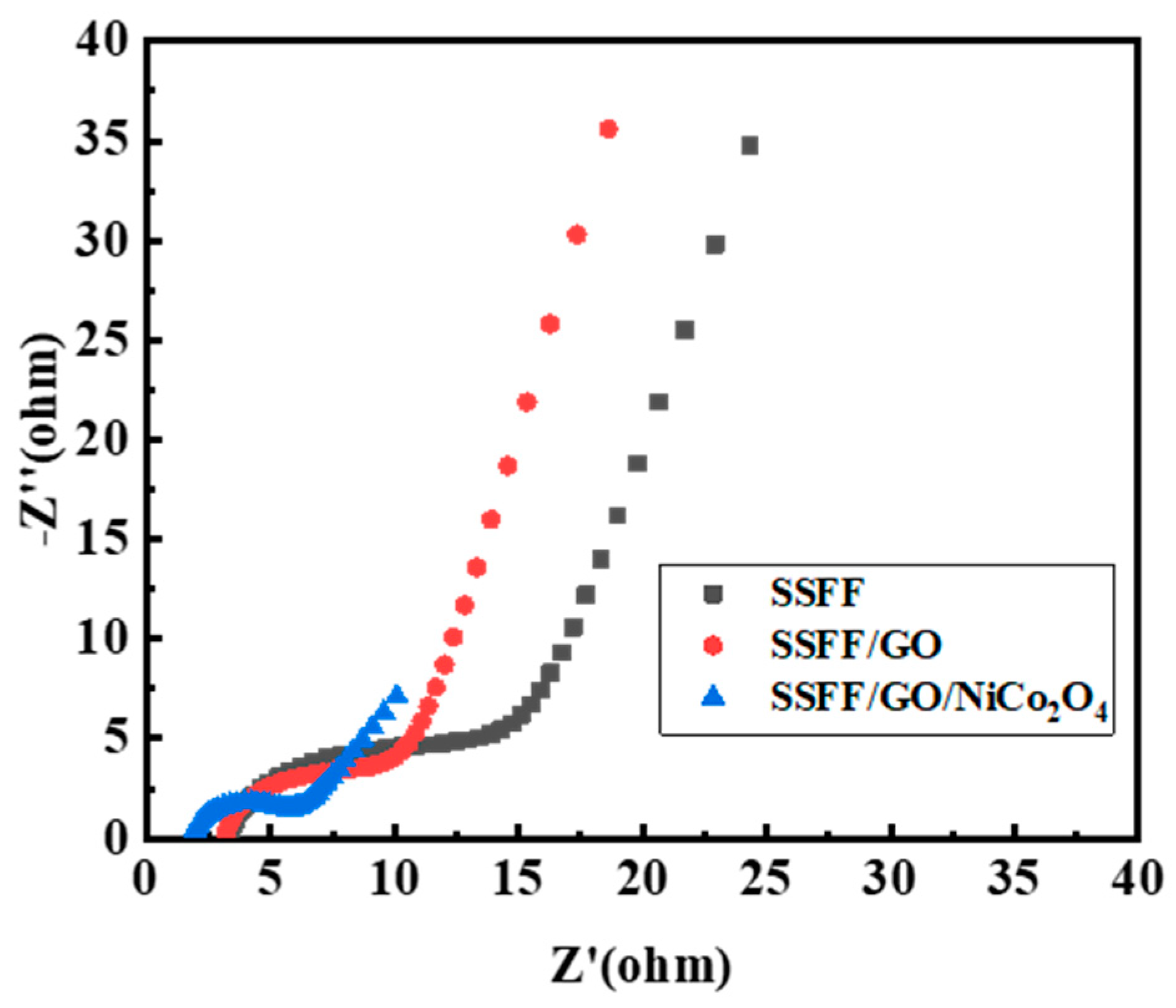
3.3. The Storage Ability of MFCs
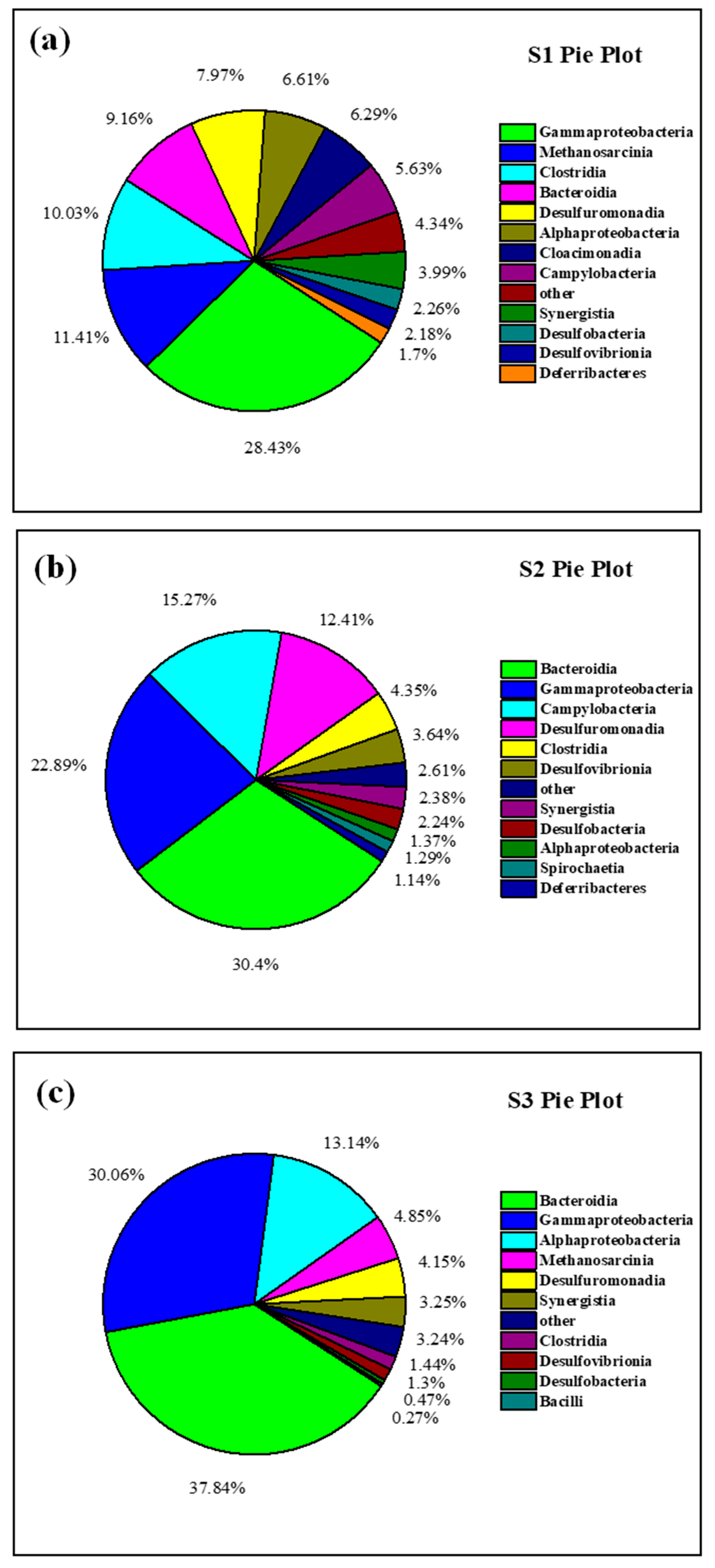
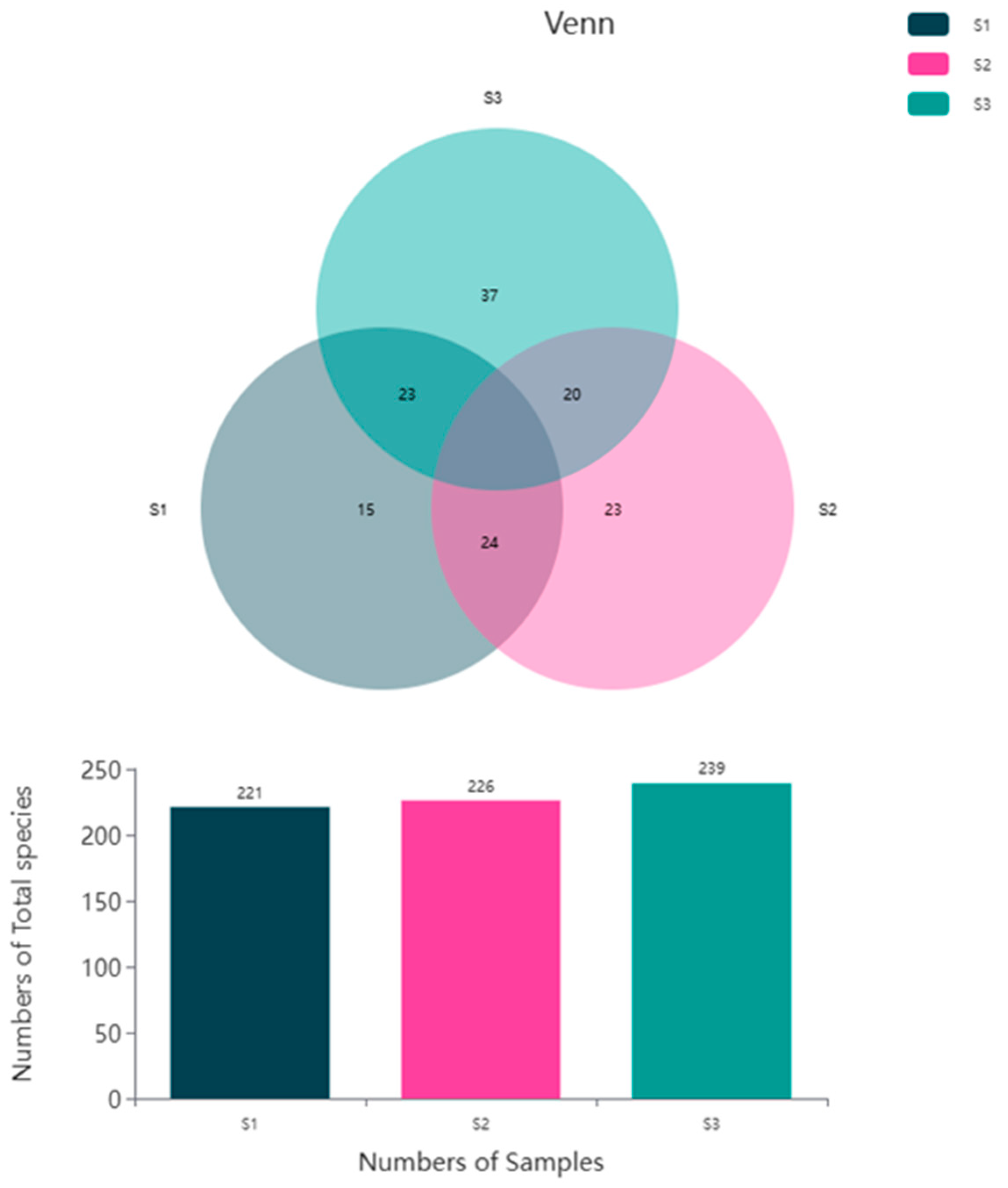
3.4. Analysis of Anode Biodiversity and Microbial Community Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A minireview. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Ahanchi, M.; Jafary, T.; Yeneneh, A.M.; Rupani, P.F.; Shafizadeh, A.; Shahbeik, H.; Pan, J.; Tabatabaei, M.; Aghbashlo, M. Review on waste biomass valorization and power management systems for microbial fuel cell application. J. Clean Prod. 2022, 380, 134994. [Google Scholar]
- Yang, W.; Wang, X.; Rossi, R.; Logan, B. Low-cost Fe–N–Ccatalyst derived from Fe (III)-chitosan hydrogel to enhance power production in microbial fuel cells. Chem. Eng. J. 2020, 380, 122522. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Zhang, Y.; Guo, Q.; Hu, T.; Xiao, H.; Lu, W.; Jia, J. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources 2023, 556, 232486. [Google Scholar] [CrossRef]
- Song, B.; Li, J.; Wang, Z.; Ali, J.; Wang, L.; Zhang, Z.; Liu, F.; Glebov, E.M.; Zhang, J.; Zhuang, X. N-doped Fe nanoparticles anchored on 3D carbonized sugarcane anode for high power density and efficient chromium (VI) removal. J. Environ. Chem. Eng. 2022, 10, 108751. [Google Scholar]
- Lu, M.; Qian, Y.; Huang, L.; Xie, X.; Huang, W. Improving the performance of microbial fuel cells through anode manipulation. Chem. Plus Chem. 2015, 80, 1216–1225. [Google Scholar]
