Abstract
The Co-CuCoMnOx coatings with varying proportions were prepared and investigated to develop a novel metal–ceramic solar selective absorption coating employed at high temperature. The CuCoMnOx powders were synthesized using the solid-phase reaction method. Subsequently, Co-CuCoMnOx coatings were deposited on the surface of 316L steels utilizing the atmospheric plasma spraying (APS) technique. The results showed that the synthesized CuCoMnOx powders were mainly composed of two phases, which were Cu1.5Mn1.5O4 and MnCo2O4. The CuCoMnOx powders had a solar absorptance of 0.929 and an infrared emittance of 0.862, which was considered a good solar absorbent. The synthesized Co-CuCoMnOx coating had a typical thermal spray layered stacking structure. The chemical phases of the coatings were mainly Co, CoO, and CoCuMnOx. Due to the addition of CuCoMnOx inhibiting the oxidation of Co during the thermal spraying process, the 95Co-5CuCoMnOx (wt%) coating exhibited the optimal quality factor (α/ε) of 2.184, with a solar absorptance α of 0.808 and an infrared emittance ε of 0.370, respectively. Moreover, this specific coating demonstrated a good thermal stability for up to 3 h when exposed to an atmospheric environment at 450 °C. The results indicate its significant potential for high-temperature solar selective absorption coating.
1. Introduction
In the contemporary world, numerous countries are actively engaged in research on photothermal power generation technology to effectively harness and utilize solar energy. As a critical component of solar collectors, solar selective absorption coatings play a key role [1], directly influencing the advancement of photothermal power generation technology. The principle underlying solar selective absorption coatings is their high absorptance (α) for solar radiation energy within the ultraviolet–visible near-infrared spectrum (0.3–2.5 μm), while simultaneously exhibiting low emittance (ε) in the mid–far-infrared range (2.5–25 μm) [2]. Solar selective absorption coatings can be categorized into three distinct types based on their operating temperature: low-temperature solar selective absorption coatings (operating at temperatures below 100 °C), medium-temperature solar selective absorption coatings (ranging from 100 °C to 400 °C), and high-temperature solar selective absorption coatings (exceeding 400 °C). Currently, research on solar selective absorption coating for mid- and low-temperature applications has reached a relatively advanced stage. In order to reduce costs and enhance the conversion efficiency of photothermal power generation, it is essential to continuously elevate the operating temperature of these solar selective absorption coatings. Therefore, there is a pressing need for the development of high-temperature solar selective absorption coatings that can endure the highest possible temperatures [3,4,5,6].
Metal–ceramic solar selective absorption coatings demonstrate exceptional optical performance and high-temperature thermal stability, making them promising candidates for the development of advanced high-temperature solar selective absorption coatings. Generally, metal–ceramic solar selective absorption coatings are composed of transition metals and their corresponding oxides, nitrides, or carbides. Examples include Ni-Al2O3 [7], Pt-Al2O3 [8], Ti-AlN [9], Ni/TiN-La2O3 [10], Co-Cr3C2-WC [11], and Ni/Mo-TiC [12]. Among these materials, the metal–ceramic solar selective absorption coatings based on Co-WC have been the subject of extensive investigation by various researchers. Chen et al. [13] investigated three types of WC-12Co cermet materials with micron, submicron, and nanometer structures to fabricate corresponding coatings using high-velocity oxygen–fuel (HVOF) spraying. It was observed that decarburization (the decomposition of WC into W2C) occurred in all three structural forms during the spraying process. Notably, the nanometer structure also resulted in the formation of new phases, such as W and W3O4. The absorptance of the coatings across these three structures ranged from 0.78 to 0.86, while their emittances varied between 0.6 and 0.7, having a quality factor (α/ε) of 1.11~1.43. The relatively high emittance observed may be attributed to the comparatively low metal content present in these coatings. Ke et al. [14] utilized a material composition of 80% Co, 10% submicron WC, and 10% nano WC to apply a coating on stainless steel (SS) via high-velocity oxygen–fuel (HVOF) spraying. The resulting Co-WC coating exhibited an absorptance of 0.821 and an emittance of 0.434 respectively, giving a quality factor of 1.89. It is evident that the quality factor of the Co-WC type metal–ceramic solar selective absorption coating is not satisfactory.
To address the challenges associated with decarburization, oxidation, and the relatively low quality factor of Co-WC type coatings, this study contemplates the selection of a novel ceramic phase. The aim is to enhance both the optical performance and high-temperature stability of Co-containing metal–ceramic solar selective absorption coatings. Metal oxides possessing a spinel structure have demonstrated remarkable optical performance and high-temperature stability [15,16,17,18,19,20,21]. Consequently, they have frequently been employed as raw materials for the fabrication of solar selective absorption coatings in recent years. Among these materials, CuCoMnOx with a spinel structure has garnered significant research attention [4,22,23,24,25,26,27]. Peng et al. [28] applied CuCoMnOx coatings onto polished aluminum plates. By optimizing the coating thickness, they achieved an absorptance of 0.928 and an emittance of 0.198 for the coated surface. Rocío Bayón et al. [15] fabricated Cu1.5Mn1.5O4 thin films on an aluminum substrate using the impregnation method, achieving an absorption rate exceeding 0.87. Subsequently, a SiO2 anti-reflection layer was deposited onto the Cu1.5Mn1.5O4 thin film via the sol–gel method, resulting in improved optical performance with an absorption rate of 0.94 and an emission rate of 0.06. George Kordas [29] incorporated CuO@SiO2 microspheres into the CuCoMnOx coating, achieving an impressive absorptance/emittance of 30. Furthermore, it was concluded that the ratio of absorptance to emittance increases linearly with the concentration of CuO@SiO2 microspheres. Ke et al. [14] developed a composite coating on a stainless steel (SS) substrate by successively depositing layers of CuCoMnOx, CuCoMnOx-SiO2, and SiO2 atop a WC-Co coating fabricated using HVOF. This resulted in the formation of a WC-Co/CuCoMnOx/CuCoMnOx-SiO2/SiO2 composite structure. The incorporation of CuCoMnOx was aimed at enhancing the absorption rate of the coating within the visible light spectrum while simultaneously filling in the grooves and pores present on the surface of the WC-Co layer, thereby reducing its surface roughness. The optical performance metrics for this composite coating were characterized by an absorption/emittance ratio of 0.915/0.29, demonstrating that it could maintain thermal stability for up to 50 h at 550 °C. Based on the aforementioned research, it is evident that CuCoMnOx represents a promising ceramic phase candidate for enhancing the optical performance of Co-containing solar selective absorption coatings, while also exhibiting excellent high-temperature stability.
To obtain a solar selective absorption coating with excellent optical performance and high-temperature thermal stability, CuCoMnOx spinel powder was synthesized using a combination of three powders—CuO, Co3O4, and MnO2—in a muffle furnace. Then, various mass ratios of Co-CuCoMnOx coatings were designed and prepared through the atmosphere plasma spraying method. The mechanism of optical property changes in plasma-sprayed coatings with different compositions and ratios was thoroughly analyzed. Finally, the coating with the best optical performance was selected to investigate its high-temperature stability, proving that it has the potential to become a candidate for high-temperature solar selective absorption coatings.
2. Experiment
2.1. Preparation of CuCoMnOx Powders
CuO powders (with purity of 99.5%), MnO2 powders (with purity of 99.5%), and Co3O4 powders (with purity of 99.9%) were sourced from Qinhuangdao Yinuo High-tech Materials Development Co., Ltd., Qinhuangdao, China. The corresponding masses of these powders were measured according to the molar ratio of Cu:Mn:Co, set at 3:3:1. Deionized water was added to the three mixed powders at a solid–liquid mass ratio of 1:0.84. Additionally, a self-prepared binder, dispersant, and several drops of defoamer (tributyl phosphate) were incorporated into the mixture. Subsequently, the mixture was introduced into a colloid mill and subjected to high-speed mixing for a duration of 2 h to ensure a homogeneous slurry. After that, the slurry was pumped into the centrifugal atomization tower to form spherical particles. The parameters for the granulation process are as follows: the air outlet temperature was set at 110 °C, while the air inlet temperature was maintained at 240 °C. The rotational speed of the atomizing disc was established at 18,000 r/min, and the feeding rate was specified as 45 mL/min. The obtained powder was sintered in a muffle furnace with a heating rate of 5 °C/min. The sinter temperature was maintained at 600 °C for 1 h to remove the binder, and at 1100 °C for 2 h to promote solid reaction. Subsequently, the material was allowed to cool within the furnace, resulting in the formation of CuCoMnOx powder.
2.2. Preparation of Thermal Spray Powders
The prepared CuCoMnOx powder was furtherly crushed by a ball mill to obtain tiny powders with a particle size of less than 5 μm. Co powder, with a particle size of 1.5 μm and a purity of 99.9%, was sourced from Shenzhen GeLinmei High-Tech Co., Ltd., Shenzhen, China. In this study, a total of five groups of coating materials were established, each featured different composition ratios as shown in Table 1. The raw powders were weight and mixed with deionized water, self-prepared adhesives, dispersants, and defoamer (tributyl phosphate) at a solid–liquid mass ratio of 1:1 though colloid milling to form a uniformly ground slurry. Then, the slurry was pumped into the centrifugal atomization tower to form spherical particles. The parameters for the granulation process are the same with those we have mentioned above. The powder obtained through granulation was subjected to high-temperature calcination in a tube furnace under an argon atmosphere. The heating rate was set at 5 °C/min, and the sintering temperature was maintained at 600 °C for 1 h to ensure complete burnout of the binder. Following this process, the binder-free powder was sieved to obtain particles within the size range of 45–75 μm, suitable for thermal spraying applications.

Table 1.
Composition and proportions of the composite powder.
2.3. Preparation of Coatings
The substrate utilized for the spraying process was 316L stainless steel, with dimensions of 30 mm × 40 mm × 1 mm. The substrate underwent ultrasonic cleaning in acetone to eliminate oil contaminants, then was followed by ultrasonic cleaning in deionized water. Finally, it was dried in a drying oven to remove any residual moisture. The cleaned substrate surface was sandblasted using 100-mesh brown alumina sand, aiming to roughen the substrate surface and enhance the bonding strength between the substrate and the coating. Before the formal spraying process, the substrate was preheated in a drying oven at 120 °C. The absorption layer was fabricated using the APS-3000K plasma spraying equipment from the Beijing Institute of Aeronautical Manufacturing Engineering under AVIC in Beijing, China. The specific parameters for the spraying process are detailed in Table 2.

Table 2.
Process parameters used for spraying.
2.4. Test of High-Temperature Stability
The coated samples were placed within a tube furnace and maintained under atmospheric conditions. The heating rate was set at 5 °C/min, and the samples were held at 450 °C for a duration of 3 h before being allowed to cool alongside the furnace.
