Adsorption of Reactive Red 120 in Decamethyl-Cyclopentasiloxane Non-Aqueous Dyeing System
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Reactive Dyeing Process
2.3. Determination of K/S Value of Dyed Cotton Fabric
2.4. Level Dyeing Property
2.5. The Calibration Curve of Reactive Red 120
2.6. Determination of the Final Uptake of Dye
2.7. Dyeing Kinetics
2.8. Color Fastness
3. Results and Discussion
3.1. Effect of Salt Concentration on the Dye Diffusion
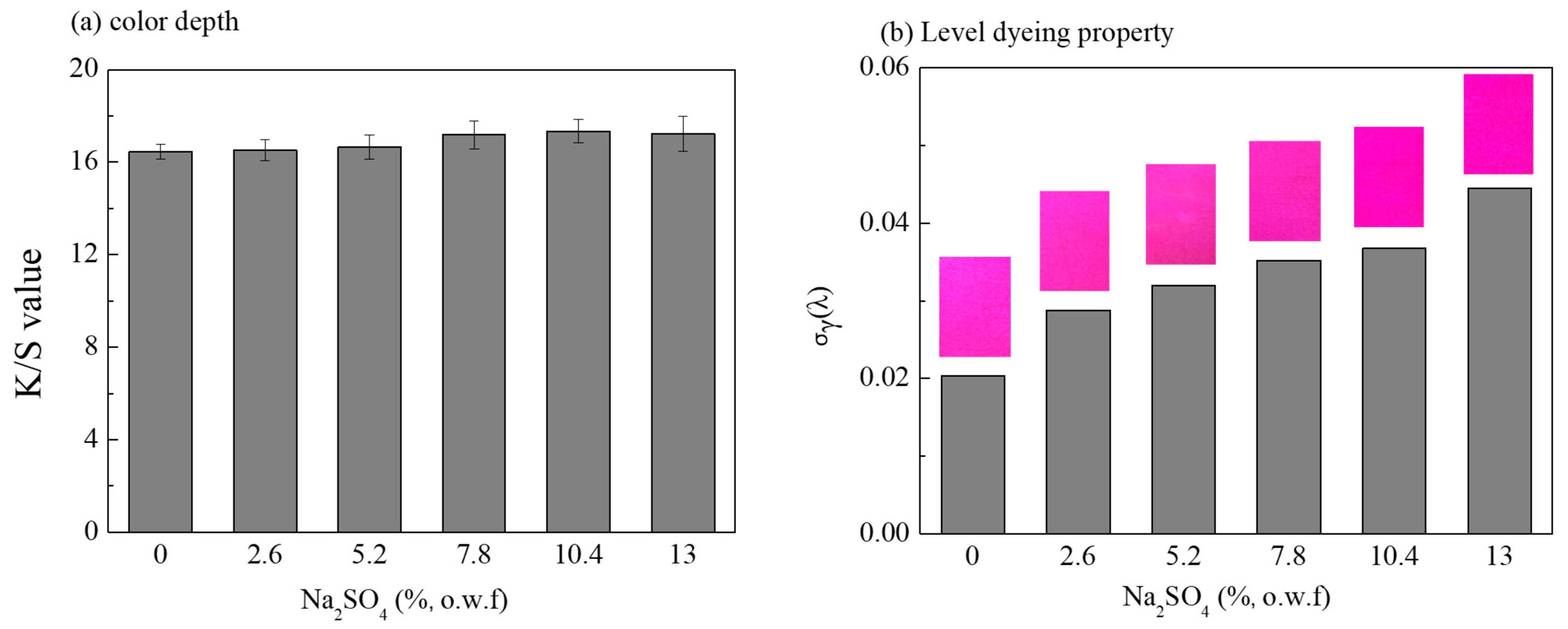
3.2. Influence of Salt Concentrations on the Dyeing Properties of Dye
3.3. Dyeing Adsorption Kinetics
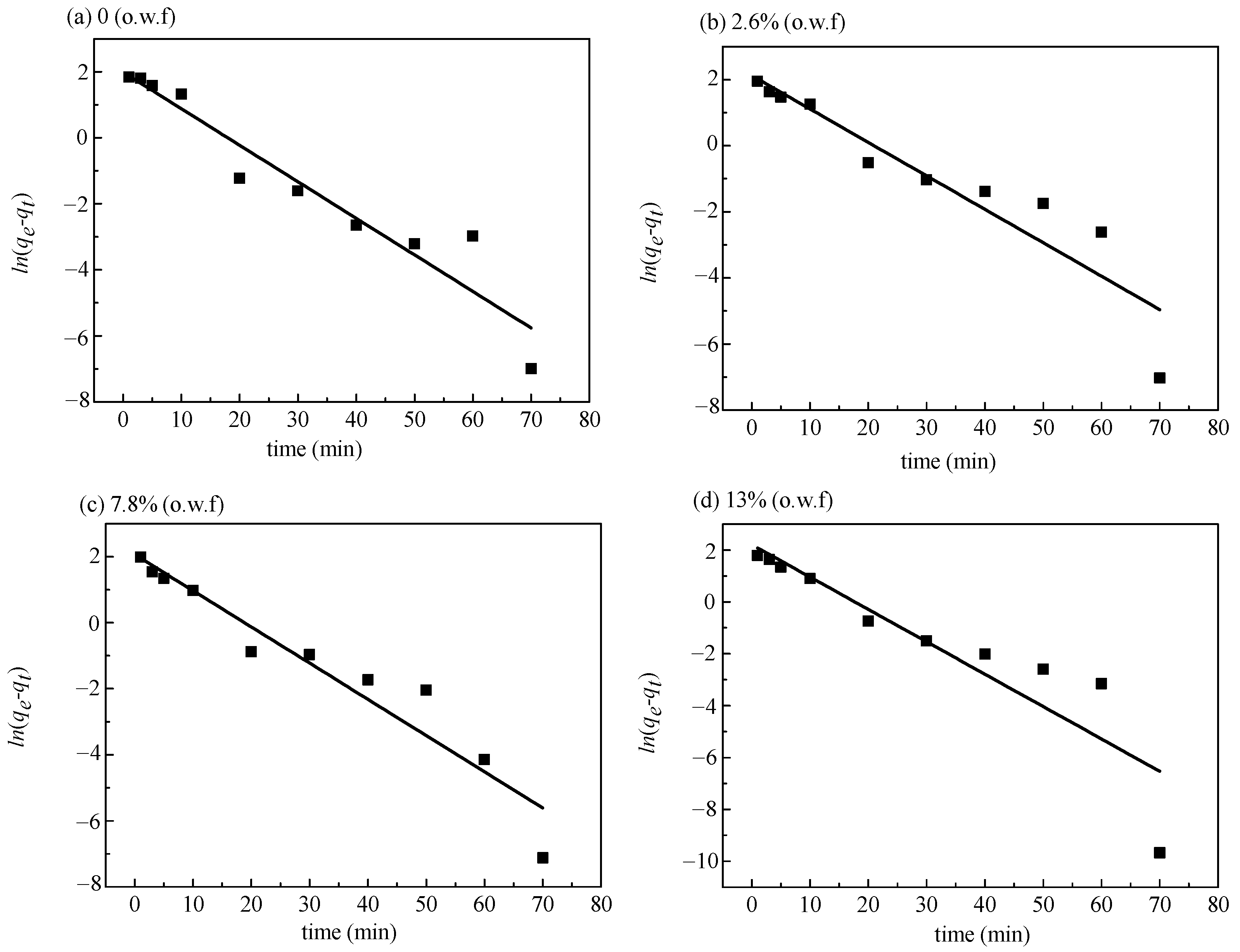
3.3.1. Fitting of Pseudo-First-Order Kinetic Model
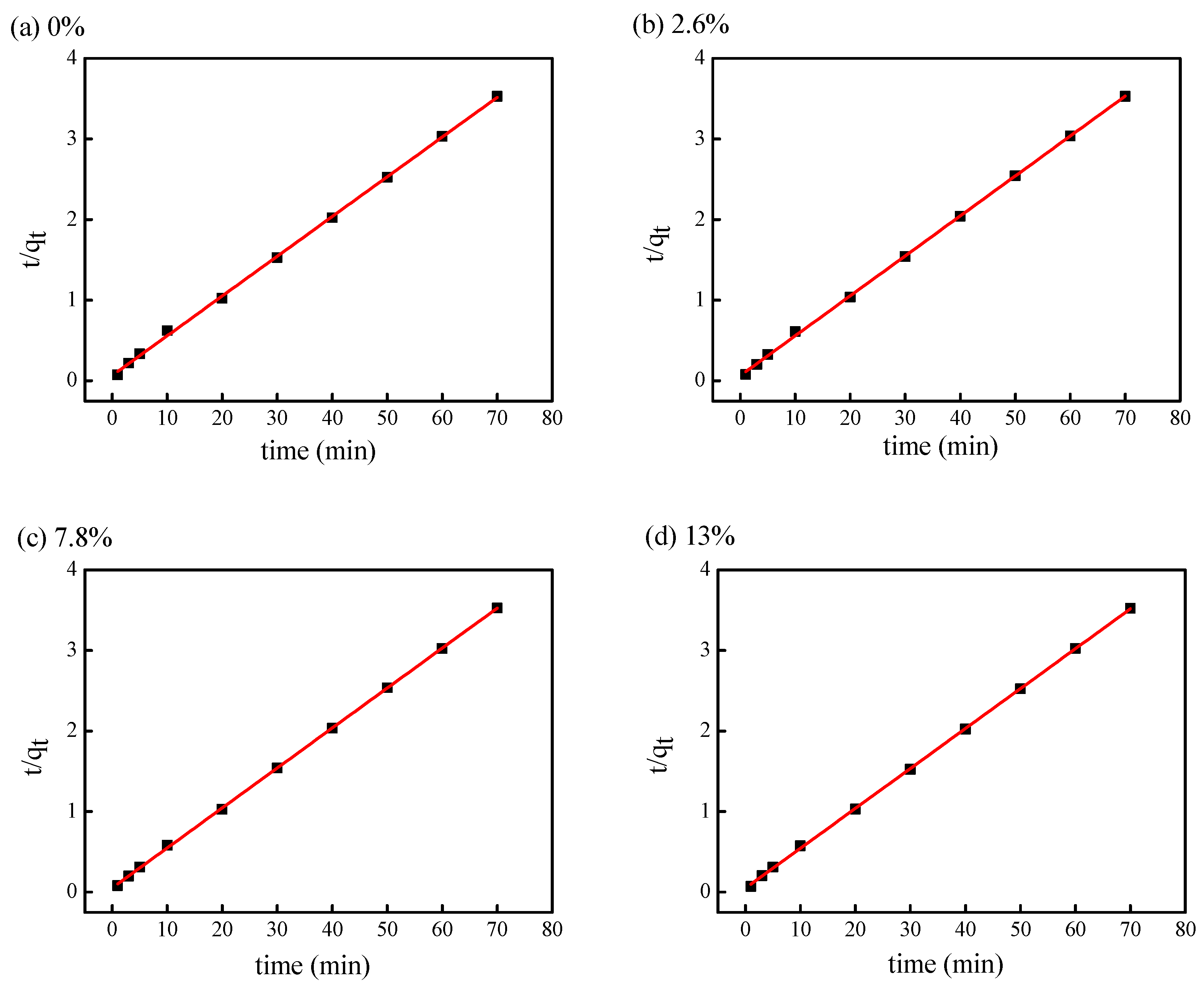
3.3.2. Fitting of Pseudo-Second-Order Kinetic Model