- Guo, K.; Soeriyadi, A.H.; Feng, H.; Prévoteau, A.; Patil, S.A.; Gooding, J.J.; Rabaey, K. Heat-treated stainless steel felt as scalable anode material for bioelectrochemical systems. Bioresour. Technol. 2015, 195, 46–50. [Google Scholar]
- Hou, J.; Liu, Z.; Li, Y. Polyaniline modified stainless steel fiber felt for high-performance microbial fuel cell anodes. J. Clean Energy Technol. 2015, 3, 165–169. [Google Scholar]
- Jayesh, M.; Al-Saadi, S.; Raman, K.; Prakash, C.; Samuel, B. Exploring the use of polyaniline-modified stainless steel plates as low-cost, high performance anodes for microbial fuel cells. Electrochim. Acta 2018, 268, 484–493. [Google Scholar]
- Hou, J.; Liu, Z.; Li, Y.; Yang, S.; Zhou, Y. A comparative study of graphenecoated stainless steel fiber felt and carbon cloth as anodes in MFCs. Bioproc. Biosyst. Eng. 2015, 38, 881–888. [Google Scholar]
- Han, D.; Yan, L.; Chen, W.; Li, W.; Bangal, P. Cellulose/graphite oxide composite films with improved mechanical properties over a wide range of temperature. Carbohyd. Polym. 2011, 83, 966972. [Google Scholar]
- Singh, A.; Chandra, A. Graphite oxide/polypyrrole composite electrodes for achieving high energy density supercapacitors. J. Appl. Electrochem. 2013, 43, 773–782. [Google Scholar]
- Liu, Q.; Wang, Y.; Zhang, Y.; Xu, S.; Wang, J. Effect of dopants on the adsorbing performance of polypyrrole/graphite electrodes for capacitive deionization process. Synth. Met. 2012, 162, 655–661. [Google Scholar]
- Zhan, D.; Ni, Z.; Chen, W.; Sun, L.; Luo, Z.; Lai, L.; Yu, T.; Shen, Z. Electronic structure of graphite oxide and thermally reduced graphite oxide. Carbon 2010, 49, 1362–1366. [Google Scholar]