2.5. Characterization
The thermogravimetric curves of the powdered mixture comprising CuO, Co3O4, and MnO2 after granulation were analyzed using TG-DTG (NETZSCH STA 2500, Netzsch Scientific Instruments Trading Co., Ltd., Shanghai, China). The phases of the synthesized CuCoMnOx powder, the thermal spray powders, and the coatings before and after the high-temperature heat treatment were analyzed using X-ray diffraction (XRD, Spectris Group in London, UK/EMPYREAN Smart Diffractometer/Cu target, Rigaku Corporation in Tokyo Japan/Smartlab SE/Co target). The surface and cross-sectional morphologies of the coatings were analyzed using a scanning electron microscope (SEM, JEOL in Tokyo Japan, JSM-IT300). The X-ray energy dispersive spectrometer (EDS, Oxford Instruments Technology Co., Ltd., X-MaxN20 EDS spectrometer, Shanghai, China) was utilized to perform point-scanning tests on both the powder and the coating. The surface roughness of the coatings was assessed using a surface roughness measurement instrument (Beijing Time Summit Technology Co., Ltd., Beijing, China, TIME®3221). The reflection spectra of the synthesized CuCoMnOx powder and the coating before and after the high-temperature heat treatment were measured within the wavelength ranges of 0.3–2.5 μm and 2.5–25 μm, using a UV–visible spectrophotometer (Shimadzu, UV-3600, Kyoto, Japan) and a Fourier-transform infrared spectrometer (Bruker, Tensor27, Karlsruhe, Germany), respectively. The absorptance (α) and emittance (ε) are determined using Equations (1) and (2), where Isol(λ) is the irradiance of the standard solar spectrum under Air Mass 1.5 (AM1.5) conditions, R(λ) denotes the spectral reflectance, and Ib(λ) indicates the radiant intensity of a blackbody at room temperature [30].
3. Results and Discussion
3.1. Synthesis Mechanism and Spectral Property of CuCoMnOx Powders
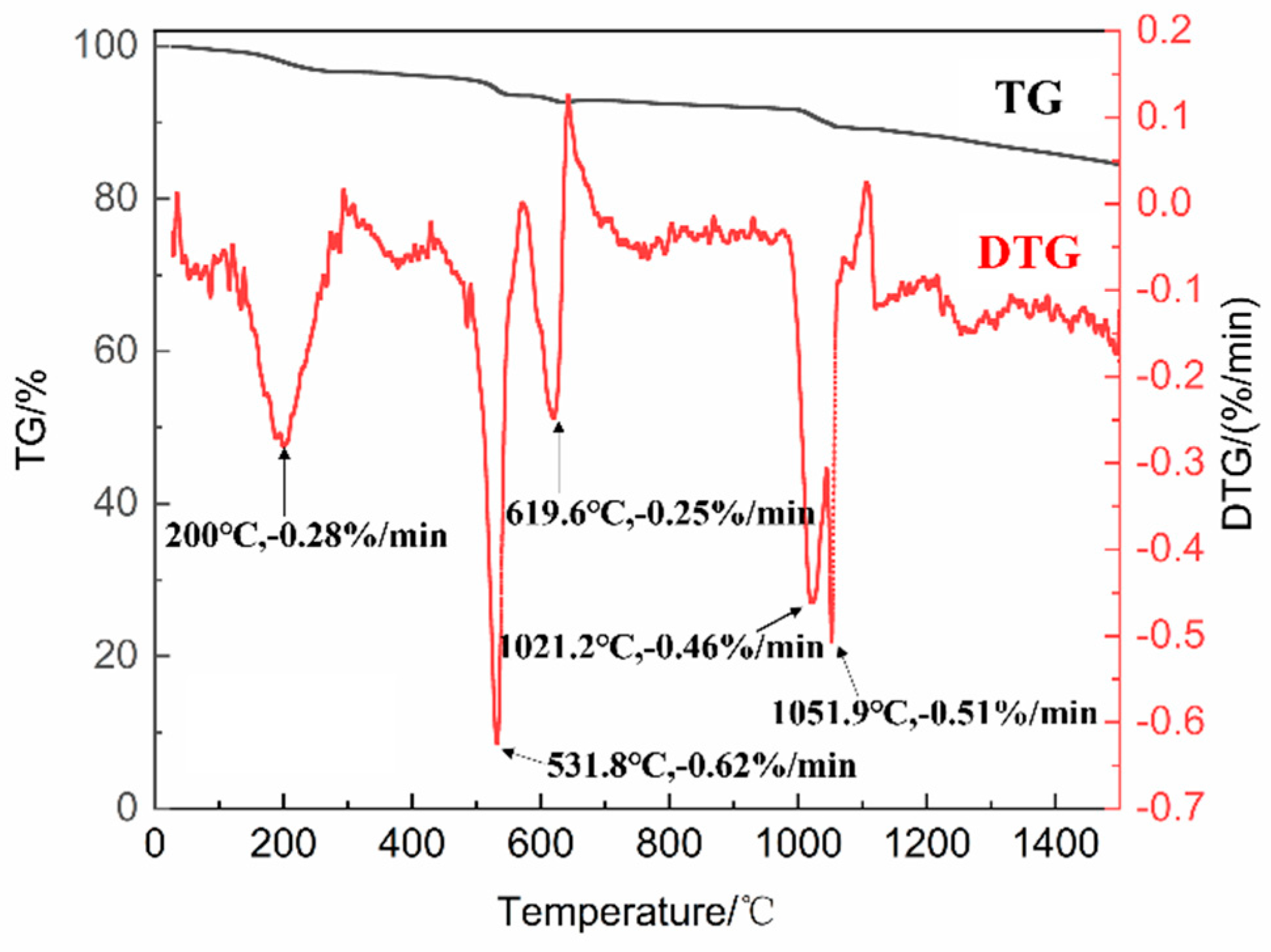
In this study, CuCoMnOx powders were synthesized using a solid-phase reaction method. To elucidate the synthesis mechanism as well as the final sintering temperature of the CuCoMnOx powders, we analyzed the TG-DTG results obtained from the granulated mixture of CuO-Co3O4-MnO2, as illustrated in Figure 1. It can be observed from Figure 1 that the granulated powder of CuO-Co3O4-MnO2 experiences mass loss at temperatures of approximately 200 °C, 531.8 °C, 619.6 °C, 1021.2 °C, and 1051.9 °C. The maximum mass loss rates corresponding to these temperature points are as follows: 0.28%/min for 200 °C, 0.62%/min for 531.8 °C, 0.25%/min for 619.6 °C, 0.46%/min for 1021.2 °C, and 0.51%/min for 1051.9 °C. It has been reported in the literature that the decomposition of MnO2 into Mn2O3 occurs at temperatures of 520 °C [31], 550 °C [32], and 596.1 °C [33]. Consequently, the weight loss observed around 531.8 °C in Figure 1 can be interpreted as the decomposition of MnO2. Based on the laboratory experience, the weight loss observed near 200 °C and 619.6 °C in Figure 1 is believed to correspond to the combustion of the binder, dispersant, and defoamer incorporated into the mixed powders of CuO-Co3O4-MnO2 during the granulation process. The primary reaction equations are presented as follows:
4MnO2→2Mn2O3 + O2 (T = 531.8 °C)
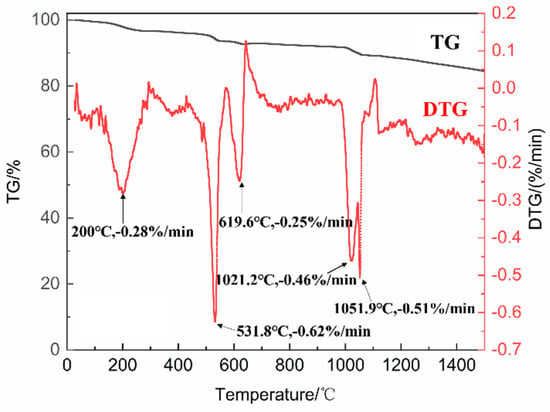
Figure 1.
The TG-DTG diagram of the granulated CuO-Co3O4-MnO2 powder.
