The Effect of Curing Temperature and Thickness of Polybutyl Methacrylate Siloxane Coatings on the Corrosion Protection of Structural Steel S355
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Preparation
2.1.1. Chemicals and Materials
2.1.2. Synthesis of Sols
2.1.3. Coating Deposition and Curing
2.2. Characterisation of Sols and Coatings
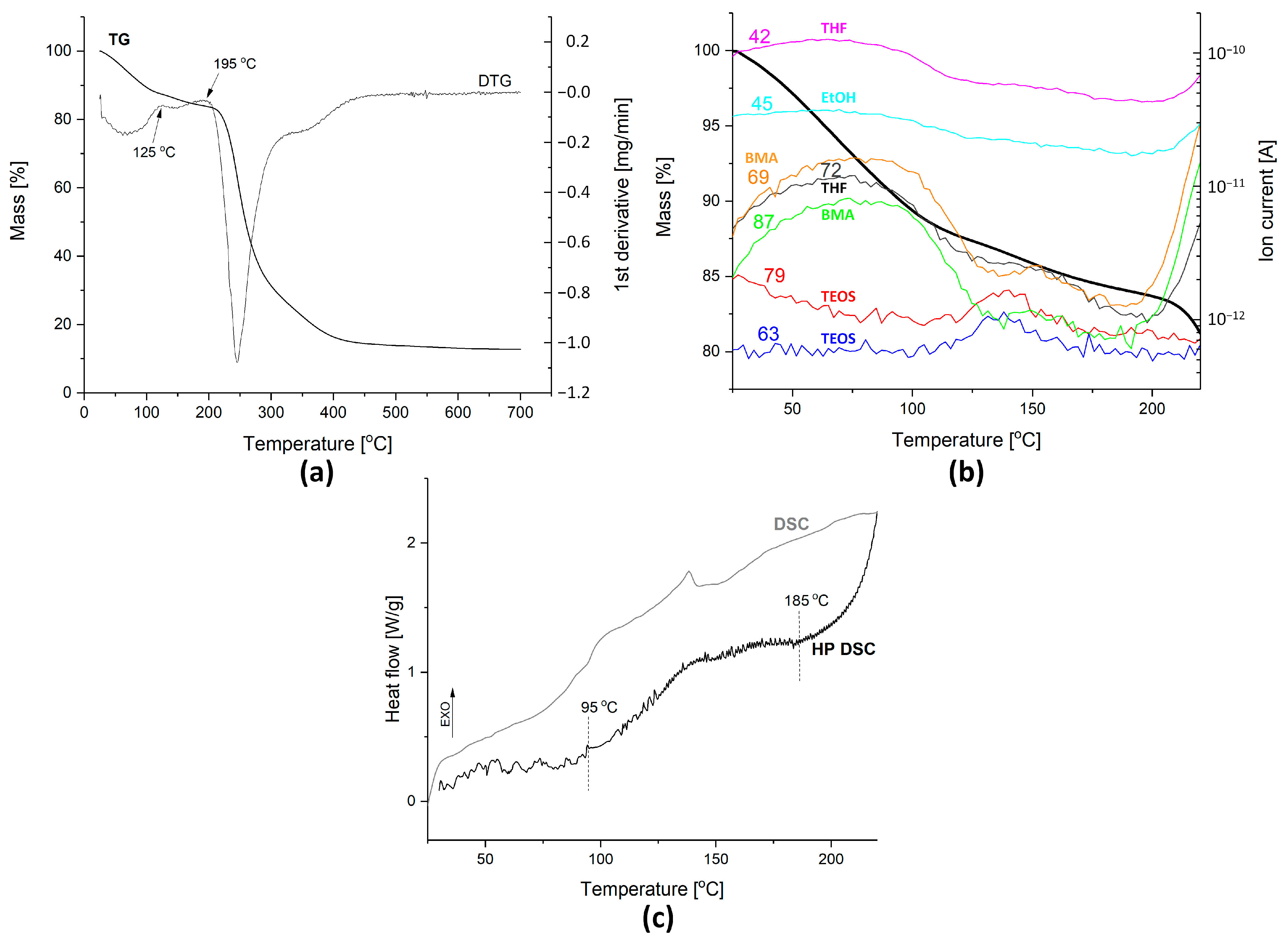
2.2.1. Thermal Analysis
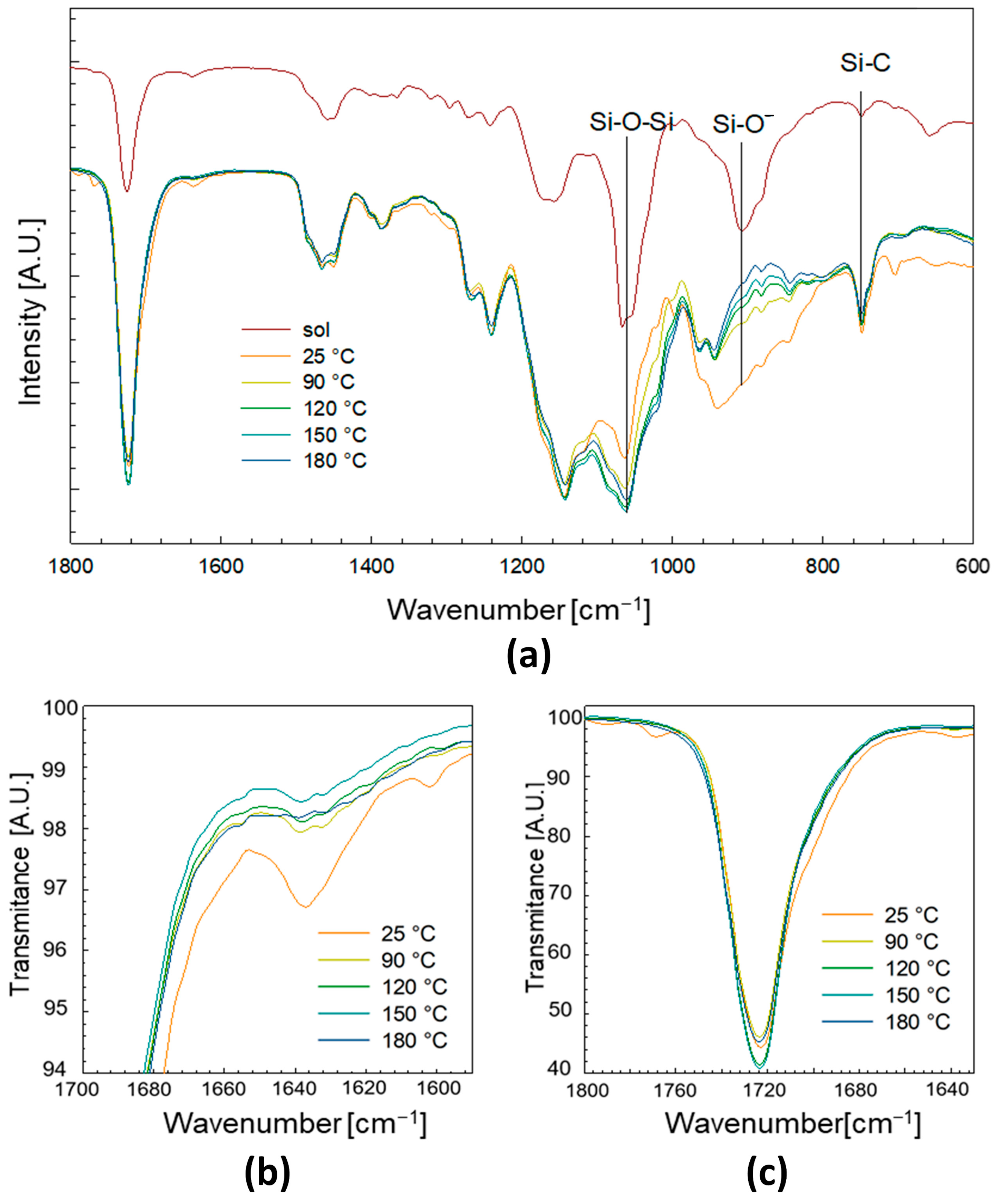
2.2.2. Chemical Composition
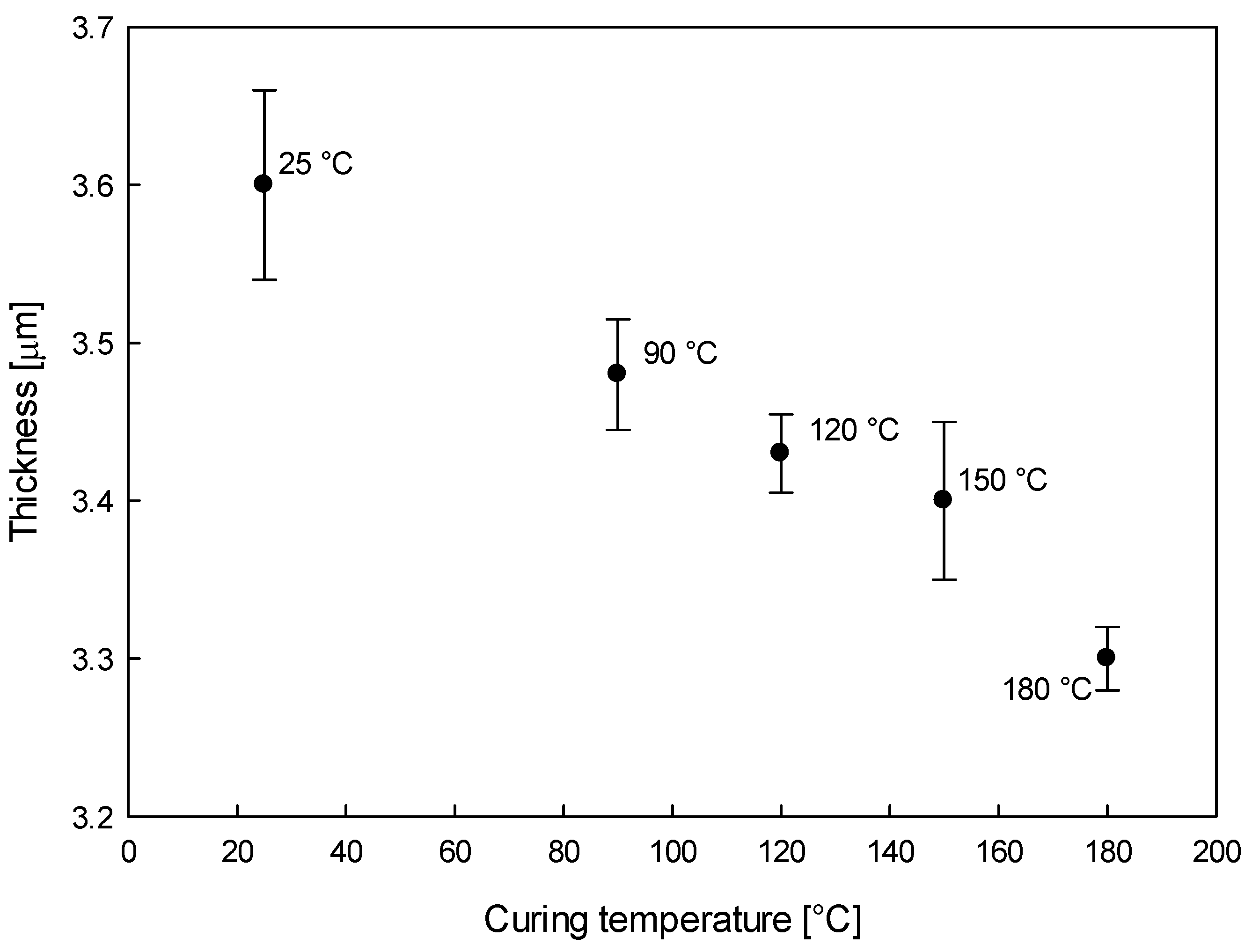
2.2.3. 3-D Topography and Coating Thickness
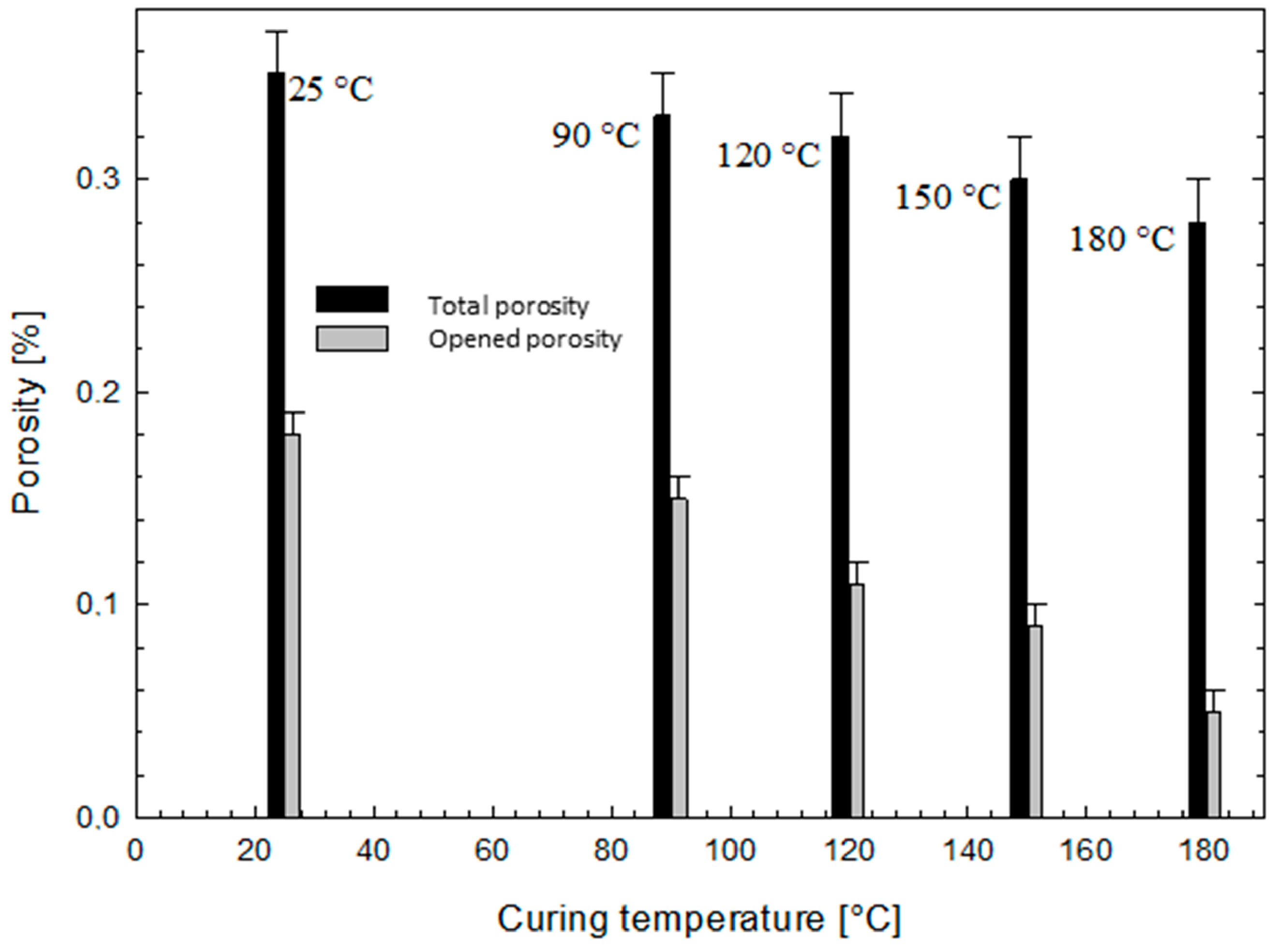
2.2.4. Coating Porosity
2.2.5. Electrochemical Measurements
2.2.6. Salt-Spray Test
2.2.7. Thickness Evaluation Graph
3. Results and Discussion
3.1. The Effect of Curing Temperature on Structure and Corrosion Resistance
3.1.1. Thermal Analysis
3.1.2. Chemical Composition and Bonding
3.1.3. Coating Thickness and Porosity
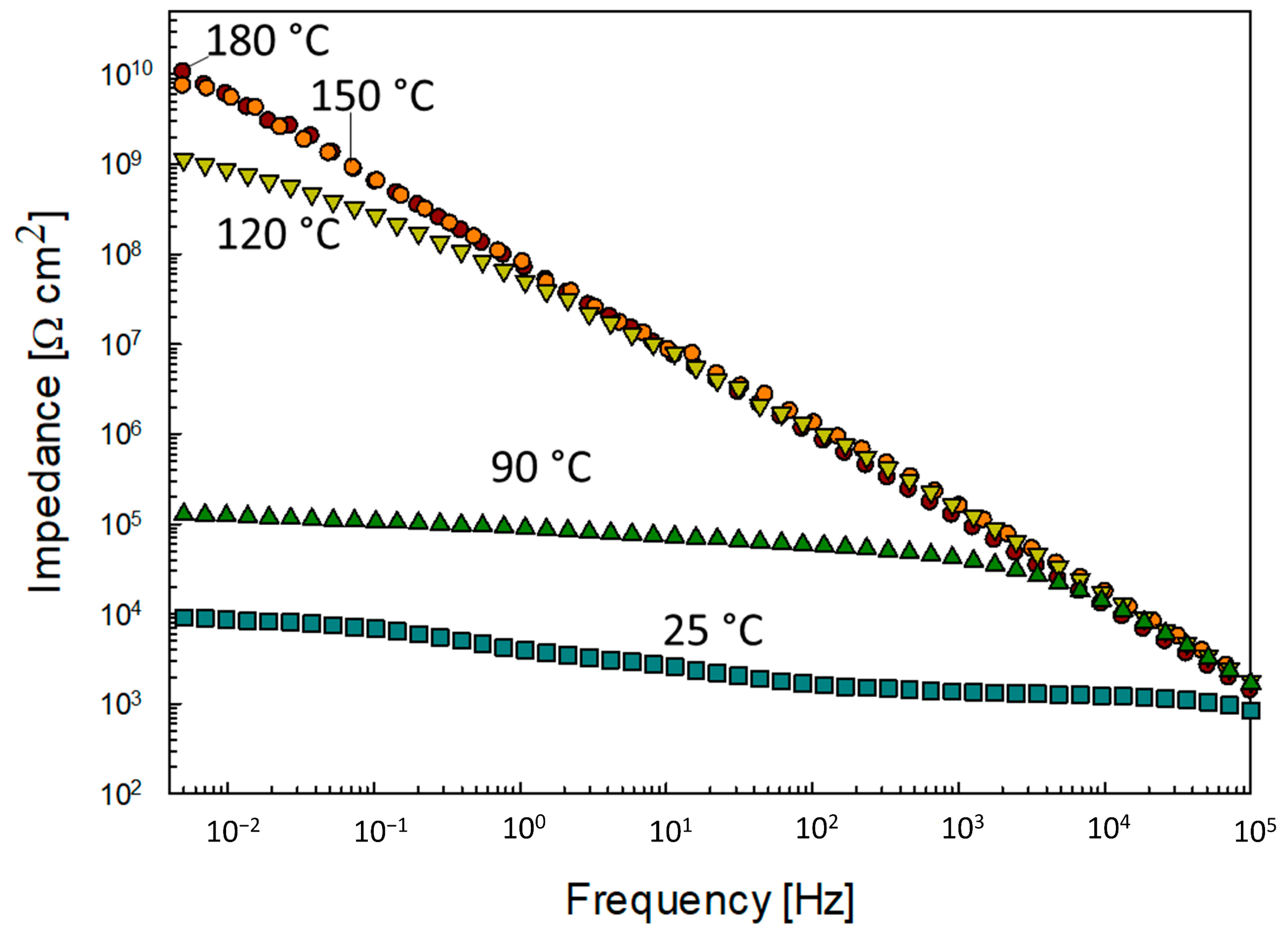
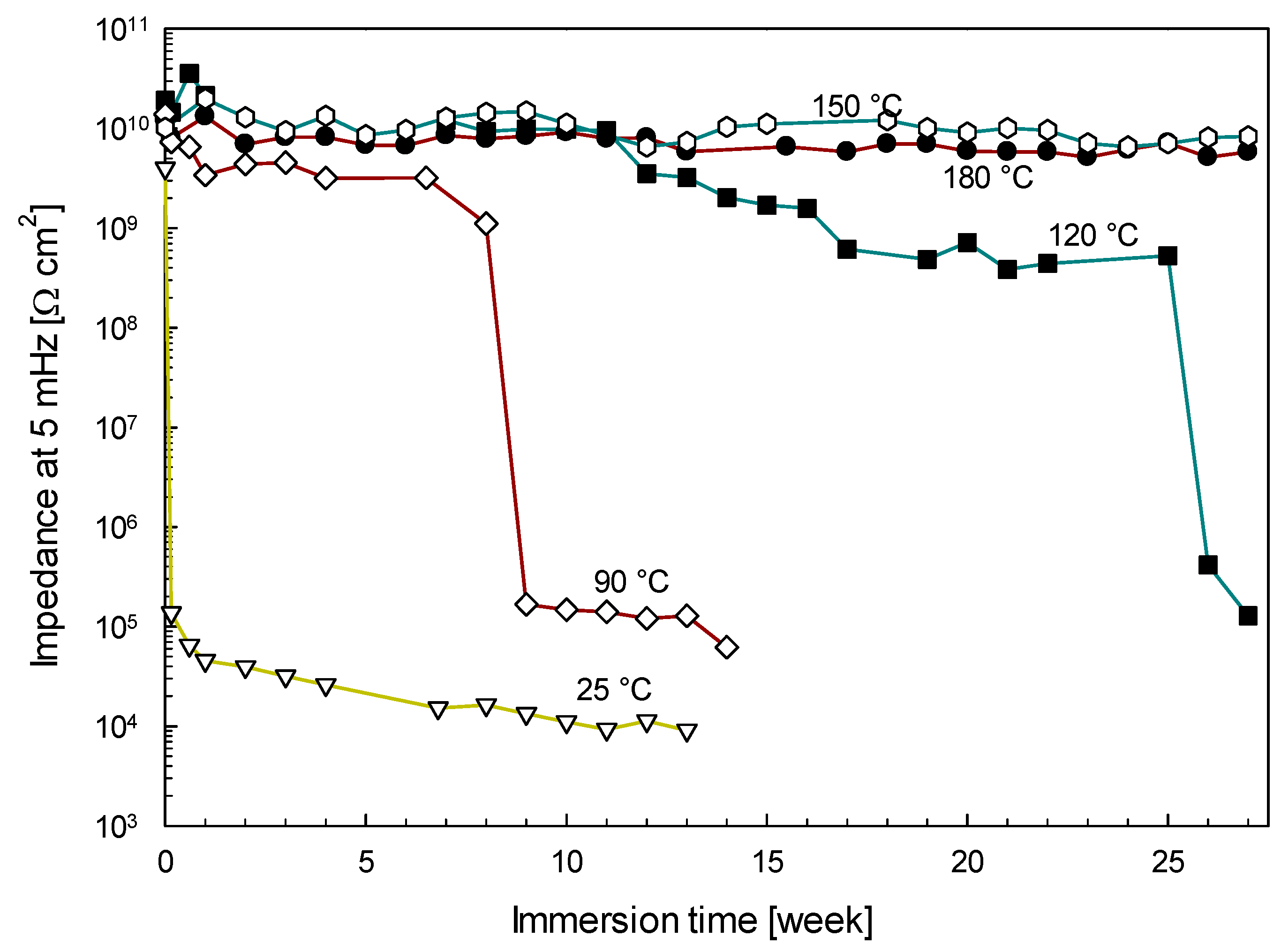
3.1.4. The Effect of Curing Temperature on the Corrosion Resistance
3.2. The Effect of Withdrawal Rate on Coating Properties
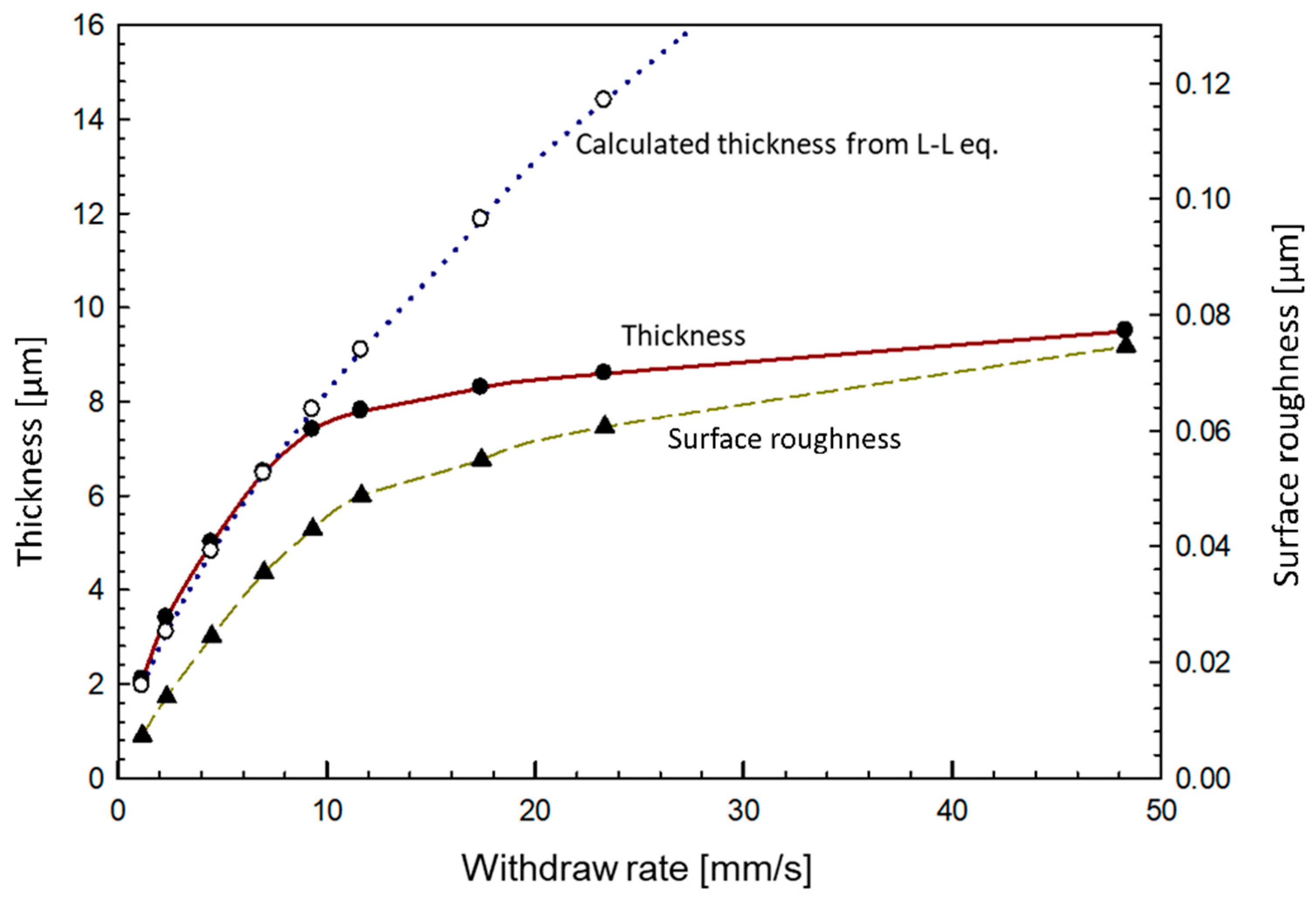
3.2.1. Coating Thickness and Topography
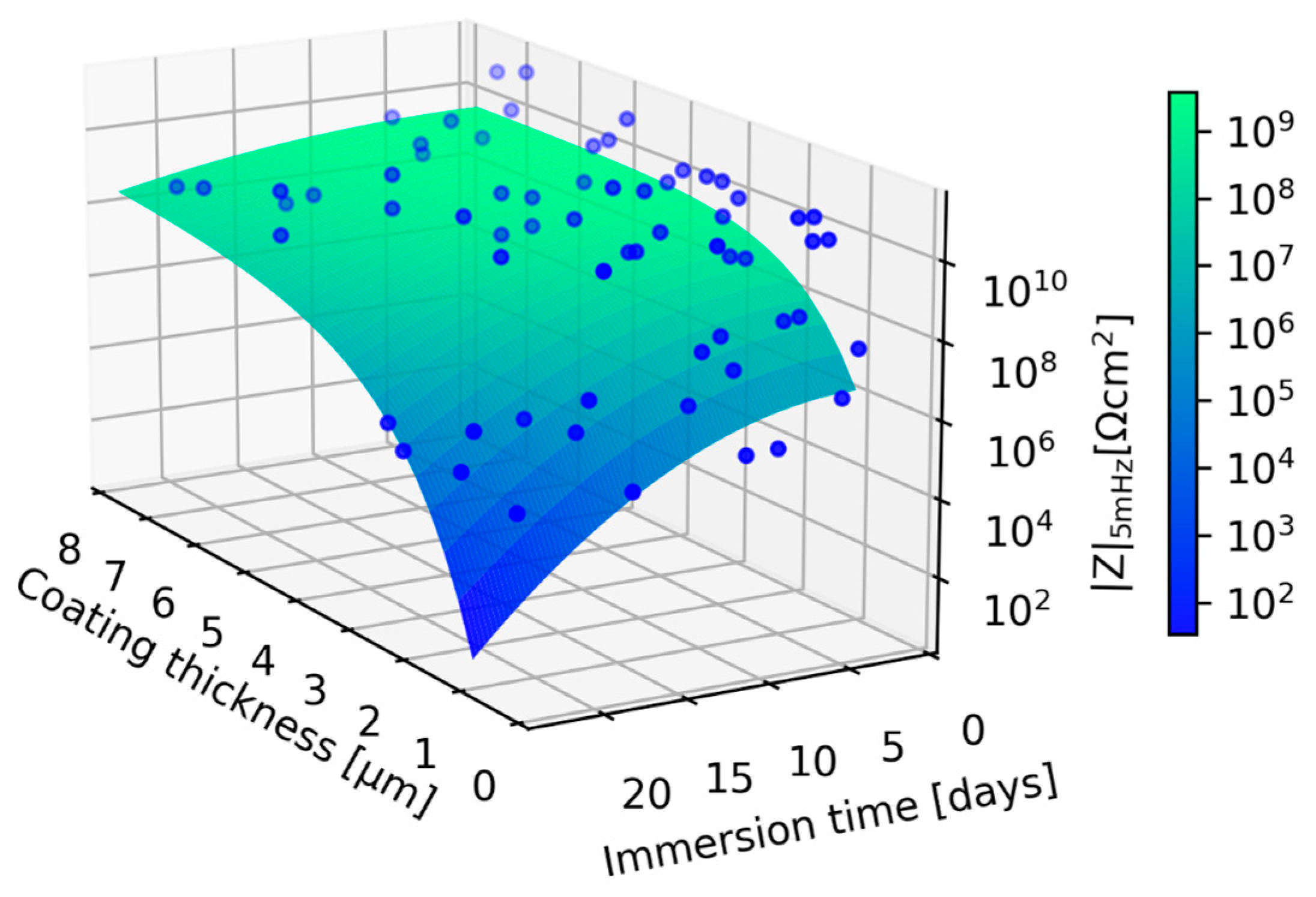
3.2.2. The Effect of Coating Thickness on the Corrosion Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. (Ed.) Corrosion: Understanding the Basics; ASM International: Materials Park, OH, USA, 2000; ISBN 978-0-87170-641-6. [Google Scholar]
- Revie, R.W. Wiley InterScience (Online service). In Uhlig’s Corrosion Handbook; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-0-470-87286-4. [Google Scholar]
- Milošev, I. Contemporary Modes of Corrosion Protection and Functionalization of Materials. Acta Chim. Slov. 2019, 66, 511–533. [Google Scholar] [CrossRef] [PubMed]
