Electrodeposition of Metallic Tungsten Coating on 9Cr-ODS Steel Substrate from Binary Oxide Molten Salt
Abstract
1. Introduction
2. Experiment and Parameters
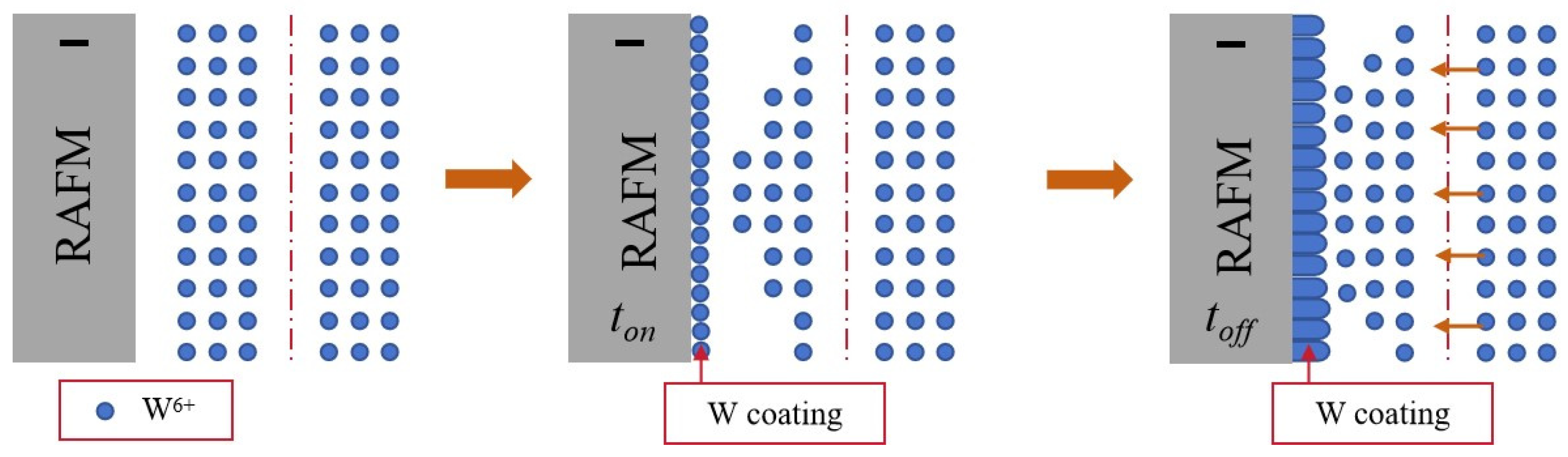
2.1. Coating Deposition
2.2. Coating Characterization
3. Results and Discussions
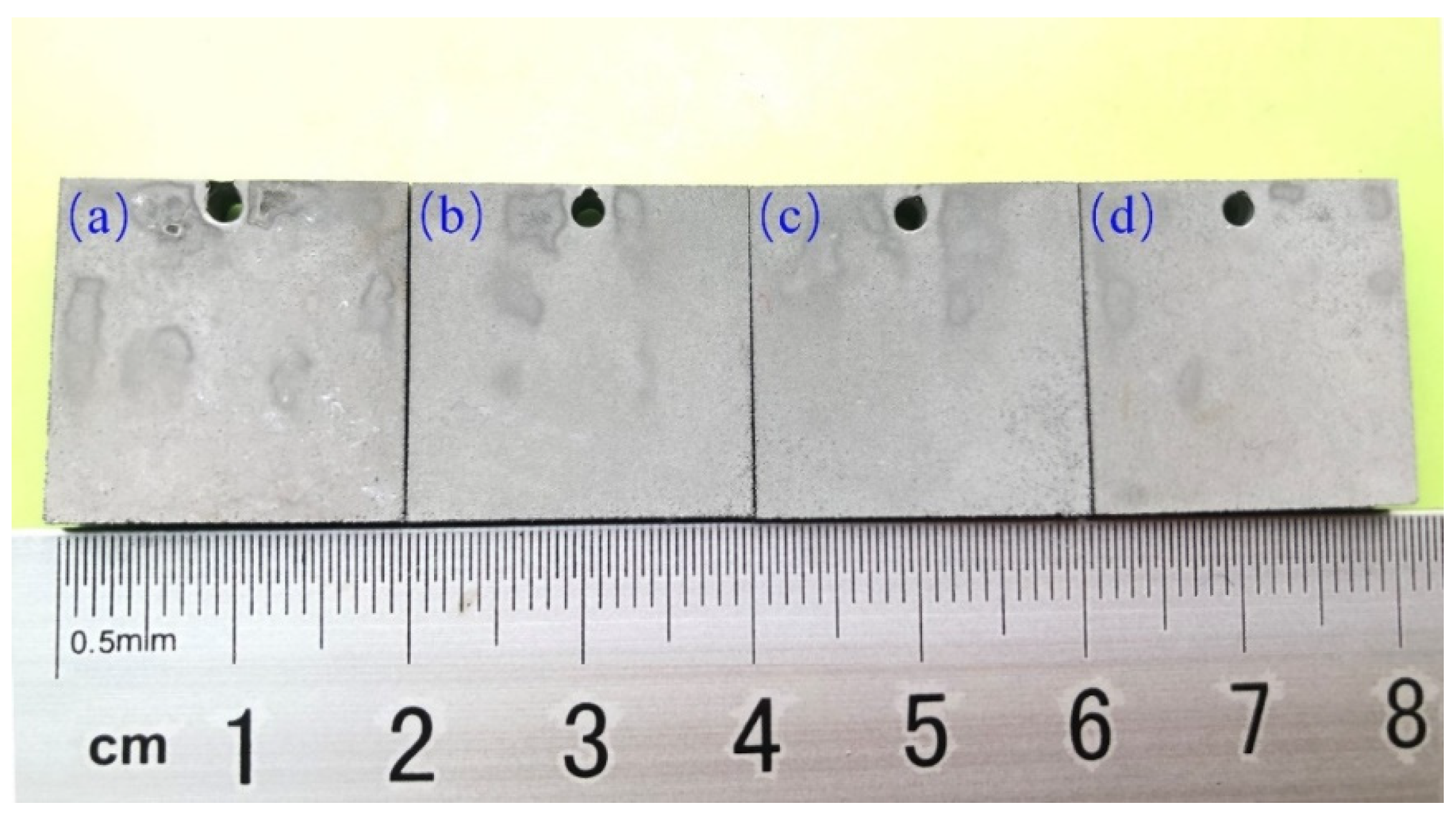
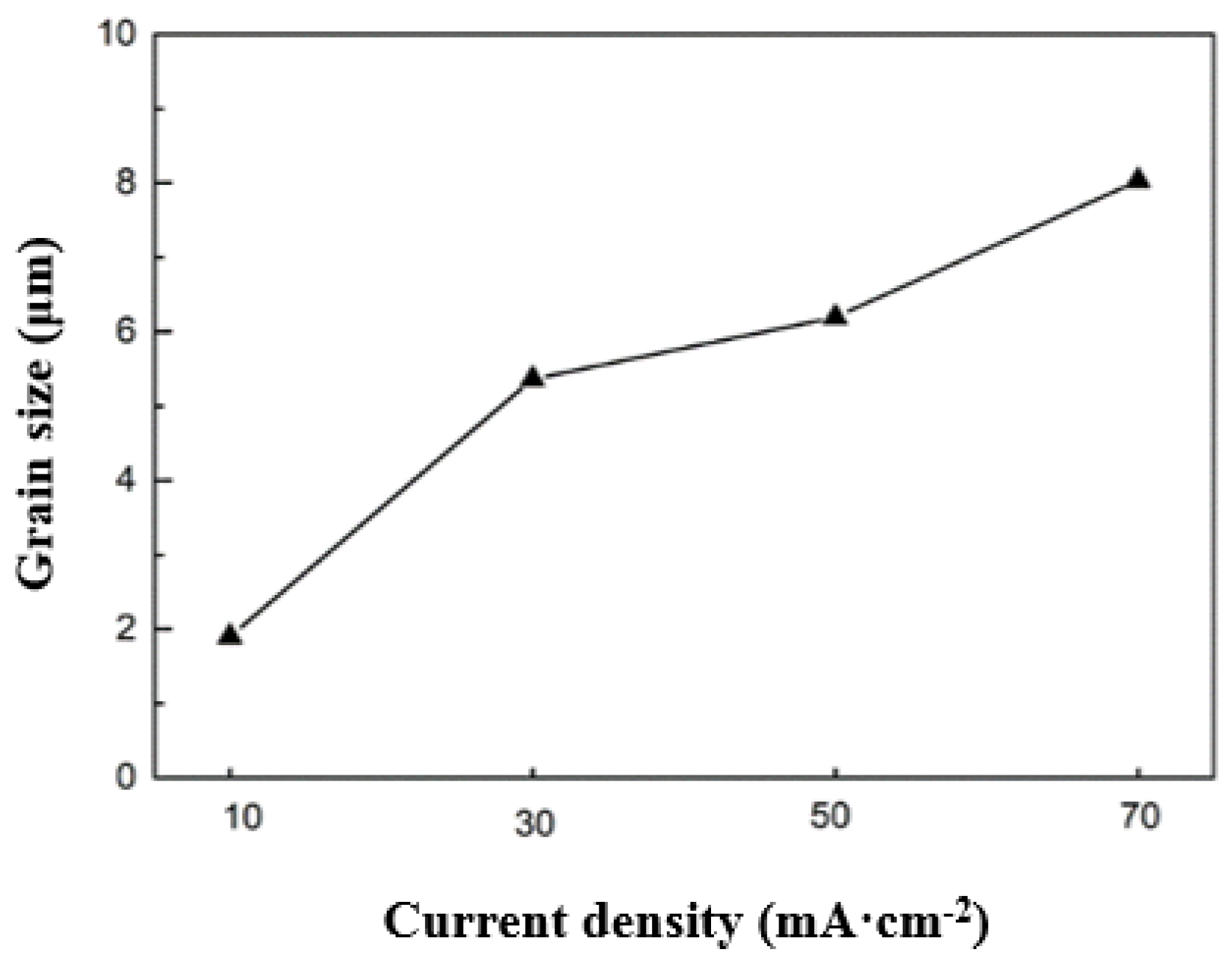
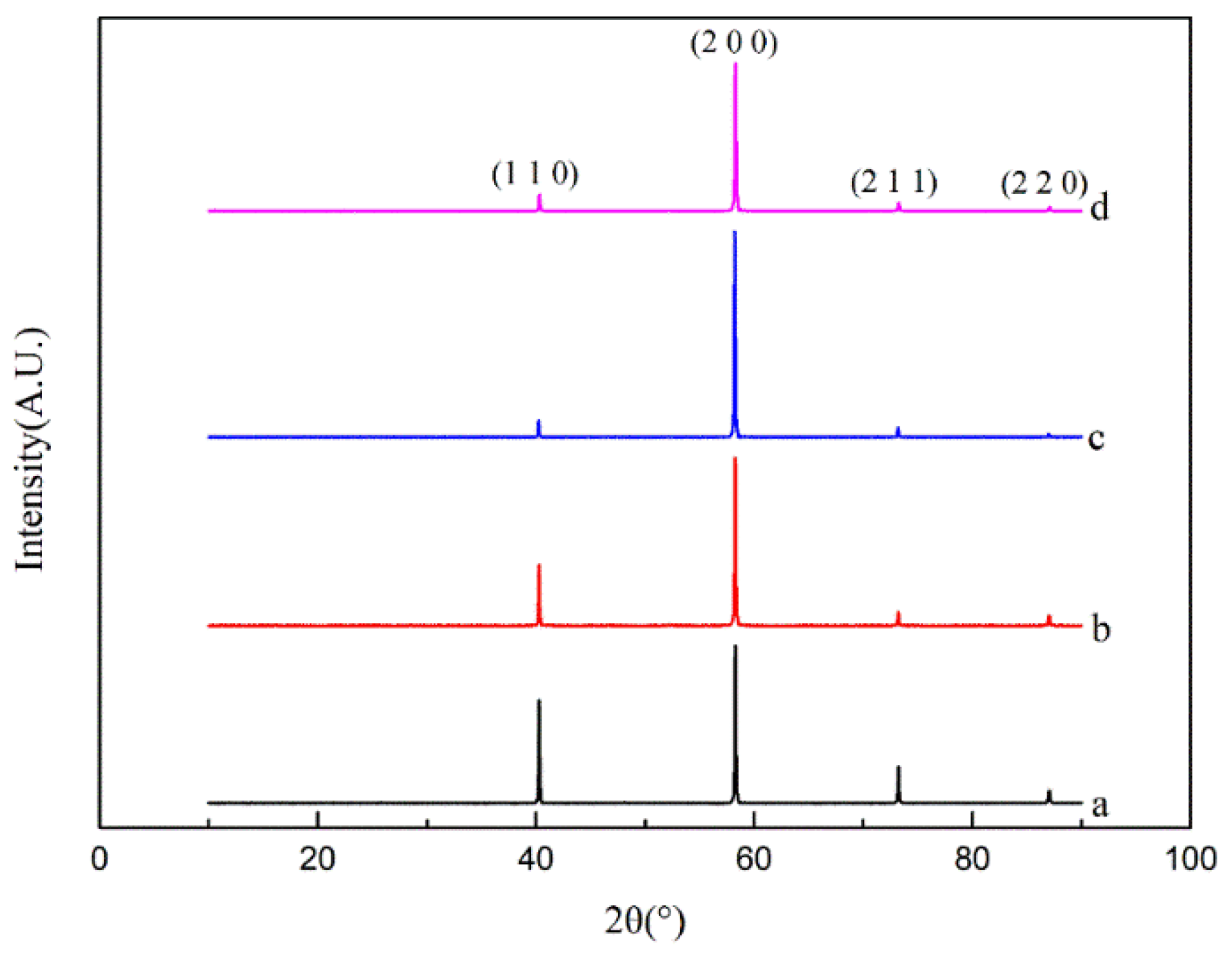
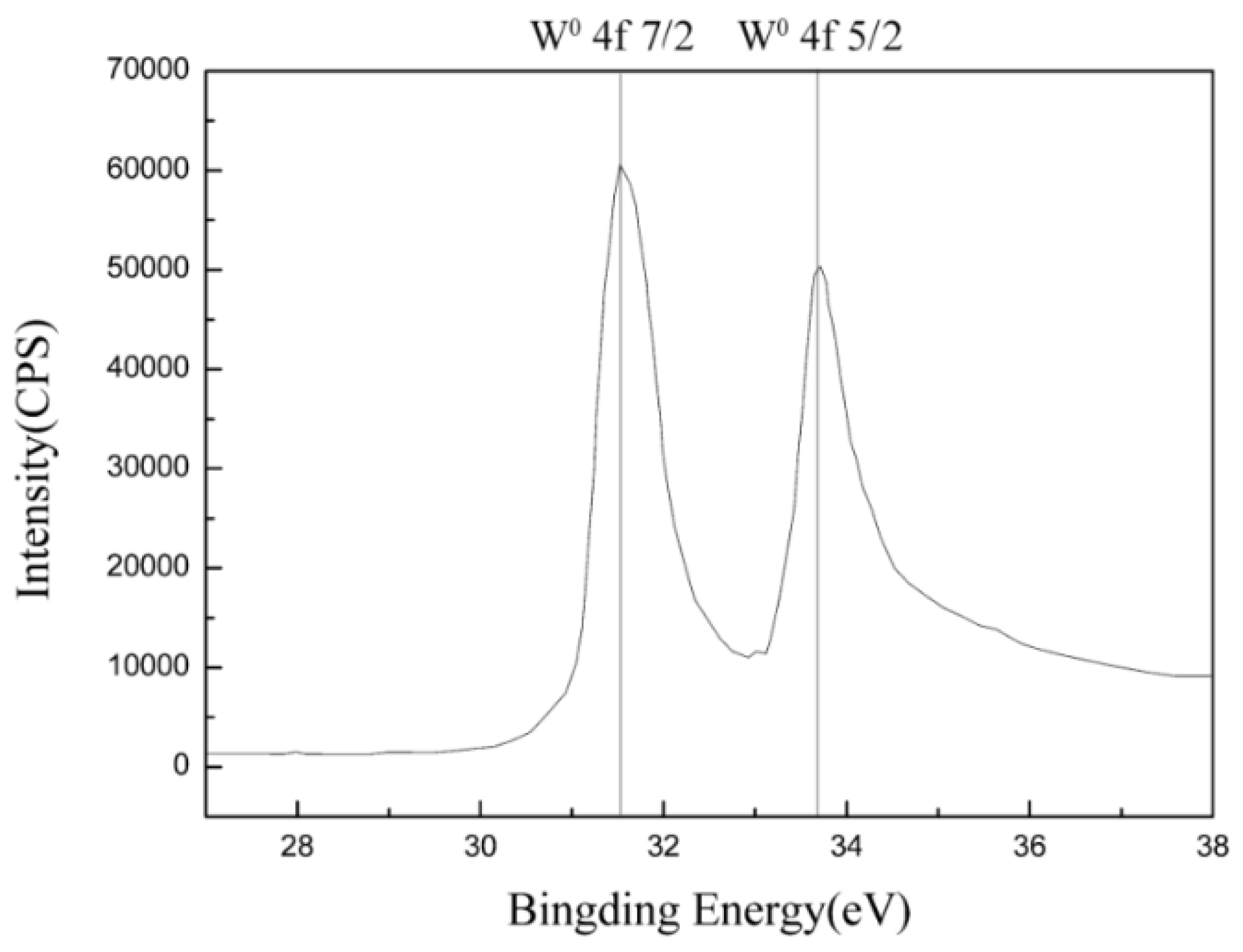
3.1. Microstructure and Crystal Structure of Thick Tungsten Coating
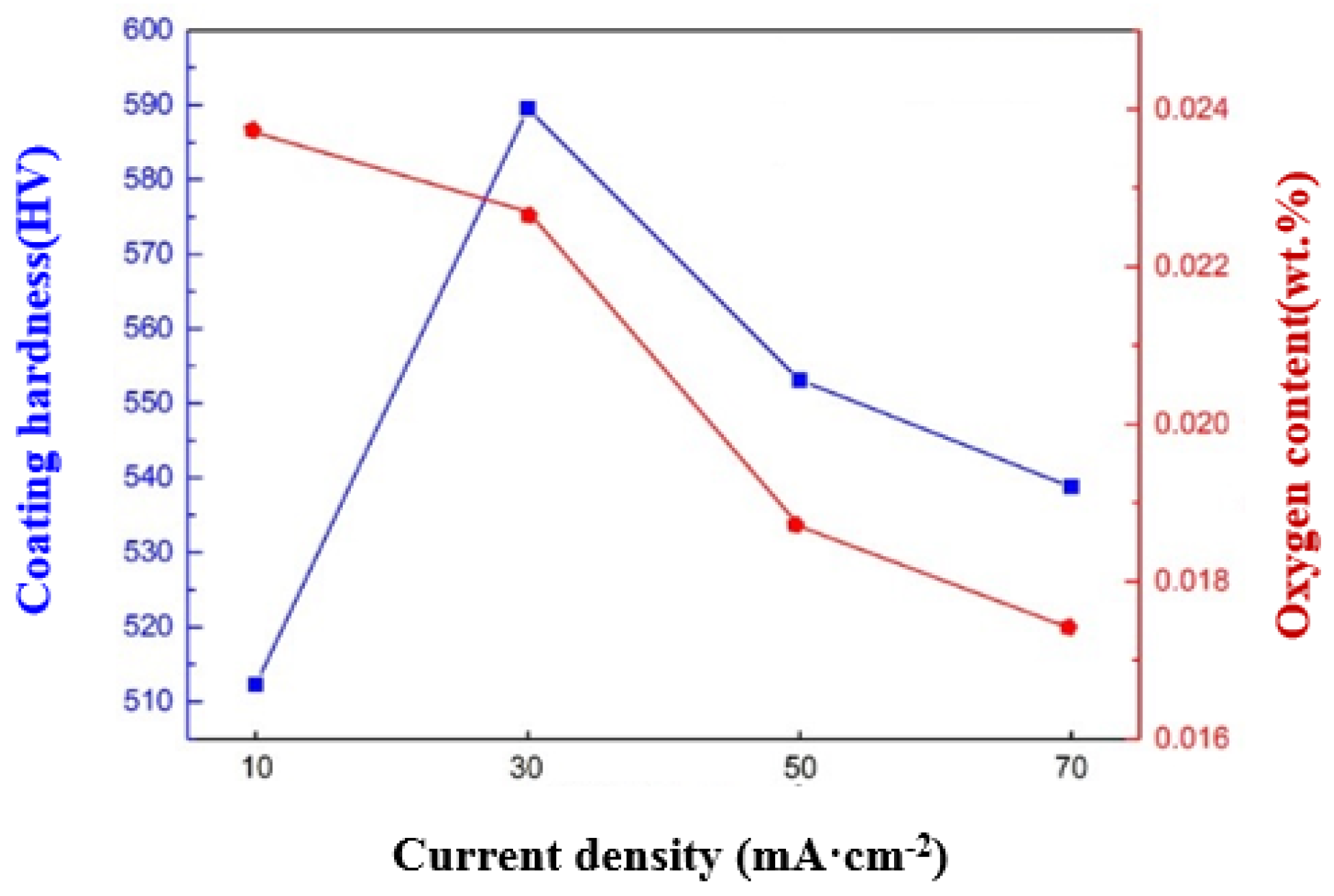
3.2. Performance Evaluation of Thick Tungsten Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez-Arrabal, R.; Mendez-González, Y.; Perlado, J.M. Sputtering fabrication of isolated W nanocolumns: A possible al-ternative as plasma facing material for nuclear fusion reactors. Nucl. Mater. Energy 2024, 40, 101704. [Google Scholar] [CrossRef]