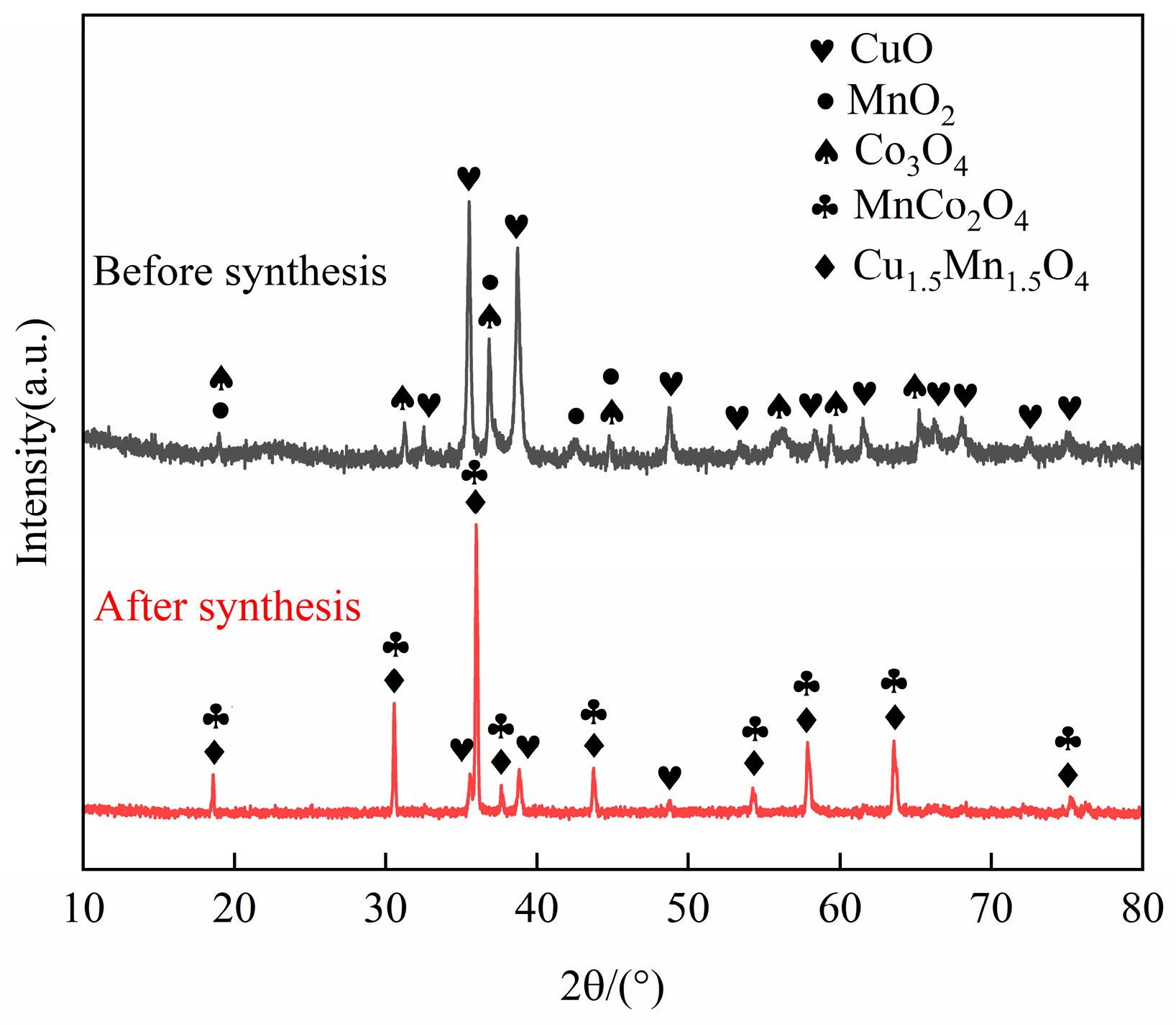
Based on the TG-DTG curve, it was concluded that the mass loss kept unchanged around 1100 °C. Therefore, the final sintering temperature of 1100 °C with a duration of 2 h were selected. The X-ray diffraction (XRD) patterns of the CuO-Co3O4-MnO2 powder before and after sintering at 1100 °C for 2 h are shown in Figure 2. It can be seen that the phase composition of the powder before sintering consists of CuO, Co3O4, and MnO2. After sintering, the powder mainly comprises Cu1.5Mn1.5O4, MnCo2O4, and a small amount of CuO. It was coincided with the target phases of CuCoMnOx powder reported in other research [24], suggesting the successful synthetization of CuCoMnOx powder. It can also be observed from Figure 2 that the diffraction peaks of Cu1.5Mn1.5O4 and MnCo2O4 are sharp, indicating Cu1.5Mn1.5O4 and MnCo2O4 possess good crystallinity. The weight loss observed around 1021.2 °C and 1051.9 °C in Figure 1 is deduced to correspond to the reaction of CuO, Co3O4, MnO2, and Mn2O3 gradually synthesizing Cu1.5Mn1.5O4 and MnCo2O4. The equation for the synthesis reactions is as follows:
6CuO + 4Co3O4 + 4MnO2 + 4Mn2O3→4Cu1.5Mn1.5O4 + 6MnCo2O4 + O2 (T = 1021.2 °C, T = 1051.9 °C)
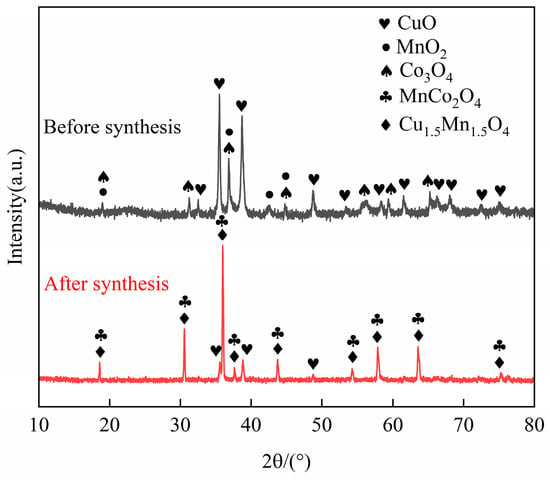
Figure 2.
XRD patterns of CuO-Co3O4-MnO2 powder before and after sintering at 1100 °C for 2 h.
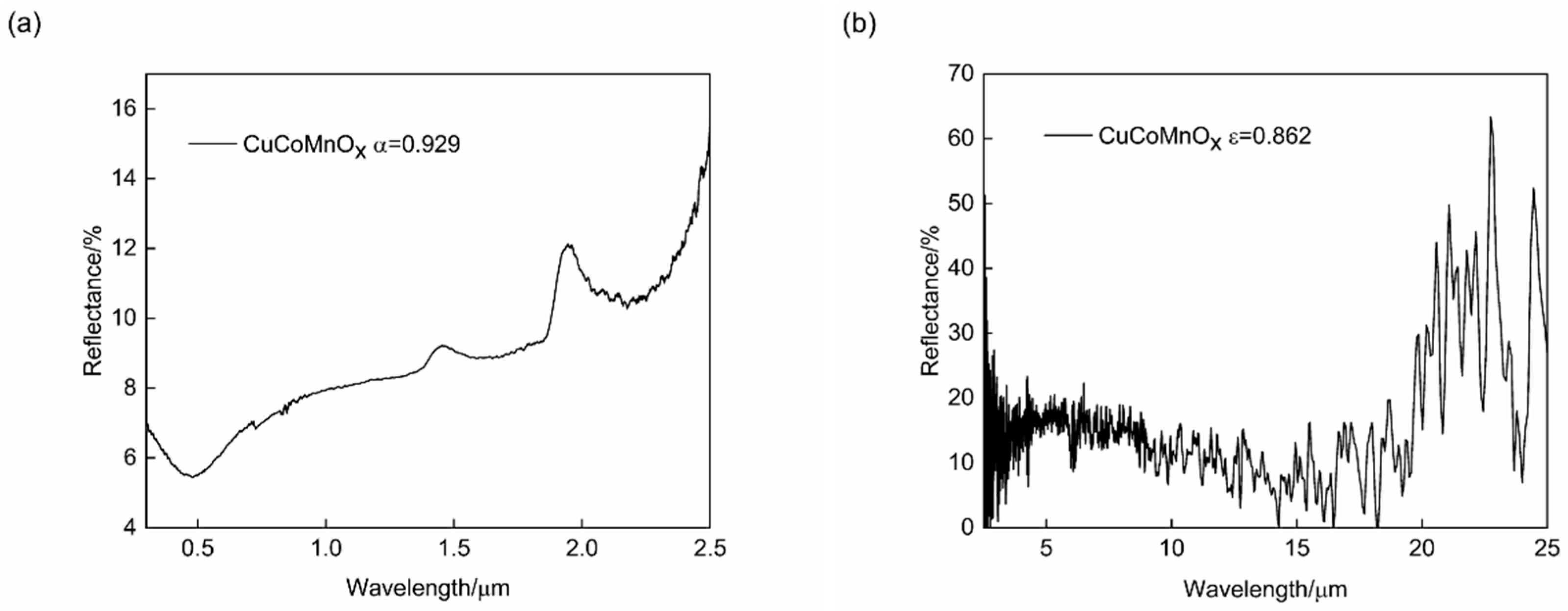
The CuCoMnOx powder serves as a fundamental raw material for the spraying coatings, and its inherent optical performance significantly impacts the final absorptance and emittance of these coatings. To investigate the optical performance of prepared CuCoMnOx powder, the reflectance spectra were measured in the wavelength ranges of 0.3–2.5 μm and 2.5–25 μm, and is presented in Figure 3. Based on results, the absorptance of the CuCoMnOx powder was 0.929, while the emittance was 0.862, giving a quality factor of α/ε equaling to 1.078. Herein, it is observed that CuCoMnOx exhibits a high absorptance, while it also has a high emittance. In other words, achieving an ideal quality factor remains impossible for single CuCoMnOx-based ceramic coating. Composite with low-absorption and low-emission metal Co to form a metal–ceramic coating might be an effective way to obtain good solar selective absorption performance. Thus, this paper attempted to optimize the composition of the Co-CuCoMnOx composite coatings, in order to obtain a higher quality factor of solar selective absorption performance.
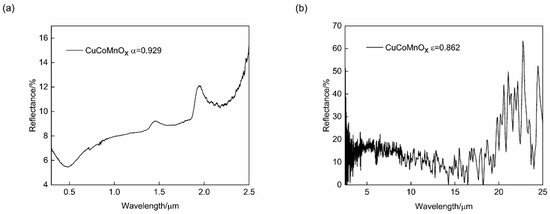
Figure 3.
Reflectance spectra of CuCoMnOx powder in the spectral ranges of (a) 0.3–2.5 μm and (b) 2.5–25 μm.
3.2. Microstructure of Co-CuCoMnOx Powders and Coatings
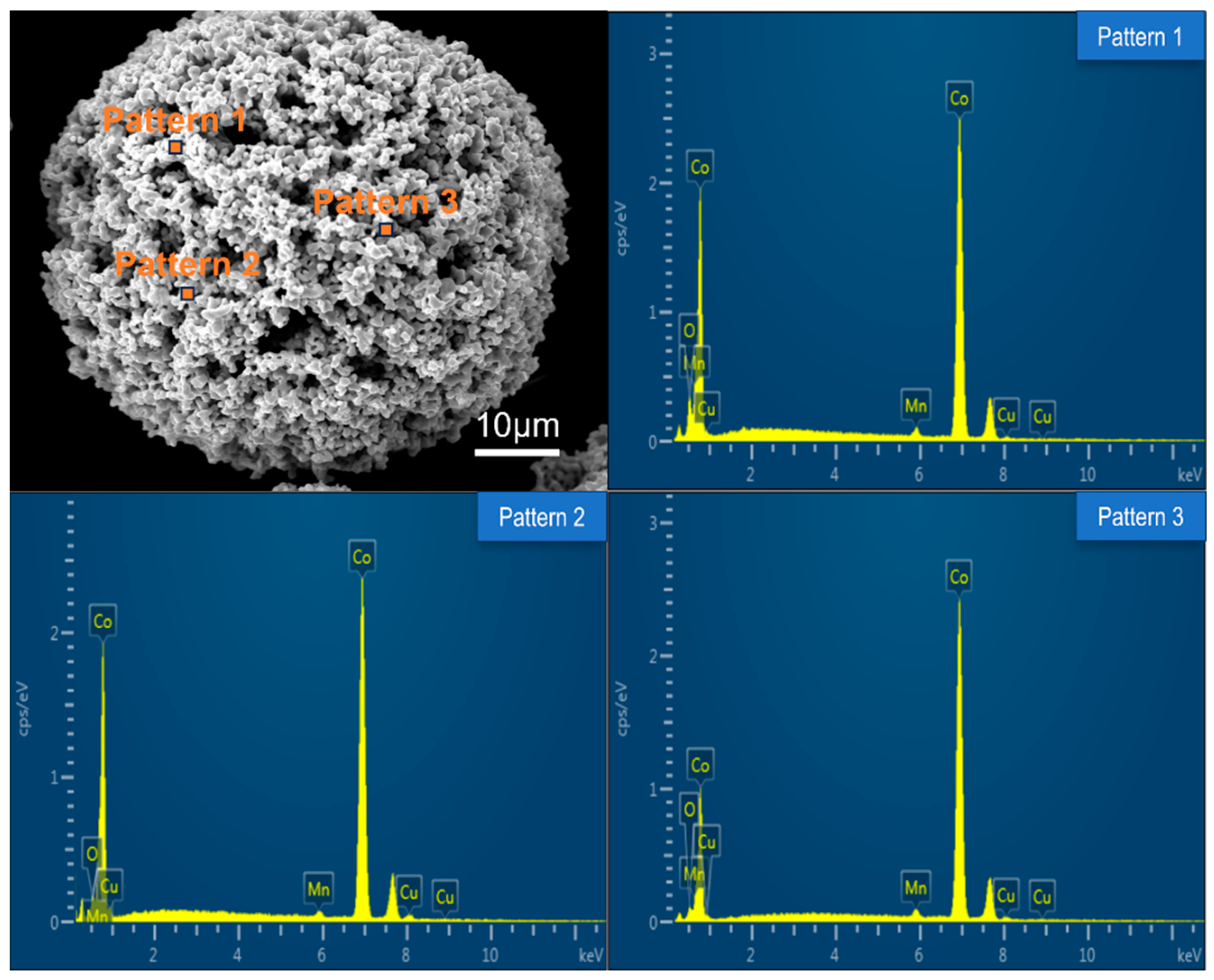
The microstructure of prepared Co-CuCoMnOx powders for coating deposition was observed. As an example, the morphology and the element content of the Co-CuCoMnOx powder in Group B was summarized and shown in Figure 4 and Table 3, correspondingly. It can be seen that the powder for thermal spraying possessed a high degree of sphericity to ensure good flowability during the deposition. Moreover, it can be found from Table 3 that the Co-CuCoMnOx powder in Group B comprised four elements: Cu, Co, Mn, and O. The weight percentages of each element were approximately consistent with the designed content.