- Nazeer, A.; Madkour, M. Potential Use of Smart Coatings for Corrosion Protection of Metals and Alloys: A Review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Zheng, S.; Li, J. Inorganic–Organic Sol Gel Hybrid Coatings for Corrosion Protection of Metals. J. Sol-Gel Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–Inorganic Hybrid Sol–Gel Coatings for Metal Corrosion Protection: A Review of Recent Progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Figueira, R.B. Hybrid Sol–Gel Coatings for Corrosion Mitigation: A Critical Review. Polymers 2020, 12, 689. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and Smart Coatings for Corrosion Protection: A Review of Recent Advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Brinker, J.; Scherer, G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1st ed.; Academic Press: Boston, MA, USA, 1990; ISBN 978-0-12-134970-7. [Google Scholar]
- Hammer, P.; dos Santos, F.C.; Cerrutti, B.M.; Pulcinelli, S.H.; Santilli, C.V. Highly Corrosion Resistant Siloxane-Polymethyl Methacrylate Hybrid Coatings. J. Sol-Gel Sci. Technol. 2012, 63, 266–274. [Google Scholar] [CrossRef]
- Hammer, P.; Schiavetto, M.G.; dos Santos, F.C.; Benedetti, A.V.; Pulcinelli, S.H.; Santilli, C.V. Improvement of the Corrosion Resistance of Polysiloxane Hybrid Coatings by Cerium Doping. J. Non-Cryst. Solids 2010, 356, 2606–2612. [Google Scholar] [CrossRef]
- dos Santos, F.C.; Harb, S.V.; Menu, M.-J.; Turq, V.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. On the Structure of High Performance Anticorrosive PMMA–Siloxane–Silica Hybrid Coatings. RSC Adv. 2015, 5, 106754–106763. [Google Scholar] [CrossRef]
- Sarmento, V.H.V.; Schiavetto, M.G.; Hammer, P.; Benedetti, A.V.; Fugivara, C.S.; Suegama, P.H.; Pulcinelli, S.H.; Santilli, C.V. Corrosion Protection of Stainless Steel by Polysiloxane Hybrid Coatings Prepared Using the Sol–Gel Process. Surf. Coat. Technol. 2010, 204, 2689–2701. [Google Scholar] [CrossRef]