- Wang, P.; Zheng, Y.; LI, B. Preparation and electrochemical properties of polypyrrole/graphite oxide composites with various feed ratios of pyrrole to graphite oxide. Synth. Met. 2013, 166, 33–39. [Google Scholar]
- Shul’ga, Y.M.; Arbuzov, A.A.; Kabachkov, E.N.; Shul’ga, N.Y.; Dremova, N.N.; Baskakov, S.A.; Blinova, L.N.; Kurkin, E.N. Composite formed upon the ultrasonication of an aqueous suspension of graphite oxide titanium dioxide. Russ. J. Phys. Chem. A 2017, 91, 189–194. [Google Scholar]
- Li, W.; Liu, J.; Yan, C. Graphite-graphite oxide composite electrode for vanadium redox flow battery. Electrochim. Acta 2011, 56, 5290–5294. [Google Scholar] [CrossRef]
- Xu, Y.; Yi, R.; Yuan, B.; Wu, X.; Dunwell, M.; Lin, Q.; Fei, L.; Deng, S.; Andersen, P.; Wang, D.; et al. High capacity MoO2/graphite oxide composite anode for lithium-ion atteries. J. Phys. Chem. Lett. 2012, 3, 309–314. [Google Scholar]
- Wang, Y.; Ma, S.; Hou, L.; Zuo, J.; Kong, X.; Song, Y.; Wang, Z.; Tian, Y.; Dong, J. Enhanced electricity generation and energy storage in a microbial fuel cell with a bimetallic-modified capacitive anode. Desalination 2025, 593, 118247. [Google Scholar]
- Cao, C.; Wei, L.; Wang, G.; Shen, J. In-situ growing NiCo2O4 nanoplatelets on carbon cloth as binder-free catalyst air-cathode for high-performance microbial fuel cells. Electrochim. Acta 2017, 231, 609–616. [Google Scholar]