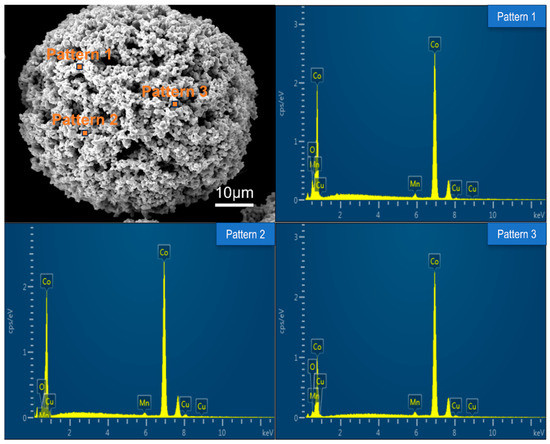
Figure 4.
Morphology and corresponding EDS spectra of Co-CuCoMnOx powder in Group B.

Table 3.
Element content in different positions of Co-CuCoMnOx powder in Group B.
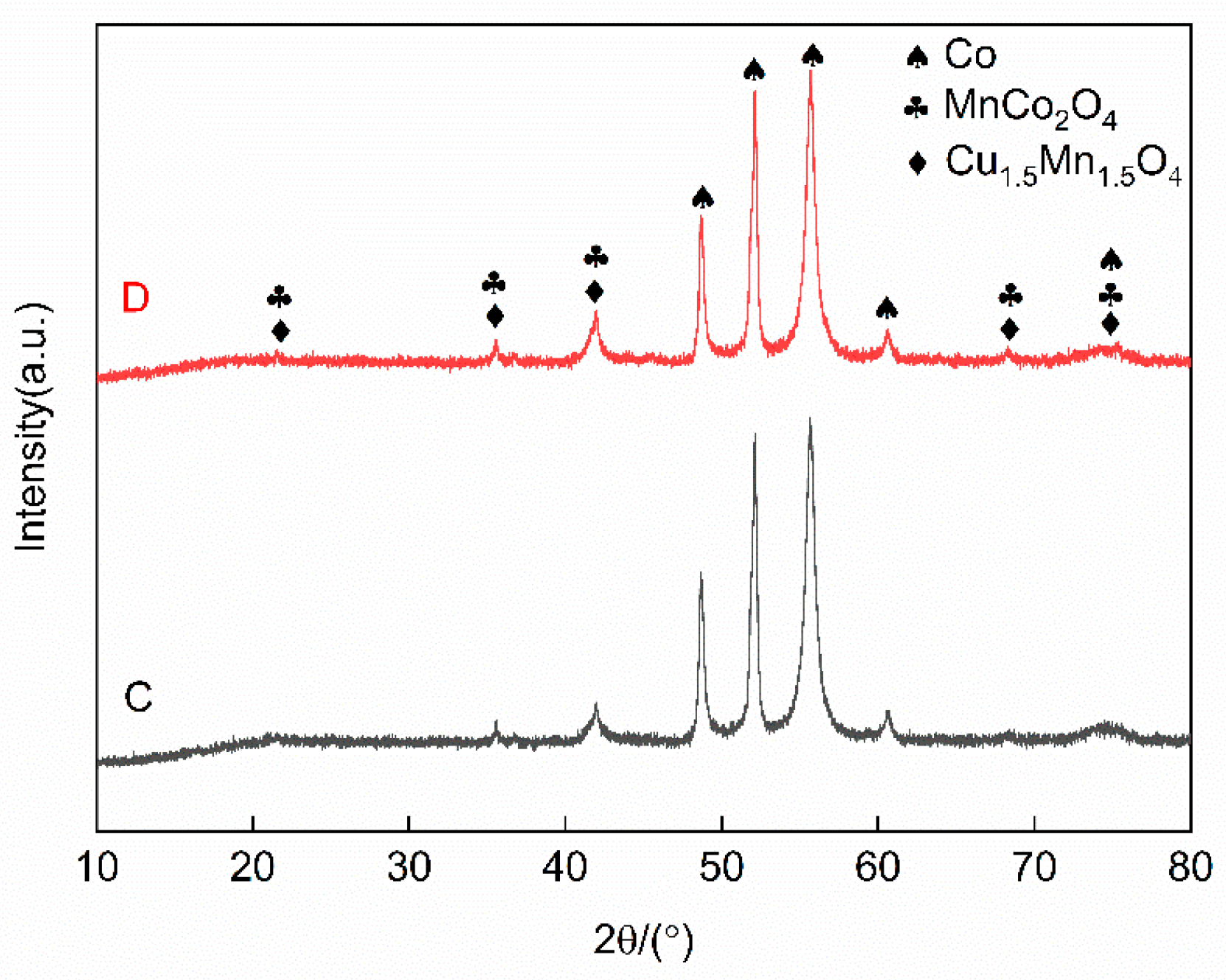
To identify the phase composition of the four-groups Co-CuCoMnOx powders, Group C (10% CuCoMnOx) and D (15% CuCoMnOx) were subjected to X-ray diffraction (XRD) analysis. The results are presented in Figure 5. Both groups of powders mainly contain three phases—Co, Cu1.5Mn1.5O4, and MnCo2O4—indicating that no chemical reactions occurred within the composite powders during the processes of granulation and degumming, and confirming that the phase composition aligned with the initial design specifications.
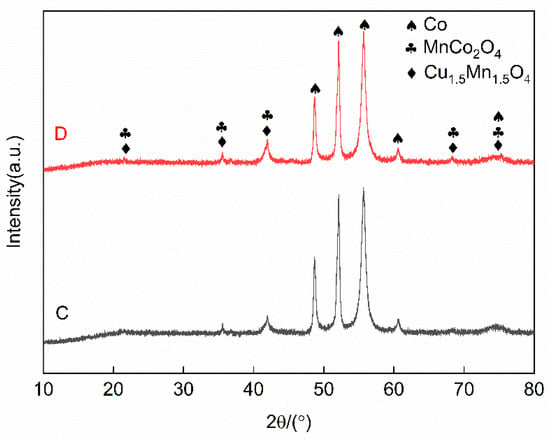
Figure 5.
XRD patterns of Co-CuCoMnOx powders in Groups C and D (Co target). Group C: 90Co-10CuCoMnOx spray powder, Group D: 85Co-15CuCoMnOx spray powder.
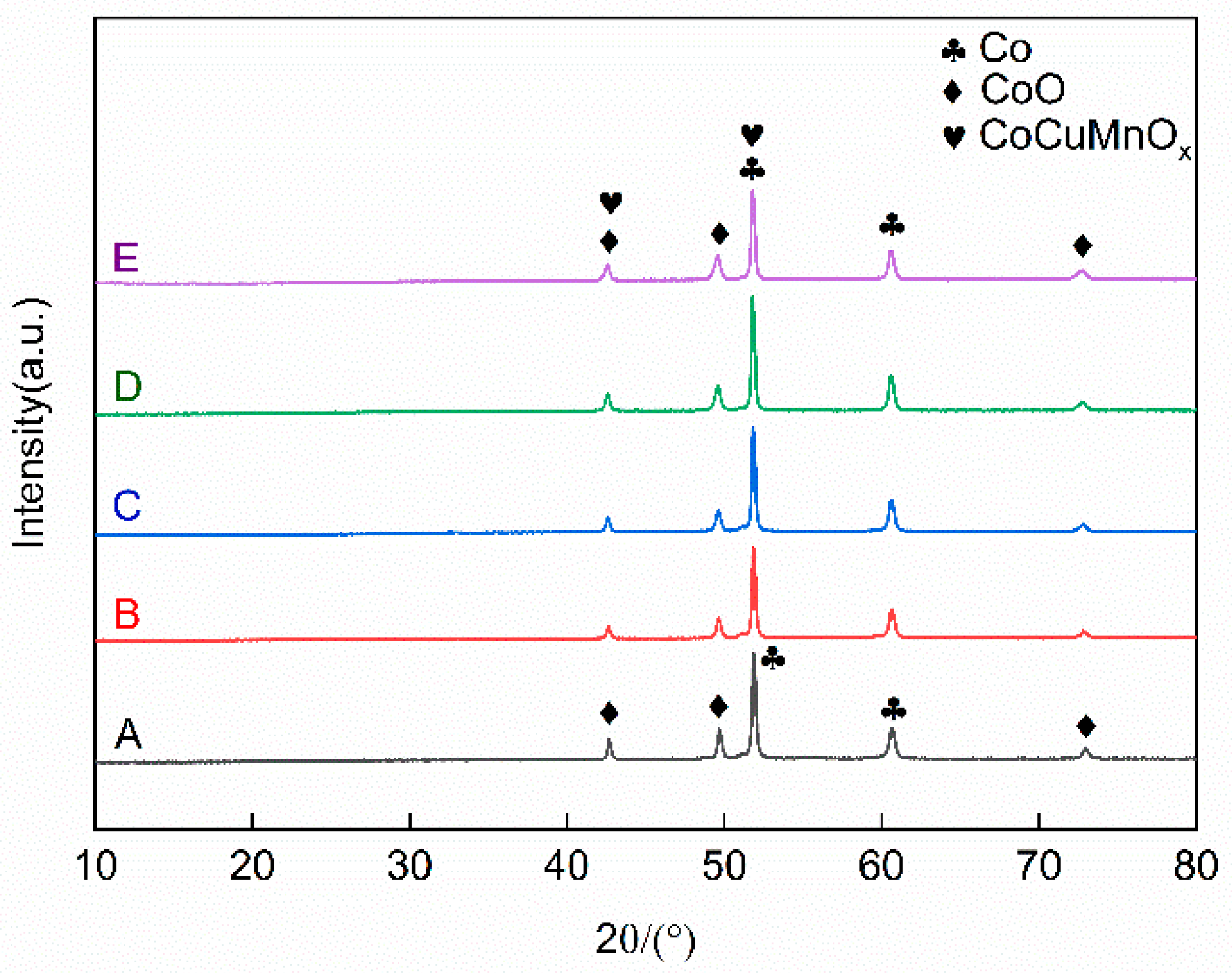
To investigate the phase composition of the Co-CuCoMnOx coatings fabricated by plasma spraying, X-ray diffraction (XRD) analysis was conducted on the coating for each group, and the results are illustrated in Figure 6. According to it, both Co and CoO phases were present in all the coating samples. Furthermore, the CoCuMnOx (#47-0324) phase was also demarcated for Groups B–E. During the plasma spraying process, the temperature of plasma flame can reach as high as 10,000 °C. A portion of cobalt (Co) absorbs oxygen from the surrounding air during its transition from the spray gun to the substrate, resulting in the formation of cobalt oxide (CoO). The high-temperature flame flow inherent in plasma spraying also facilitates the mutual solubility of Cu1.5Mn1.5O4 and MnCo2O4, leading to the generation of the CoCuMnOx phase. By comparing the relative intensities of the two diffraction peaks at 49.64° and 60.64° in Groups A–E as illustrated in Figure 6, it is evident that the intensity ratio of CoO/Co of group A is the highest. This phenomenon suggested that oxidation within the pure Co group is most pronounced. In contrast, for the other four groups, it was inferred that the addition of the CuCoMnOx powders may inhibit the oxidation of Co, since the intensity ratio of CoO/Co apparently decreased. It was thought that the added CoCuMnOx powders might consume the heat for Co oxidation, which reduced the oxidation of Co.