- Detty, M.R.; Ciriminna, R.; Bright, F.V.; Pagliaro, M. Environmentally Benign Sol–Gel Antifouling and Foul-Releasing Coatings. Acc. Chem. Res. 2014, 47, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Wu, X.; Fu, Q.; Ho, J.W.C.; Kanhere, P.D.; Li, L.; Chen, Z. Development of Durable Self-Cleaning Coatings Using Organic–Inorganic Hybrid Sol–Gel Method. Appl. Surf. Sci. 2015, 344, 205–212. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Kumar, A.M.; Kumar Sadasivuni, K.; Xing, R.; Liu, S. Self—Cleaning Superhydrophobic Coatings: Potential Industrial Applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Trentin, A.; Harb, S.V.; Uvida, M.C.; Pulcinelli, S.H.; Santilli, C.V.; Marcoen, K.; Pletincx, S.; Terryn, H.; Hauffman, T.; Hammer, P. Dual Role of Lithium on the Structure and Self-Healing Ability of PMMA-Silica Coatings on AA7075 Alloy. ACS Appl. Mater. Interfaces 2019, 11, 40629–40641. [Google Scholar] [CrossRef]
- Minami, T. Advanced Sol–Gel Coatings for Practical Applications. J. Sol-Gel Sci. Technol. 2013, 65, 4–11. [Google Scholar] [CrossRef]
- Pathak, S.S.; Khanna, A.S. Sol–Gel Nanocoatings for Corrosion Protection. In Corrosion Protection and Control Using Nanomaterials; Elsevier: Cambridge, UK, 2012; pp. 304–329. ISBN 978-1-84569-949-9. [Google Scholar]
- Schubert, U.; Huesing, N.; Lorenz, A. Hybrid Inorganic-Organic Materials by Sol-Gel Processing of Organofunctional Metal Alkoxides. Chem. Mater. 1995, 7, 2010–2027. [Google Scholar] [CrossRef]
- Zha, J.; Roggendorf, H. Sol–Gel Science, the Physics and Chemistry of Sol–Gel Processing; Academic Press: Boston, MA, USA, 1991; Volume 3, ISBN 0-12-134970-5. [Google Scholar]