- Piip, K.; Paris, P.; Hakola, A.; Bystrov, K.; De Temmerman, G.; Aints, M.; Jõgi, I.; Kozlova, J.; Laan, M.; Likonen, J.; et al. Influence of He/d2 Plasma Fluxes on the Morphology and Crystallinity of Tungsten Coatings. Phys. Scr. 2014, 89, 044009. [Google Scholar] [CrossRef]
- Ribis, J.; Bordas, E.; Trocellier, P.; Serruys, Y.; de Carlan, Y.; Legris, A. Comparison of the neutron and ion irradiation response of nano-oxides in oxide dispersion strengthened materials. J. Mater. Res. 2015, 30, 2210–2221. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, X.P.; Tan, H.Y.; Fu, H.Y.; Ni, J.J.; Luo, L.M.; Wu, Y.C. Effects of helium irradiation damage on deuterium plasma-driven permeation through tungsten coated RAFM steel. Nucl. Fusion 2025, 65, 026056. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y.C. Crack-free tungsten coating on ods plate via a modified laser-based additive technique: Offset laser cladding. Surf. Coat. Technol. 2024, 476, 130285. [Google Scholar] [CrossRef]
- Matthews, G.F.; Coad, P.; Greuner, H.; Hill, M.; Hirai, T.; Likonen, J.; Maier, H.; Mayer, M.; Neu, R.; Philipps, V.; et al. Development of divertor tungsten coatings for the JET ITER-like wall. J. Nucl. Mater. 2009, 390–391, 934–937. [Google Scholar] [CrossRef]
- Wan, B.; Ding, S.; Qian, J.; Li, G.; Xiao, B.; Xu, G. Physics Design of CFETR: Determination of the Device Engineering Parameters. IEEE Trans. Plasma Sci. 2014, 42, 495–502. [Google Scholar] [CrossRef]
- Matějíček, J.; Vilémová, M.; Mušálek, R.; Sachr, P.; Horník, J. The Influence of Interface Characteristics on the Adhesion/Cohesion of Plasma Sprayed Tungsten Coatings. Coatings 2013, 3, 108–125. [Google Scholar] [CrossRef]