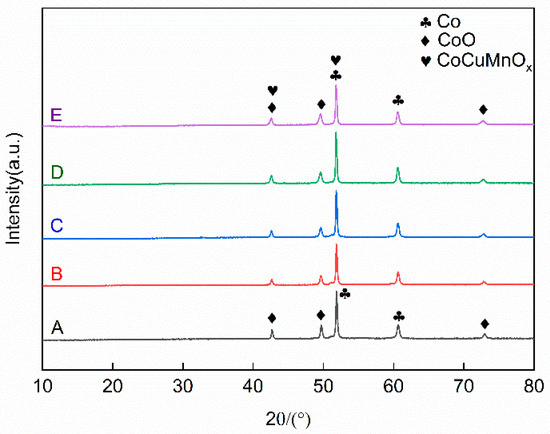
Figure 6.
XRD diffraction patterns of the absorption coating prepared by plasma spraying (Co target). Group A: 100Co coating, Group B: 95Co-5CuCoMnOx coating, Group C: 90Co-10CuCoMnOx coating, Group D: 85Co-15CuCoMnOx coating, Group E: 80Co-20CuCoMnOx coating.
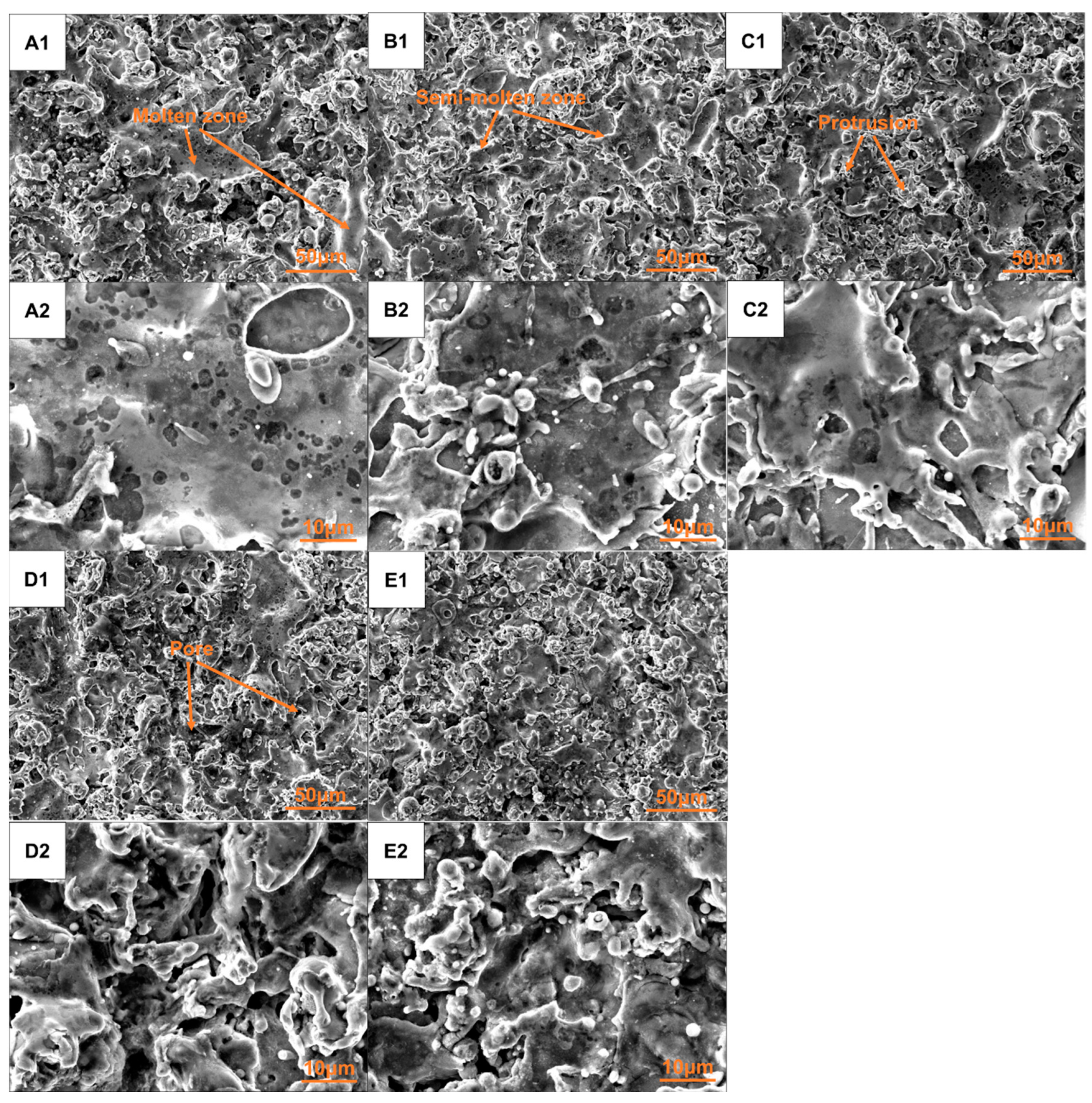
The surface morphologies of the five groups of coatings produced by plasma spraying are illustrated in Figure 7. All the coatings exhibited similar surface morphology, which consisted of molten zones, semi-molten zones, protrusions, and pores. The molten zone came from the fully melted droplets in the flame flow flying towards the surface of the substrate and spreading out, resulting in a relatively smooth and dense area. However, there was still a portion of the composite powder that did not undergo complete heating within the plasma flame flow prior to impacting the substrate. This incomplete heating, along with some molten materials presenting at the edge of the molten zone, collectively formed the semi-molten zone. The formation of protrusions and pores may be attributed to the rapid cooling and contraction of the droplet impacted onto the substrate. The surface roughness (Ra) of these five groups of coatings was similar, which ranged from 3.19 to 4.25 μm. When observed under high-magnification views, it was found that the local spreading conditions of the coatings of Groups A–C were relatively favorable, characterized by a smoother surface with fewer protrusions and smaller pores. In contrast, the local spreading conditions of the coatings of Groups D and E were comparatively poor, exhibiting a rougher surface with more protrusions and larger pores. The surface produced by plasma spraying was a micro-uneven structure, which appeared macroscopically flat while exhibiting microscopic irregularities. Incident light with wavelengths that are less than or equal to the dimensions of the pores or the spacing between protrusions will undergo multiple reflections within these pores or among the protrusions, leading to absorption by the coating. Conversely, incident light with wavelengths exceeding the size of the pores or the distance between protrusions will be directly reflected into the surrounding environment, akin to specular reflection. Consequently, these coatings of Groups A–C might demonstrate a lower absorption rate for ultraviolet–visible near-infrared solar radiation and a lower absorption in the mid–far-infrared range, than the coatings of Groups D–E based on the surface morphology.
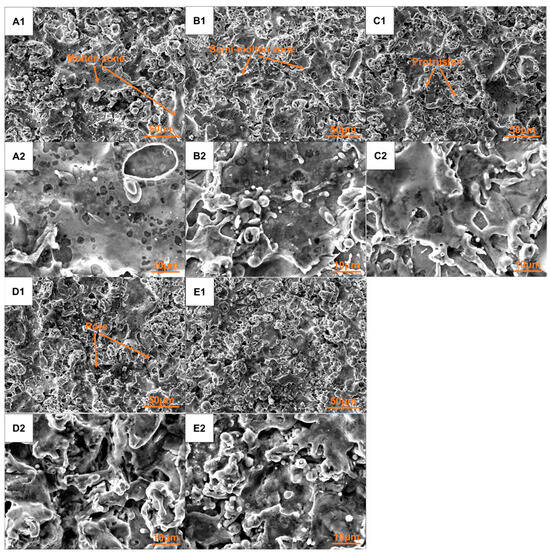
Figure 7.
Surface morphology of plasma sprayed Co-CuCoMnOx coatings under low-magnification view (A1–E1) and local high-magnification view (A2–E2).