- Rodič, P.; Iskra, J.; Milošev, I. Study of a Sol–Gel Process in the Preparation of Hybrid Coatings for Corrosion Protection Using FTIR and 1H NMR Methods. J. Non-Cryst. Solids 2014, 396, 25–35. [Google Scholar] [CrossRef]
- Rodič, P.; Iskra, J.; Milošev, I. A Hybrid Organic–Inorganic Sol–Gel Coating for Protecting Aluminium Alloy 7075-T6 against Corrosion in Harrison’s Solution. J. Sol-Gel Sci. Technol. 2014, 70, 90–103. [Google Scholar] [CrossRef]
- Rodič, P.; Zanna, S.; Milošev, I.; Marcus, P. Degradation of Sol-Gel Acrylic Coatings Based on Si and Zr Investigated Using Electrochemical Impedance, Infrared and X-Ray Photoelectron Spectroscopies. Front. Mater. 2021, 8, 756447. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I.; Lekka, M.; Andreatta, F.; Fedrizzi, L. Corrosion Behaviour and Chemical Stability of Transparent Hybrid Sol-Gel Coatings Deposited on Aluminium in Acidic and Alkaline Solutions. Prog. Org. Coat. 2018, 124, 286–295. [Google Scholar] [CrossRef]
- Harb, S.V.; Pulcinelli, S.H.; Santilli, C.V.; Knowles, K.M.; Hammer, P. A Comparative Study on Graphene Oxide and Carbon Nanotube Reinforcement of PMMA-Siloxane-Silica Anticorrosive Coatings. ACS Appl. Mater. Interfaces 2016, 8, 16339–16350. [Google Scholar] [CrossRef]
- Harb, S.V.; dos Santos, F.C.; Caetano, B.L.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Structural Properties of Cerium Doped Siloxane–PMMA Hybrid Coatings with High Anticorrosive Performance. RSC Adv. 2015, 5, 15414–15424. [Google Scholar] [CrossRef]
- Trentin, A.; de L. Gasparini, A.; Faria, F.A.; Harb, S.V.; dos Santos, F.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Barrier Properties of High Performance PMMA-Silica Anticorrosion Coatings. Prog. Org. Coat. 2020, 138, 105398. [Google Scholar] [CrossRef]
- Sakai, R.T.; da Cruz, M.; de Melo, H.G.; Benedetti, A.V.; Santilli, C.V.; Suegama, P.H. Electrochemical Study of TEOS, TEOS/MPTS, MPTS/MMA and TEOS/MPTS/MMA Films on Tin Coated Steel in 3.5% NaCl Solution. Prog. Org. Coat. 2012, 74, 288–301. [Google Scholar] [CrossRef]