- Strekalina, D.; Dushik, V.; Shaporenkov, A.; Tatiana, L.; Bukatin, T. Prospects of tungsten CVD technology for electric vehicles. AIP Conf. Proc. 2024, 3154, 020018. [Google Scholar] [CrossRef]
- Han, X.Y.; Liu, X.L.; Wang, D.Z.; Wu, Z.; Duan, B. Copper-based tungsten coating by CVD: Microstructure, thermal shock resistance and interfacial bond force. Surf. Coat. Technol. 2021, 426, 127778. [Google Scholar] [CrossRef]
- Sun, N.B.; Zhang, Y.C.; Lang, S.T.; Jiang, F.; Wang, L.L. Tungsten coatings electro-deposited on CFC substrates from oxide molten salt. J. Nucl. Mater. 2014, 455, 450–453. [Google Scholar] [CrossRef]
- Tokunaga, K.; Yoshida, N.; Kubota, Y.; Noda, N.; Imamura, Y.; Oku, T.; Kurumada, A.; Sogabe, T.; Kato, T.; Plöchl, L. High heat flux test of actively cooled tungsten-coated carbon divertor mock-ups. Fusion Eng. Des. 2000, 49–50, 371–376. [Google Scholar] [CrossRef]
- Matějíček, J.; Koza, Y.; Weinzettl, V. Plasma sprayed tungsten-based coatings and their performance under fusion relevant conditions. Fusion Eng. Des. 2005, 75–79, 395–399. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zhang, Y.C.; Liu, Q.Z.; Li, X.L.; Jiang, F. The effects of electro-deposition current parameters on performance of tungsten coating. Int. J. Refract. Met. Hard Mater. 2012, 35, 241–245. [Google Scholar] [CrossRef]
- Lian, Y.Y.; Liu, X.; Xu, Z.Y.; Song, J.P.; Yu, Y. Preparation and properties of CVD-W coated W/Cu FGM mock-ups. Fusion Eng. Des. 2013, 88, 1694–1698. [Google Scholar] [CrossRef]
- Horník, J.; Tondl, D.; Sachr, P.; Anisimov, E.; Puchnin, M.; Chraska, T. The Effect of PVD Tungsten-Based Coatings on Improvement of Hardness and Wear Resistance. Key Eng. Mater. 2014, 606, 163–166. [Google Scholar] [CrossRef]