- Ge, B.; Li, K.; Fu, Z.; Pu, L.; Zhang, X.; Liu, Z.; Huang, K. The performance of nano urchin-like NiCo2O4 modified activated carbon as air cathode for microbial fuel cell. J. Power Sources 2016, 303, 325–332. [Google Scholar] [CrossRef]
- Padmaraj, O.; Austin, S.; Yesuraj, J.; Yashwant, P.; Venkateswaran, C. A study of synergistic effect on oxygen reduction activity and capacitive performance of NiCo2O4/rGO hybrid catalyst for rechargeable metal-air batteries and supercapacitor applications. Compos. Part B-Eng. 2019, 176, 107327. [Google Scholar]
- Li, J.; Liu, Y.; Zhan, D.; Zou, Y.; Xu, F.; Sun, L.; Xiang, C.; Zhang, J. Electrospinning synthesis of NiCo2O4 embedded N-doped carbon for high-performance supercapacitors. J. Energy Storage 2021, 39, 102665. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Kong, X.; Song, Y.; Tian, Y.; Lin, J. Capacitive and biocompatibility composite anode material for enhanced renewable energy conversion for MFCs. Fuel 2024, 376, 132736. [Google Scholar] [CrossRef]
- Wang, Y. Carbon felt electrode modified with RGO/PANI composite material for enhancing renewable energy storage in microbial fuel cells. Renew. Energy 2024, 232, 121109. [Google Scholar] [CrossRef]
- Shabnam, S.; Nazila, M. Engineered Heterostructure Photocatalyst: Chitosan-Coated Chromium Ferrite/Graphite Oxide Synthesized Hydrothermally for Environmental Remediation. J. Polym. Environ. 2024, 33, 794–813. [Google Scholar]
- Dewan, A.; Beyenal, H.; Lewandowski, Z. Intermittent energy harvesting improves the performance of microbial fuel cells. Environ. Sci. Technol. 2009, 43, 4600–4605. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Q.; Chen, Y.; Qi, L. A novel polyaniline interlayer manganese dioxide composite anode for high-performance microbial fuel cell. J. Taiwan Inst. Chem. 2017, 75, 112–118. [Google Scholar] [CrossRef]