- Hamulić, D.; Rodič, P.; Poberžnik, M.; Jereb, M.; Kovač, J.; Milošev, I. The Effect of the Methyl and Ethyl Group of the Acrylate Precursor in Hybrid Silane Coatings Used for Corrosion Protection of Aluminium Alloy 7075-T6. Coatings 2020, 10, 172. [Google Scholar] [CrossRef]
- Suegama, P.H.; Sarmento, V.H.V.; Montemor, M.F.; Benedetti, A.V.; de Melo, H.G.; Aoki, I.V.; Santilli, C.V. Effect of Cerium (IV) Ions on the Anticorrosion Properties of Siloxane-Poly(Methyl Methacrylate) Based Film Applied on Tin Coated Steel. Electrochim. Acta 2010, 55, 5100–5109. [Google Scholar] [CrossRef]
- Milošev, I.; Hamulić, D.; Rodič, P.; Carrière, C.; Zanna, S.; Budasheva, H.; Korte, D.; Franko, M.; Mercier, D.; Seyeux, A.; et al. Siloxane Polyacrylic Sol-Gel Coatings with Alkyl and Perfluoroalkyl Chains: Synthesis, Composition, Thermal Properties and Long-Term Corrosion Protection. Appl. Surf. Sci. 2022, 574, 151578. [Google Scholar] [CrossRef]
- Hamulić, D.; Rodič, P.; Milošev, I. The Influence of Length of Alkyl Chain on the Chemical Structure and Corrosion Resistance of Silica-Polyacrylic Hybrid Coatings on Structural Steel. Prog. Org. Coat. 2021, 150, 105982. [Google Scholar] [CrossRef]
- Hamulić, D.; Putna-Nimane, I.; Liepina-Leimane, I.; Dimante-Deimantovica, I.; Rodič, P.; Milošev, I. Field Testing and Ecotoxicity of Acrylate-Based Sol–Gel Coatings in Fresh and Seawater. J. Coat. Technol. Res. 2022, 1–55. [Google Scholar] [CrossRef]
- Xu, Y.; Dayo, A.Q.; Wang, J.; Wang, A.; Lv, D.; Zegaoui, A.; Derradji, M.; Liu, W. Mechanical and Thermal Properties of a Room Temperature Curing Epoxy Resin and Related Hemp Fibers Reinforced Composites Using a Novel In-Situ Generated Curing Agent. Mater. Chem. Phys. 2018, 203, 293–301. [Google Scholar] [CrossRef]