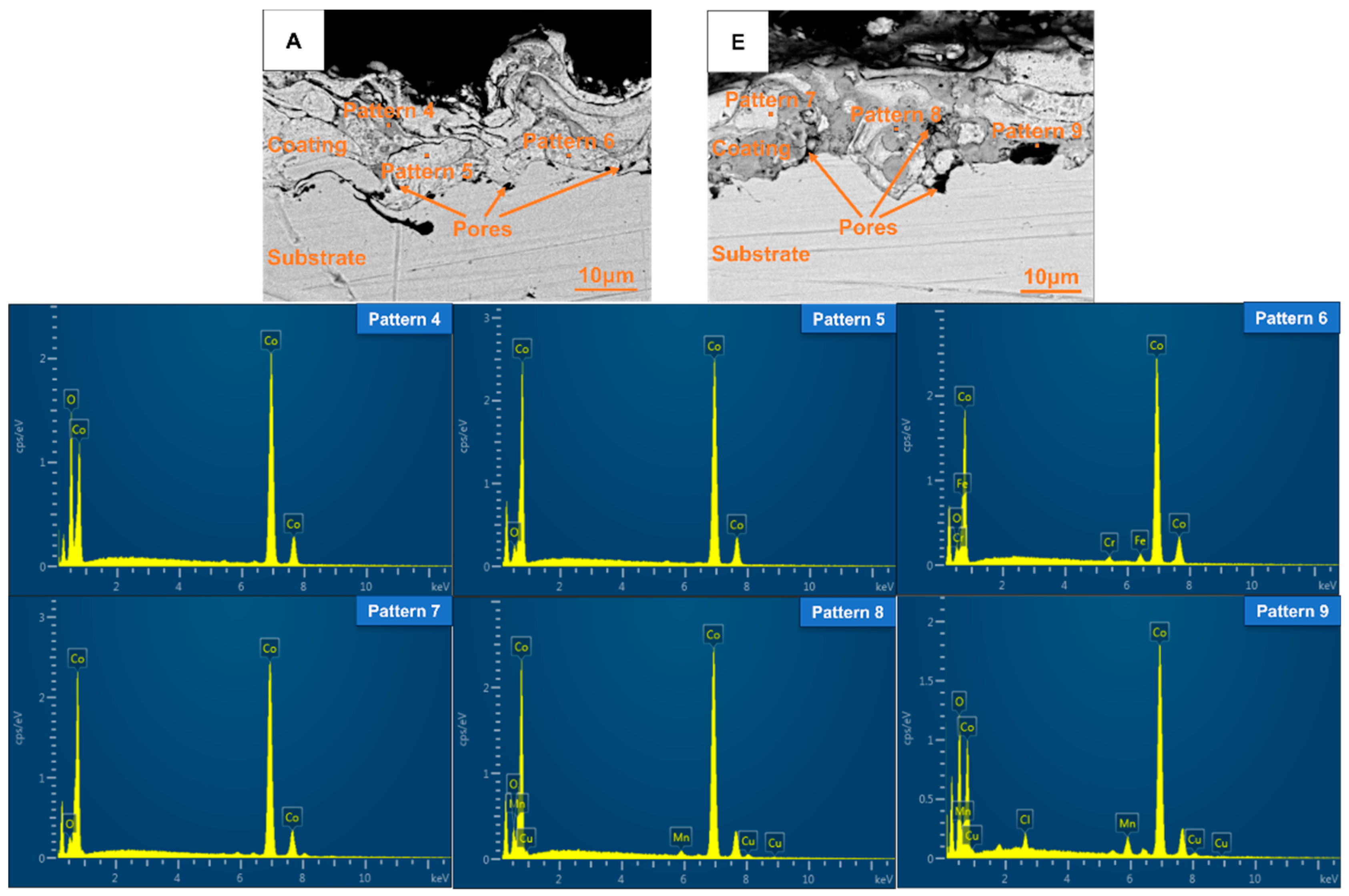
As an example, the cross-sectional morphology of the plasma-sprayed coating of Group A and E is illustrated in Figure 8. The coating thickness ranged from 10 to 20 μm. The interaction between the plasma-sprayed coating and the substrate manifested as mechanical interlocking, demonstrating a robust bond. The coating exhibited a layered structure, which resulted from the molten and semi-molten droplets interlacing and overlapping with one another. It could also be observed from Figure 8 that the plasma sprayed coating contained pores of varying sizes distributed throughout its structure.
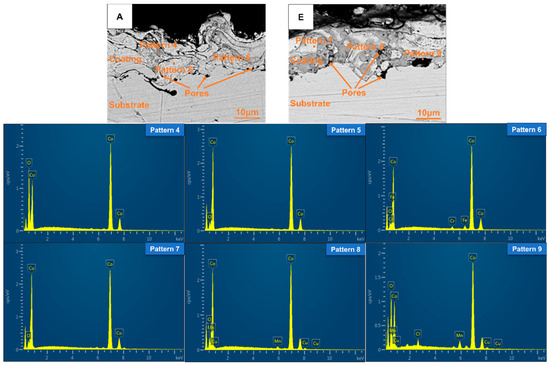
Figure 8.
Cross-sectional morphology and corresponding EDS energy spectrum diagrams of the coatings of Groups A and E.
To investigate the chemical composition differences among coatings containing CoCuMnOx and those without, the corresponding EDS point analysis of the coatings of Groups A and E was selected. The results are summarized in Table 4 and Figure 8. In the backscattering images, the brighter the phase, the larger the corresponding atomic number. Based on the results of Patterns 5 and 6, it could be inferred that the grayish-white regions within the coating of Group A mainly consisted of cobalt (Co) along with a minor presence of cobalt oxide (CoO). In addition, A small amount of matrix elements Fe and Cr are diffused in the part of Group A coating near the substrate, and there are pores inside the coating. In contrast, according to Pattern 4, the gray areas in the coating resulted from a more extensive oxidation of Co. In conjunction with the XRD results, it was inferred that the grayish-white regions marked by Pattern 7 within the coating of Group E consist of Co and a small amount of CoO. The grayish-black areas were identified as containing Co, CoCuMnOx, and CoO, while the black regions not only exhibited porosity but also comprised a mixture of Co, CoCuMnOx, and CoO. In addition, the presence of Cl in Group E coating might be due to a very small amount of Cl impurity in the granulation tower, which was mixed into the spherical spray powder during the spray granulation process, resulting in the inclusion of the Cl element in the coating.

Table 4.
Element content in different positions of Groups A and E coatings (wt%).
3.3. Optical Properties of Co-CuCoMnOx Coatings
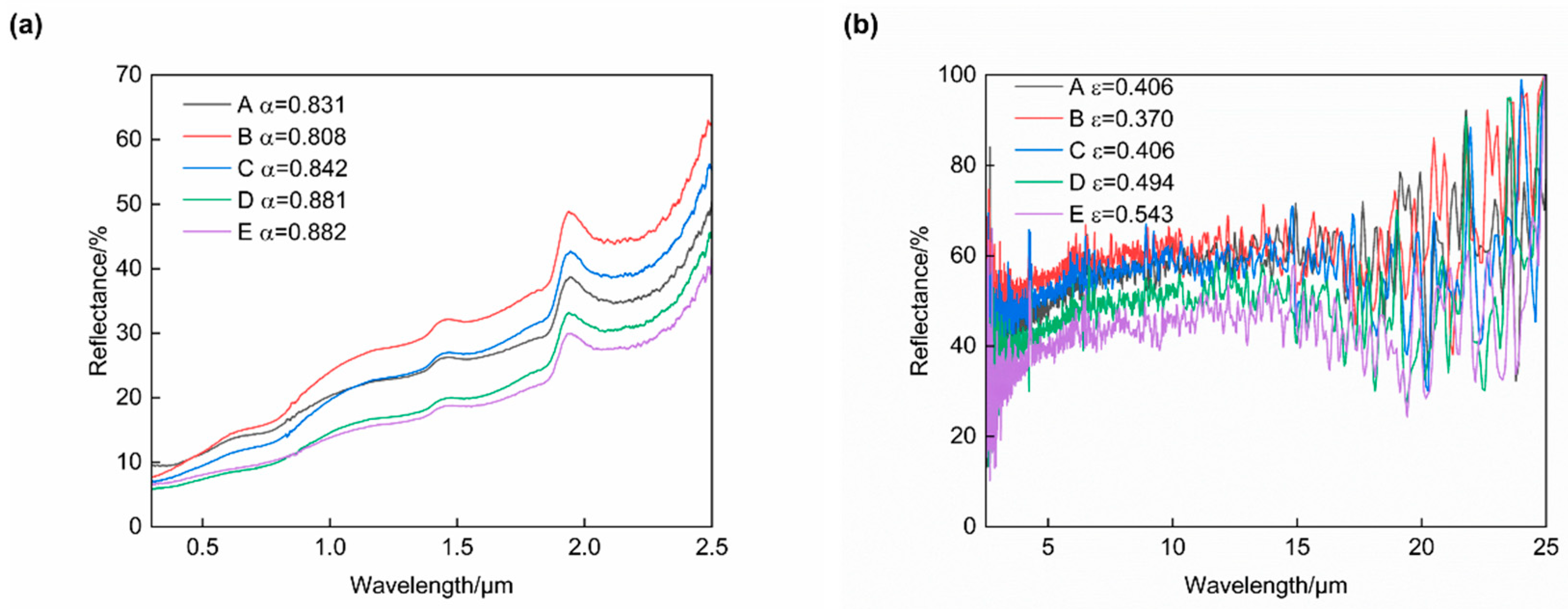
The reflectance spectra for the five groups of plasma-sprayed coatings are illustrated in Figure 9, and the optical properties derived from these reflectance spectra are summarized in Table 5. According to the results, it was found that both the solar absorptance and infrared emittance of those coatings first decreased and then increased when increasing the addition amount of CuCoMnOx. The highest quality factor (α/ε) of 2.184 was obtained in the coating of Group B, whose solar absorptance and infrared emittance were 0.808 and 0.370, respectively.
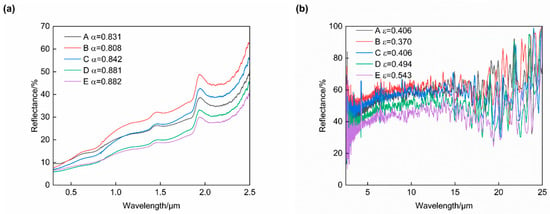
Figure 9.
Reflectance spectra of Co-CuCoMnOx coatings in the ranges of (a) 0.3–2.5 μm and (b) 2.5–25 μm. Group A: 100Co coating, Group B: 95Co-5CuCoMnOx coating, Group C: 90Co-10CuCoMnOx coating, Group D: 85Co-15CuCoMnOx coating, Group E: 80Co-20CuCoMnOx coating.

Table 5.
Optical properties of Co-CuCoMnOx coatings.