- Mehdinia, A.; Ziaei, E.; Jabbari, A. Multi-walled carbon nanotube/SnO2 nanocomposite: A novel anodematerial for microbial fuel cells. Electrochim. Acta 2014, 130, 512–518. [Google Scholar] [CrossRef]
- Park, I.; Christy, M.; Kim, P.; Nahm, K. Enhanced electrical contact of microbes using Fe3O4/CNT nanocomposite anode in mediator-less microbial fuel cell. Biosens Bioelectron. 2014, 58, 75–80. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, H.; Chen, Y.; Wen, Q.; Wu, J. Macroporous composite capacitive bioanode applied in microbial fuel cells. Chin. Chem. Lett. 2020, 31, 205–209. [Google Scholar] [CrossRef]
- Zolfaghari, A.; Ataherian, F.; Ghaemi, M.; Gholami, A. Capacitive behavior of nanostructured MnO2 prepared by sonochemistry method. Electrochim. Acta 2007, 52, 2806–2814. [Google Scholar]
- Cui, H.; Du, L.; Guo, P.; Zhu, B.; Luong, J. Controlled modification of carbon nanotubes and polyaniline on macroporous graphite felt for high-performance microbial fuel cell anode. J. Power Sources 2015, 283, 46–53. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Li, B.; Li, W. Novelly developed three-dimensional carbon scaffold anodes from polyacrylonitrile for microbial fuel cells. J. Mater. Chem. A 2015, 3, 5110–5118. [Google Scholar]
- Qiao, Y.; Bao, S.; Li, C.; Cui, X.; Lu, Z.; Guo, J. Nanostructured polyaniline/titanium dioxide composite anode for microbial fuel cells. ACS Nano 2008, 2, 113–119. [Google Scholar]