- Parker, R.E.; Isaacs, N.S. Mechanisms Of Epoxide Reactions. Chem. Rev. 1959, 59, 737–799. [Google Scholar] [CrossRef]
- Hammer, P.; dos Santos, F.C.; Cerrutti, B.M.; Pulcinelli, S.H.; Santilli, C.V. Carbon Nanotube-Reinforced Siloxane-PMMA Hybrid Coatings with High Corrosion Resistance. Prog. Org. Coat. 2013, 76, 601–608. [Google Scholar] [CrossRef]
- Trentin, A.; Harb, S.V.; Uvida, M.C.; Marcoen, K.; Pulcinelli, S.H.; Santilli, C.V.; Terryn, H.; Hauffman, T.; Hammer, P. Effect of Ce(III) and Ce(IV) Ions on the Structure and Active Protection of PMMA-Silica Coatings on AA7075 Alloy. Corros. Sci. 2021, 189, 109581. [Google Scholar] [CrossRef]
- Rodič, P.; Korošec, R.C.; Kapun, B.; Mertelj, A.; Milošev, I. Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA7075-T6 in Aircraft Applications: The Effect of Copolymerization Time. Polymers 2020, 12, 948. [Google Scholar] [CrossRef]
- Rodič, P.; Lekka, M.; Andreatta, F.; Fedrizzi, L.; Milošev, I. The Effect of Copolymerisation on the Performance of Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA2024-T3. Prog. Org. Coat. 2020, 147, 105701. [Google Scholar] [CrossRef]
- Criado, M.; Sobrados, I.; Sanz, J. Polymerization of Hybrid Organic–Inorganic Materials from Several Silicon Compounds Followed by TGA/DTA, FTIR and NMR Techniques. Prog. Org. Coat. 2014, 77, 880–891. [Google Scholar] [CrossRef]
- dos Santos, F.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Protective PMMA-Silica Coatings for Aluminum Alloys: Nanostructural Control of Elevated Thermal Stability and Anticorrosive Performance. Prog. Org. Coat. 2021, 152, 106129. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; Uvida, M.C.; Magnani, M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. A Comparative Study on PMMA-TiO2 and PMMA-ZrO2 Protective Coatings. Prog. Org. Coat. 2020, 140, 105477. [Google Scholar] [CrossRef]