Compared to the ceramic phase, pure cobalt exhibits a low solar absorptance as well as low infrared emittance [11]. However, the deposited cobalt coating (Group A) exhibited a solar selective absorption performance, which might be attributed to the oxidation and the formation of CoO during the plasma spraying. The t2 state of Co2+ ions in CoO is characterized by unstable electrons. The interactions arising from this instability lead to intrinsic absorption, which may result in an increased absorption rate and emittance of the coating [34]. Since the two Jahn–Teller ions, Cu2+ and Mn3+, are present in Cu1.5Mn1.5O4, MnCo2O4, and CoCuMnOx during plasma spraying, the composite powder is rapidly heated to 10,000 °C within the plasma flame stream. The phases of Cu1.5Mn1.5O4 and MnCo2O4 undergo mutual dissolution to form the CoCuMnOx phase. At this juncture, alterations occur in the molecular vibration and rotational modes, which subsequently induce lattice distortion and enhance the intrinsic absorption characteristics of the material while simultaneously reducing reflectivity in the infrared spectrum [35]. Therefore, it is likely that the formation of CoCuMnOx within the coating will further augment both absorption and emission rates compared to those exhibited by Cu1.5Mn1.5O4 and MnCo2O4. Combining the XRD analysis of the coatings presented in Figure 6, it was evident that Group A contained cobalt (Co) and cobalt oxide (CoO), and also exhibits the highest concentration of cobalt oxide (CoO). The optical performance of the coating in Group A was a result of the combination of Co and CoO. However, the addition of 5 wt% CuCoMnOx to Group B resulted in a hindrance of Co oxidation; which, in other words, means that the content of Co increased. Since the added content of CuCoMnOx was relatively low, the optical performance of the coating was primarily affected by the Co content, and exhibited characteristics associated with reduced solar absorption and infrared emission. However, when a proportion of 10 wt% or more of CuCoMnOx was incorporated into the coating, the solar absorptance and infrared emittance of Groups C–E exhibited a gradual increase. This phenomenon can be attributed to the increase in CoCuMnOx, which possessed high solar absorptance and high infrared emittance.
3.4. High Temperature Stability of Co-CuCoMnOx Coatings
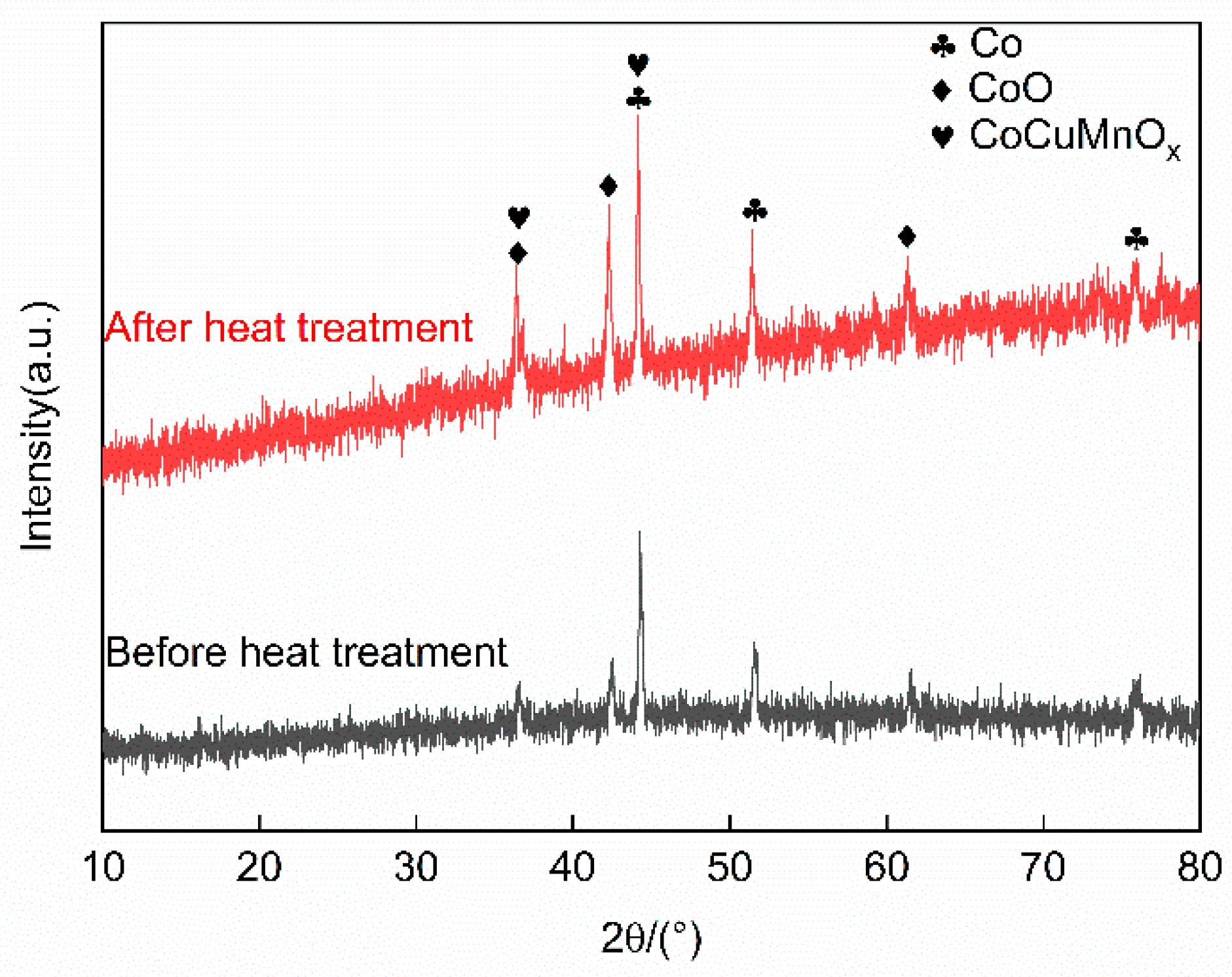
To evaluate the high temperature stability of the Co-CuCoMnOx coatings, the coatings of Group B underwent a high-temperature heat treatment at 450 °C in an atmospheric environment for a duration of 3 h. The optical performance prior to and after the high-temperature heat treatment are presented in Table 6. Additionally, the X-ray diffraction (XRD) patterns of the coatings before and after the heat treatment are illustrated in Figure 10. Based on the variations in diffraction peak intensity before and after the heat treatment, it could be inferred that the content of CoO increased after the heat treatment. Consequently, the solar absorptance and infrared emittance of the coating increased after the heat treatment. The performance criterion (PC) to evaluate the high temperature stability of the coating is if the PC value described as Equation (5) [36] is below 0.05, it can be concluded that the degradation performance of the coating at elevated temperatures is not significant. Furthermore, this suggests that the coating exhibits relatively good thermal stability under such conditions [37]. After the heat treatment, it was found that no cracking or shedding happened on the coating surface. The solar absorptance of the coating reached 0.89, while its emissivity increased by 0.168 compared to pre-treatment values. The PC value of the coating after the heat treatment at 450 °C for 3 h in an atmospheric environment was calculated as 0.002, which was far below the threshold of 0.05. The attenuation of the optical performance of the coating was negligible. Therefore, it could be concluded that the coating exhibited excellent thermal stability when maintained at 450 °C in an atmospheric environment for a duration of 3 h.
PC = (αbefore heat treatment − αafter heat treatment) − 0.5 × (εbefore heat treatment − εafter heat treatment)

Table 6.
The absorptivity and emissivity values of Group B coatings before and after the heat treatment.
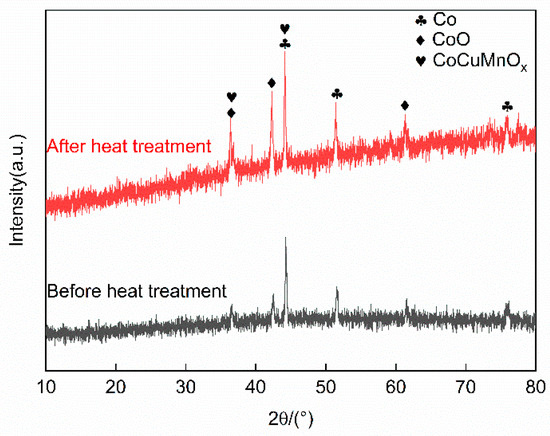
Figure 10.
XRD patterns of the coatings in Group B before and after the heat treatment.
4. Conclusions
By employing the solid-phase reaction method, CuO-Co3O4-MnO2 composite materials were successfully synthesized into CuCoMnOx powder at 1100 °C in a muffle furnace for 2 h. The CuCoMnOx powder predominantly consisted of Cu1.5Mn1.5O4 and MnCo2O4. Furthermore, the CuCoMnOx powder demonstrated a solar absorptance of 0.929 and an infrared emittance of 0.862, which was considered a good absorbent. Co-CuCoMnOx spherical spray powders were prepared using spray granulation and de-gelling processes, with the phase composition of the spray powder containing CuCoMnOx being Co, Cu1.5Mn1.5O4, and MnCo2O4, maintaining consistency with the initially designed components. Subsequently, Co-CuCoMnOx coatings were deposited on the surface of 316L steels utilizing the APS technique, aiming at developing a novel metal–ceramic solar selective absorption coating employed at high temperature. The chemical phase, microstructure, and optical performance, as well as high temperature stability of the prepared coatings, were systematically studied. The results showed that the Co-CuCoMnOx coating possessed a solar selective absorption capacity after being compound with cobalt. The synthesized Co-CuCoMnOx coating had a typical thermal spray layered stacking structure with molten zones, semi-molten zones, protrusions, and pores distributed on the coating surface, and had a thickness of 10~20 μm. The chemical phases of the coatings were mainly Co, CoO, and CoCuMnOx. Here, CoO was formed by the partial oxidation of Co as it absorbed oxygen from the surrounding air during plasma spraying. The inherent high-temperature flame flow in plasma spraying promoted the mutual solubility of Cu1.5Mn1.5O4 and MnCo2O4, leading to the formation of the CoCuMnOx phase. As the amount of CuCoMnOx added increased, both the solar absorption rate and infrared emissivity of the five groups of coatings produced by plasma spraying showed a trend of first decreasing and then increasing, while the quality factor showed a trend of first increasing and then decreasing. Due to the addition of CuCoMnOx inhibiting the oxidation of Co during the thermal spraying process, the 95Co-5CuCoMnOx (wt%) coating exhibited the optimal quality factor (α/ε) of 2.184, with a solar absorptance α of 0.808 and an infrared emittance ε of 0.370, respectively. Moreover, this specific coating demonstrated a good thermal stability for up to 3 h, in which the PC value was far less than 0.05 when it was exposed to an atmospheric environment at 450 °C. This finding holds significant implications for the research and development of high-temperature solar selective absorption coatings.