- Li, Q.; Liu, J.; Zou, J.; Chunder, A.; Chen, Y.; Zhai, L. Synthesis and electrochemical performance of multiwalled carbon nanotube/polyaniline/MnO2 ternary coaxial nanostructures for supercapacitors. J. Power Sources 2011, 196, 565–572. [Google Scholar]
- Grondin, F.; Perrier, M.; Tartakovsky, B. Microbial fuel cell operation with intermittent connection of the electrical load. J. Power Sources 2012, 208, 18–23. [Google Scholar]
- Yuan, Y.; Zhou, S.; Liu, Y.; Tang, J. Nanostructured macroporous bioanode based on polyaniline-modified natural loofah sponge for high-performance microbial fuel cells. Environ. Sci. Technol. 2013, 47, 14525–14532. [Google Scholar] [CrossRef]
- Liang, P.; Wu, W.; Wei, J.; Yuan, L.; Xia, X.; Huang, X. Alternate charging and discharging of capacitor to enhance the electron production of bioelectrochemical systems. Environ. Sci. Technol. 2011, 45, 6647–6653. [Google Scholar] [CrossRef]
- Peng, X.; Yu, H.; Yu, H.; Wang, X. Lack of anodic capacitance causes power overshoot in microbial fuel cells. Bioresour. Technol. 2013, 138, 353–358. [Google Scholar]
- Zhang, B.; Zhang, J.; Liu, Y.; Hao, C.; Tian, C.; Feng, C.; Lei, Z.; Huang, W.; Zhang, Z. Identification of removal principles and involved bacteria in microbial fuel cells for sulfide removal and electricity generation. Int. J. Hydrogen Energy 2013, 38, 14348–14355. [Google Scholar]