- Available online: Https://Webbook.Nist.Gov/Chemistry/Form-Ser (accessed on 3 February 2023).
- Leach, R. Chapter 8—Surface Topography Characterisation. In Fundamental Principles of Engineering Nanometrology, 2nd ed.; Leach, R., Ed.; Micro and Nano Technologies; William Andrew Publishing: Oxford, UK, 2014; pp. 241–294. ISBN 978-1-4557-7753-2. [Google Scholar]
- Korte, D.; Franko, M. Application of Complex Geometrical Optics to Determination of Thermal, Transport, and Optical Parameters of Thin Films by the Photothermal Beam Deflection Technique. J. Opt. Soc. Am. A 2015, 32, 61. [Google Scholar] [CrossRef]
- Pawlak, M.; Pal, S.; Ludwig, A.; Wieck, A.D. On the Infrared Absorption Coefficient Measurement of Thick Heavily Zn Doped GaAs Using Spectrally Resolved Modulated Photothermal Infrared Radiometry. J. Appl. Phys. 2017, 122, 135109. [Google Scholar] [CrossRef]
- Chrobak, Ł.; Korte, D.; Budasheva, H.; Maliński, M.; Rodič, P.; Milošev, I.; Janta-Lipińska, S. Investigations of the Thermal Parameters of Hybrid Sol–Gel Coatings Using Nondestructive Photothermal Techniques. Energies 2022, 15, 4122. [Google Scholar] [CrossRef]
- Cabrera, H.; Korte, D.; Budasheva, H.; Abbasgholi, N.; Asbaghi, B.; Bellucci, S. Through-Plane and In-Plane Thermal Diffusivity Determination of Graphene Nanoplatelets by Photothermal Beam Deflection Spectrometry. Materials 2021, 14, 7273. [Google Scholar] [CrossRef]
- Cernuschi, F.; Bianchi, P.; Leoni, M.; Scardi, P. Thermal Diffusivity/Microstructure Relationship in Y-PSZ Thermal Barrier Coatings. J. Therm. Spray Technol. 1999, 8, 102–109. [Google Scholar] [CrossRef]
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM: West Conshohocken, PA, USA, 2019. Available online: https://www.astm.org/b0117-19.html (accessed on 3 February 2023).