Author Contributions
X.Z. and X.C. conceived the idea. Investigation, Z.L. (Ziqiang Long) and Z.L. (Ziyong Liu). X.Z., Z.L. (Ziqiang Long) and Q.C. designed the experiment. Z.L. (Ziqiang Long) analyzed the data. X.Z. and Z.L. (Ziqiang Long) participated in the writing of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No.52473280) and Guangxi Science and Technology Major Project (GUIKE AA23062025-3, China).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kalogirou, S.A. Solar thermal collectors and applications. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Zäll, E.; Segervald, J.; Mahmoodi, H.; Perivoliotis, D.; Edman, L.; Wågberg, T. Achieving optically selective coatings of silica fixated carbon nanotubes for solar energy applications. Sol. Energy Mater. Sol. Cells 2024, 278, 113202. [Google Scholar] [CrossRef]
- Zheng, L.Q.; Gao, F.Y.; Zhao, S.X.; Zhou, F.Y.; Nshimiyimana, J.P.; Diao, X.G. Optical design and co-sputtering preparation of high performance Mo–SiO2 cermet solar selective absorbing coating. Appl. Surf. Sci. 2013, 280, 240–246. [Google Scholar] [CrossRef]
- Farchado, M.; Vicente, G.S.; Barandica, N.; Sánchez-Señorán, D.; Morales, Á. High performance selective solar absorber stable in air for high temperature applications. Sol. Energy Mater. Sol. Cells 2024, 271, 112849. [Google Scholar] [CrossRef]
- Kant, K.; Sibin, K.P.; Pitchumani, R. Novel fractal-textured solar absorber surfaces for concentrated solar power. Sol. Energy Mater. Sol. Cells 2022, 248, 112010. [Google Scholar] [CrossRef]
- Elahi, A.N.M.T.; Crist, R.; Francoeur, M.; Park, K.; Rao, S. Characterization of high-temperature figure of merit for solar-thermal absorbers. Sol. Energy Mater. Sol. Cells 2023, 257, 112378. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhao, J.X.; Ren, L.H. Aqueous solution-chemical derived Ni–Al2O3 solar selective absorbing coatings. Sol. Energy Mater. Sol. Cells 2012, 105, 90–95. [Google Scholar] [CrossRef]
- Tsegay, M.G.; Gebretinsae, H.G.; Sackey, J.; Arendse, C.J.; Nuru, Z.Y. Structural and optical properties of Pt-Al2O3 double cermet as selective solar absorber. Mater. Today Proc. 2021, 36, 571–575. [Google Scholar] [CrossRef]
- Wu, Y.W.; Zheng, W.F.; Lin, L.M.; Qu, Y.; Lai, F.C. Colored solar selective absorbing coatings with metal Ti and dielectric AlN multilayer structure. Sol. Energy Mater. Sol. Cells 2013, 115, 145–150. [Google Scholar] [CrossRef]
- Pang, X.M.; Jiang, J.X.; Li, B.; Zhao, D.Y.; Zhou, J.X. Study of wide temperature range and hard protective La2O3 doped cermet based single-layer solar selective absorbing coating by laser cladding. Surf. Interfaces 2021, 27, 101544. [Google Scholar] [CrossRef]
- Shao, H.; Zeng, X.; Li, Q.Y.; Liu, F.S.; Liu, T.Z.; Cheng, X.D. Preparation and Properties of Co-Cr3C2-WC/Al2O3 Solar Selective Absorbing Coatings. Surf. Technol. 2020, 49, 138–145. (In Chinese) [Google Scholar]
- Pang, X.M. High temperature solar selective absorber coating deposited by laser cladding. Mater. Res. Express 2017, 4, 095503. [Google Scholar] [CrossRef]
- Chen, W.; Ding, Z.X.; Gao, R.Y.; Cheng, X.D. Performance Study of WC-12Co Solar Energy Selective Absorption Coatings Deposited by HVOF. Therm. Spray Technol. 2011, 3, 40–46. (In Chinese) [Google Scholar]
- Ke, C.Z.; Zhang, X.M.; Guo, W.Y.; Li, Y.J.; Gong, D.Q.; Cheng, X.D. Solar selective coatings with multilayered structure based on thermal spraying WC-Co solar absorption layer. Vacuum 2018, 152, 114–122. [Google Scholar] [CrossRef]
- Bayón, R.; Vicente, G.S.; Maffiotte, C.; Morales, Á. Preparation of selective absorbers based on CuMn spinels by dip-coating method. Renew. Energy 2008, 33, 348–353. [Google Scholar] [CrossRef]
- Atchuta, S.R.; Sakthivel, S.; Barshilia, H.C. Transition metal based CuxNiyCoz−x−yO4 spinel composite solar selective absorber coatings for concentrated solar thermal applications. Sol. Energy Mater. Sol. Cells 2019, 189, 226–232. [Google Scholar] [CrossRef]
- Pakzad, E.; Ranjbar, Z.; Ghahari, M. Synthesized of octahedral cupper chromite spinel for spectrally selective absorber (SSA) coatings. Prog. Org. Coat. 2019, 132, 21–28. [Google Scholar] [CrossRef]
- Atchuta, S.R.; Sakthivel, S.; Barshilia, H.C. Selective properties of high-temperature stable spinel absorber coatings for concentrated solar thermal application. Sol. Energy 2020, 199, 453–459. [Google Scholar] [CrossRef]
- Deng, X.Q.; Xue, M.M.; Lv, Y.L.; Li, R.H.; Tong, J.M.; Shi, G.H.; Yang, Y.; Dong, Y.C. Study on spectral selective absorbing coatings with spinel structures fabricated via plasma spraying. Vacuum 2020, 174, 109214. [Google Scholar] [CrossRef]
- Atchuta, S.R.; Sakthivel, S.; Barshilia, H.C. Nickel doped cobaltite spinel as a solar selective absorber coating for efficient photothermal conversion with a low thermal radiative loss at high operating temperatures. Sol. Energy Mater. Sol. Cells 2019, 200, 109917. [Google Scholar] [CrossRef]
- Wei, S.C.; Xie, J.; Gao, W.; Zhan, Z.L.; Li, Z.L. Characteristics and Failure Behaviors of an MnCo2O4 Spinel Coating in High-Temperature Oxidation Processes. Trans. Indian Inst. Met. 2022, 75, 797–804. [Google Scholar] [CrossRef]
- Aslan, E.; Emir, Ö.; Arslan, F.; Goktas, A.; Tumbul, A.; Durgun, M.; Kilic, A.; Aktacir, M.A.; Aslan, F. Improving the optical properties of CuCoMnOx spinel absorber using ZnO nanorod arrays for thermal collector and photocatalytic applications. Ceram. Int. 2024, 50, 9169–9176. [Google Scholar] [CrossRef]
- El Mahallawy, N.; Shoeib, M.; Ali, Y. Application of CuCoMnOx coat by sol gel technique on aluminum and copper substrates for solar absorber application. J. Coat. Technol. Res. 2014, 11, 979–991. [Google Scholar] [CrossRef]
- Vince, J.; Vuk, A.Š.; Krašovec, U.O.; Orel, B.; Köhl, M.; Heck, M. Solar absorber coatings based on CoCuMnOx spinels prepared via the sol–gel process: Structural and optical properties. Sol. Energy Mater. Sol. Cells 2003, 79, 313–330. [Google Scholar] [CrossRef]
- Bayón, R.; Vicente, G.S.; Maffiotte, C.; Morales, Á. Characterization of copper–manganese-oxide thin films deposited by dip-coating. Sol. Energy Mater. Sol. Cells 2008, 92, 1211–1216. [Google Scholar] [CrossRef]
- Geng, Q.F.; Zhao, X.; Gao, X.H.; Liu, G. Sol–Gel Combustion-Derived CoCuMnOx Spinels as Pigment for Spectrally Selective Paints. J. Am. Ceram. Soc. 2010, 94, 827–832. [Google Scholar] [CrossRef]
- Farchado, M.; Vicente, G.S.; Barandica, N.; Morales, A. Novel highly performing tandem selective solar absorber for industrial heat applications. Sol. Energy Mater. Sol. Cells 2025, 279, 113249. [Google Scholar] [CrossRef]
- Peng, Y.J.; Fang, Z.; Hu, J.P.; Wang, B.W.; Xie, P.K. Test Study on Performance of Endothermic Highly Hydrophobic Anti-Icing Materials. J. Phys. Conf. Ser. 2020, 1659, 012007. [Google Scholar] [CrossRef]
- Kordas, G. Incorporation of spherical shaped CuO@SiO2 light microtraps into CuCoMnOx spinels to enhance solar absorbance. J. Am. Ceram. Soc. 2019, 103, 1536–1541. [Google Scholar] [CrossRef]
- Liu, H.D.; Fu, T.R.; Duan, M.H.; Wan, Q.; Chen, Y.M.; Fu, D.J.; Ren, F.; Li, Q.Y.; Cheng, X.D.; Yang, B.; et al. Structure and thermal stability of spectrally selective absorber based on AlCrON coating for solar-thermal conversion applications. Sol. Energy Mater. Sol. Cells 2016, 157, 108–116. [Google Scholar] [CrossRef]
- He, X.M.; Pu, W.H.; Cai, Y.; Jiang, C.Y.; Wan, C.R.; Xia, D.G. Preparation of spherical LiMn2O4 for Li-ion batteries based on controlled crystallization. J. Nonferrous Met. 2005, 15, 1390–1395. (In Chinese) [Google Scholar]
- Zhang, H.X. Desulfurization reactivity of zinc based composite oxides. J. Fuel Chem. Technol. 2007, 35, 619–623. (In Chinese) [Google Scholar]
- Du, J.J.; Liu, Y.H.; Yao, G.C.; Zhang, X. Effect of MnO2 Addition on Sintering Behavior and Magnetic Property of Nickel Ferrite Spinel. J. Northeast Univ. 2011, 32, 1452–1455. (In Chinese) [Google Scholar]
- Zhang, X.M.; Wang, X.B.; Zhang, X.; Li, Y.J.; Cheng, X.D. Effect of multilayered CoO-CoAl2O4 films on improving solar absorptance of Co-WC solar selective absorbing coatings. Vacuum 2018, 155, 185–192. [Google Scholar] [CrossRef]
- Geng, Q.F.; Zhao, X.; Gao, X.H.; Yu, H.C.; Yang, S.R.; Liu, G. Optimization design of CuCrxMn2−xO4-based paint coatings used for solar selective applications. Sol. Energy Mater. Sol. Cells 2012, 105, 293–301. [Google Scholar] [CrossRef]
- Köhl, M.; Heck, M.; Brunold, S.; Frei, U.; Carlsson, B.; Möller, K. Advanced procedure for the assessment of the lifetime of solar absorber coatings. Sol. Energy Mater. Sol. Cells 2004, 84, 275–289. [Google Scholar] [CrossRef]
- Yu, H.W.; Li, J.K.; Zhang, Q.; Pang, W.; Yan, H.; Li, G.Y. Thermal Stability of Chromium-Iron Oxidation Mixture Cermet-Based Solar Selective Absorbing Coatings. Molecules 2020, 25, 1178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).