- Dunaj, S.; Vallino, J.; Hines, M.; Gay, M.; Kobyljanec, C.; Rooney-Varga, J. Relationships between soil organic matter nutrients bacterial community structure, and the performance of microbial fuel cells. Environ. Sci. Technol. 2012, 46, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, L.; Ding, L.; Ren, H.; Cui, H. Effect of conductive polymers coated anode on the performance of microbial fuel cells (MFCs) and its biodiversity analysis. Biosens. Bioelectron. 2011, 26, 4169–4176. [Google Scholar]
- Ermete, A. Composite materials for polymer electrolyte membrane microbial fuel cells. Biosens. Bioelectron. 2015, 69, 54–70. [Google Scholar]
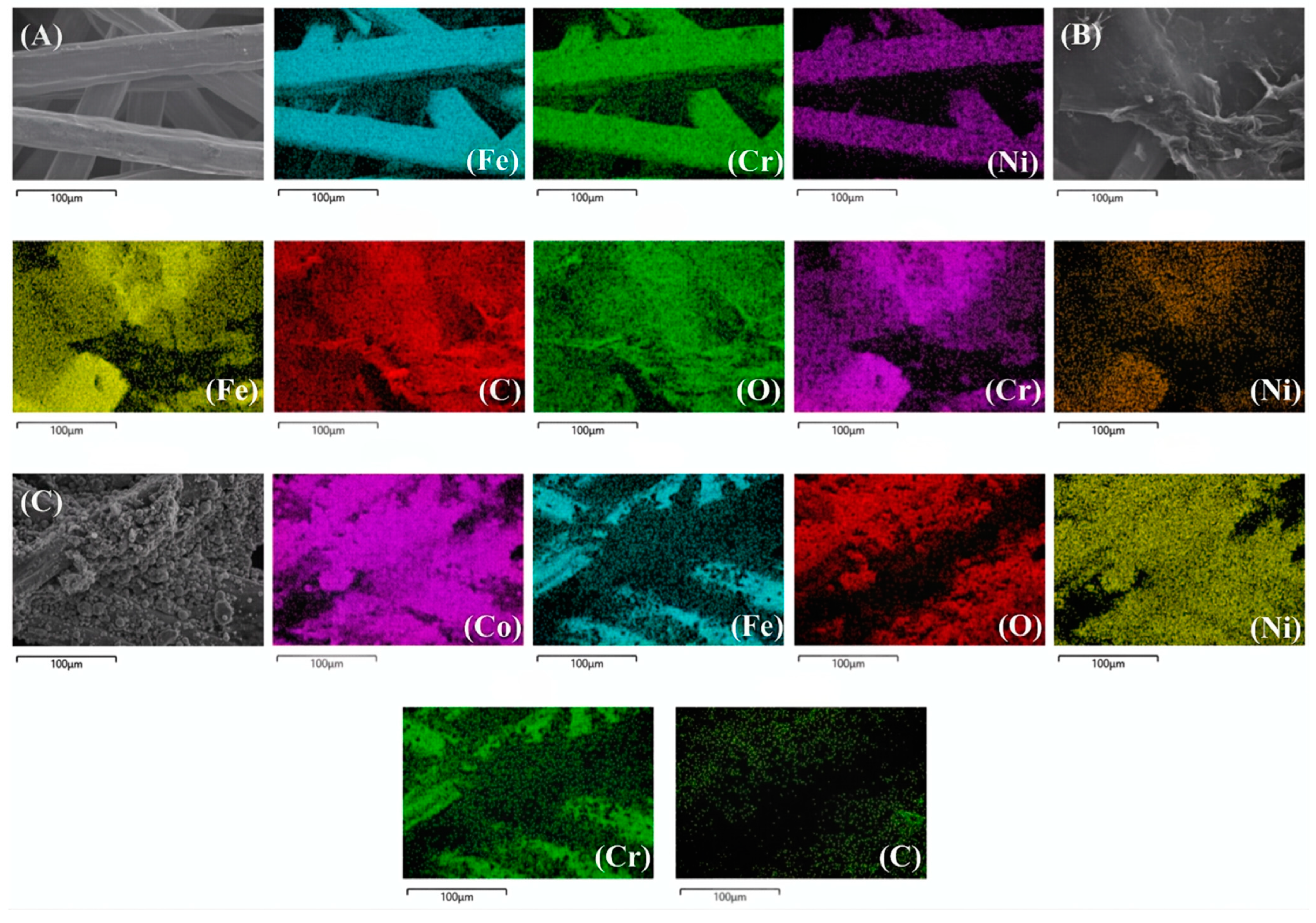


| Anodes | Element | Welght % | Atomic % |
|---|---|---|---|
| SSFF | Fe | 61.36 | 47.56 |
| Cr | 17.09 | 14.22 | |
| Ni | 9.38 | 6.92 | |
| SSFF/GO | C | 31.32 | 57.95 |
| O | 14.21 | 19.74 | |
| Fe | 36.68 | 14.59 | |
| Cr | 10.36 | 4.43 | |
| Ni | 5.36 | 2.03 | |
| SSFF/GO/NiCo2O4 | C | 7.15 | 20.13 |
| O | 16.64 | 35.15 | |
| Co | 37.10 | 21.28 | |
| Ni | 19.21 | 11.06 | |
| Fe | 14.75 | 8.93 | |
| Cr | 4.42 | 2.88 |
| References | Anode | Power Density | |
|---|---|---|---|
| 1 | This study | SSFF/GO/NiCo2O4 | 1267.5 mW/m2 |
| 2 | Hou et al. [8] | SSFF/PANI | 360 mW/m2 |
| 3 | Mehdinia et al. [29] | MWCNT/SnO2/GC | 1421 mW/m2 |
| 4 | Park et al. [30] | CP/CNT/Fe3O4 | 830 mW/m2 |
| 5 | Wang et al. [31] | S/N-CNT/PANI/MnO2 | 1019.5 mW/m2 |
| 6 | Wang et al. [28] | CF/MnO2/PANI/MnO2 | 1124.8 mW/m2 |
| Anodes | Parameter | C15/D15 |
|---|---|---|
| SSFF anode | ih (A/m2) | 10.31 |
| is (A/m2) | 0.24 | |
| Qs (C/m2) | 39.27 | |
| Qt (C/m2) | 427.08 | |