- Levenberg, K. A Method for the Solution of Certain Non-Linear Problems in Least Squares. Q. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Rubio, F.; Rubio, J.; Oteo, J.L. A FT-IR Study of the Hydrolysis of Tetraethylorthosilicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- Liu, S.-H.; Hou, H.-Y.; Shu, C.-M. Thermal Hazard Evaluation of the Autocatalytic Reaction of Benzoyl Peroxide Using DSC and TAM III. Thermochim. Acta 2015, 605, 68–76. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Achilias, D.S.; Karava, O. Reactivity of Benzoyl Peroxide/Amine System as an Initiator for the Free Radical Polymerization of Dental and Orthopaedic Dimethacrylate Monomers: Effect of the Amine and Monomer Chemical Structure. Macromolecules 2006, 39, 2072–2080. [Google Scholar] [CrossRef]
- Sun, D.-X.; Miao, X.; Xie, C.-X.; Gu, J.; Li, R. Study on Thermal Properties and Kinetics of Benzoyl Peroxide by ARC and C80 Methods. J. Therm. Anal. Calorim. 2012, 107, 943–948. [Google Scholar] [CrossRef]
- Verganelakis, V.; Nicolaou, P.D.; Trapalis, C.; Kordas, G. Evaluation of the Critical Processing Parameters of Ormosil Coatings on the Increase of the Strength of Glass. J. Non-Cryst. Solids 2000, 265, 265–275. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W.; Roth, E.P. Sol → Gel → Glass: II. Physical and Structural Evolution during Constant Heating Rate Experiments. J. Non-Cryst. Solids 1985, 72, 345–368. [Google Scholar] [CrossRef]
- Rebrov, E.V.; Schouten, J.C. Limiting Withdrawal Rate and Maximum Film Thickness during Dip-Coating of Titania Sols onto a Si Substrate. Chem. Eng. Process. Process Intensif. 2011, 50, 1063–1068. [Google Scholar] [CrossRef]
- Faustini, M.; Louis, B.; Albouy, P.A.; Kuemmel, M.; Grosso, D. Preparation of Sol−Gel Films by Dip-Coating in Extreme Conditions. J. Phys. Chem. C 2010, 114, 7637–7645. [Google Scholar] [CrossRef]
- Matur, U.C.; Akyol, S.; Baydoğan, N.; Cimenoglu, H. The Optical Properties of CIGS Thin Films Derived by Sol-Gel Dip Coating Process at Different Withdrawal Speed. Procedia—Soc. Behav. Sci. 2015, 195, 1762–1767. [Google Scholar] [CrossRef]
- Bile, J.; Bolzinger, M.-A.; Valour, J.-P.; Fessi, H.; Chevalier, Y. Antimicrobial Films Containing Microparticles for the Enhancement of Long-Term Sustained Release. Drug Dev. Ind. Pharm. 2016, 42, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J. Dip Coating. In Chemical Solution Deposition of Functional Oxide Thin Films; Schneller, T., Waser, R., Kosec, M., Payne, D., Eds.; Springer Vienna: Vienna, Austria, 2013; pp. 233–261. ISBN 978-3-211-99310-1. [Google Scholar]
- Landau, L.; Levich, B. Dragging of a Liquid by a Moving Plate. Acta Physicochim. URSS 1988, 17, 141–153. [Google Scholar] [CrossRef]
- Schneller, T.; Waser, R.; Kosec, M.; Payne, D. Chemical Solution Deposition of Functional Oxide Thin Films; Springer Science & Business Media: Vienna, Austria, 2014; ISBN 978-3-211-99311-8. [Google Scholar]
- Castro, Y.; Ferrari, B.; Moreno, R.; Durán, A. Corrosion Behaviour of Silica Hybrid Coatings Produced from Basic Catalysed Particulate Sols by Dipping and EPD. Surf. Coat. Technol. 2005, 191, 228–235. [Google Scholar] [CrossRef]
- Ono, S.; Tsuge, H.; Nishi, Y.; Hirano, S. Improvement of Corrosion Resistance of Metals by an Environmentally Friendly Silica Coating Method. J. Sol-Gel Sci. Technol. 2004, 29, 147–153. [Google Scholar] [CrossRef]
- Birnbaum, L.S. Preservation Coatings for Ships in Service. Bur. Ships J. 1957, 6. [Google Scholar]
- Knudsen, O.Ø.; Forsgren, A.; Forsgren, A. Corrosion Control through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-15318-6. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamulić, D.; Medoš, G.; Korte, D.; Rodič, P.; Milošev, I. The Effect of Curing Temperature and Thickness of Polybutyl Methacrylate Siloxane Coatings on the Corrosion Protection of Structural Steel S355. Coatings 2023, 13, 675. https://doi.org/10.3390/coatings13040675
Hamulić D, Medoš G, Korte D, Rodič P, Milošev I. The Effect of Curing Temperature and Thickness of Polybutyl Methacrylate Siloxane Coatings on the Corrosion Protection of Structural Steel S355. Coatings. 2023; 13(4):675. https://doi.org/10.3390/coatings13040675
Chicago/Turabian StyleHamulić, Damir, Gregor Medoš, Dorota Korte, Peter Rodič, and Ingrid Milošev. 2023. "The Effect of Curing Temperature and Thickness of Polybutyl Methacrylate Siloxane Coatings on the Corrosion Protection of Structural Steel S355" Coatings 13, no. 4: 675. https://doi.org/10.3390/coatings13040675
APA StyleHamulić, D., Medoš, G., Korte, D., Rodič, P., & Milošev, I. (2023). The Effect of Curing Temperature and Thickness of Polybutyl Methacrylate Siloxane Coatings on the Corrosion Protection of Structural Steel S355. Coatings, 13(4), 675. https://doi.org/10.3390/coatings13040675