- Deng, C.; Liu, M.; Yang, Z.; Deng, C.; Zhou, K.; Kuang, Z.; Zhang, J. Manufacture of thick VPS W coatings on relatively large CuZrCr substrate and its steady high heat load performance. J. Nucl. Mater. 2014, 455, 145–150. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Liu, Z.Q.; Cai, Y.K.; Wang, B.; Song, Q.H. Effect of Coating Thickness on the Bond Strength of Cold-Sprayed Ni-Based cBN Coating on 42CrMo Substrate. J. Therm. Spray Technol. 2023, 32, 1554–1569. [Google Scholar] [CrossRef]




| Current Density | Bonding Strength (MPa) |
|---|---|
| 10 mA/cm2 | 45.87 |
| 30 mA/cm2 | 47.56 |
| 50 mA/cm2 | 51.22 |
| 70 mA/cm2 | 49.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Li, Y.; You, Y.; Gao, Z.; Zhang, Y. Electrodeposition of Metallic Tungsten Coating on 9Cr-ODS Steel Substrate from Binary Oxide Molten Salt. Coatings 2025, 15, 257. https://doi.org/10.3390/coatings15030257
Dong X, Li Y, You Y, Gao Z, Zhang Y. Electrodeposition of Metallic Tungsten Coating on 9Cr-ODS Steel Substrate from Binary Oxide Molten Salt. Coatings. 2025; 15(3):257. https://doi.org/10.3390/coatings15030257
Chicago/Turabian StyleDong, Xiaoxu, Yusha Li, Yajie You, Zeyu Gao, and Yingchun Zhang. 2025. "Electrodeposition of Metallic Tungsten Coating on 9Cr-ODS Steel Substrate from Binary Oxide Molten Salt" Coatings 15, no. 3: 257. https://doi.org/10.3390/coatings15030257
APA StyleDong, X., Li, Y., You, Y., Gao, Z., & Zhang, Y. (2025). Electrodeposition of Metallic Tungsten Coating on 9Cr-ODS Steel Substrate from Binary Oxide Molten Salt. Coatings, 15(3), 257. https://doi.org/10.3390/coatings15030257