| SSFF/GO anode | ih (A/m2) | 37.55 |
| is (A/m2) | 3.31 | |
| Qs (C/m2) | 538.65 | |
| Qt (C/m2) | 4537.42 | |
| SSFF/GO/NiCo2O4 anode | ih (A/m2) | 70.93 |
| is (A/m2) | 8.67 | |
| Qs (C/m2) | 1405.35 | |
| Qt (C/m2) | 11,836.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Kong, X.; Wang, Z.; Zhang, D.; Song, Y.; Ma, S.; Duan, Y.; Vyshnikin, A.; Palchykov, V.; Zuo, J. Investigation into the Preparation and Electrochemical Energy Storage Performance of Nickel Cobalt Oxide-Based Composite Anode Materials. Coatings 2025, 15, 373. https://doi.org/10.3390/coatings15040373
Wang Y, Kong X, Wang Z, Zhang D, Song Y, Ma S, Duan Y, Vyshnikin A, Palchykov V, Zuo J. Investigation into the Preparation and Electrochemical Energy Storage Performance of Nickel Cobalt Oxide-Based Composite Anode Materials. Coatings. 2025; 15(4):373. https://doi.org/10.3390/coatings15040373
Chicago/Turabian StyleWang, Yuyang, Xiangquan Kong, Zhijie Wang, Dongming Zhang, Yu Song, Su Ma, Ying Duan, Andrii Vyshnikin, Vitalii Palchykov, and Jinlong Zuo. 2025. "Investigation into the Preparation and Electrochemical Energy Storage Performance of Nickel Cobalt Oxide-Based Composite Anode Materials" Coatings 15, no. 4: 373. https://doi.org/10.3390/coatings15040373
APA StyleWang, Y., Kong, X., Wang, Z., Zhang, D., Song, Y., Ma, S., Duan, Y., Vyshnikin, A., Palchykov, V., & Zuo, J. (2025). Investigation into the Preparation and Electrochemical Energy Storage Performance of Nickel Cobalt Oxide-Based Composite Anode Materials. Coatings, 15(4), 373. https://doi.org/10.3390/coatings15040373