3.4. Dyeing Thermodynamic of Reactive Red 120 in Non-Aqueous Medium Dyeing System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahabub, H.M.; Khandakar, A.N.; Mohammad, A.A.A.Y.; Chandra, G.N. Application of Purified Lawsone as Natural Dye on Cotton and Silk Fabric. J. Text. 2015, 2015, 1–7. [Google Scholar]
- Garcia, S.; Cordeiro, A.; de Alencar Nääs, I.; Neto, P.L.D.O.C. The sustainability awareness of Brazilian consumers of cotton clothing. J. Clean. Prod. 2019, 215, 1490–1502. [Google Scholar] [CrossRef]
- Khatri, A.; Peerzada, M.H.; White, M.M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Dong, X.; Gu, Z.; Hang, C.; Ke, G.; Jiang, L.; He, J. Study on the salt-free low-alkaline reactive cotton dyeing in high concentration of ethanol in volume. J. Clean. Prod. 2019, 226, 316–323. [Google Scholar] [CrossRef]
- Bomgardner, M.M. These new textile dyeing methods could make fashion more sustainable. Chem. Eng. News 2018, 96, 31–33. [Google Scholar]
- Wang, J.; Gao, Y.; Zhu, L.; Gu, X.; Dou, H.; Pei, L. Dyeing property and adsorption kinetics of reactive dyes for cotton textiles in salt-free non-aqueous dyeing systems. Polymers 2018, 10, 1030. [Google Scholar] [CrossRef]
- Madan, G.L.; Shrivastava, S.K. Physical chemistry of dyeing of cellulosic fibers with reactive dyes: Part I: The role of electrolytes in the sorption of hydrolyzed reactive dyes. Text. Res. J. 1979, 49, 322–325. [Google Scholar] [CrossRef]
- Rabiei, N.; Kish, M.H.; Amirshahi, S.H.; Radjabian, M. The kinetic and thermodynamic parameters of dyeing of polypropylene/clay composite fibers using disperse dye. Dye. Pigment. 2012, 94, 386–392. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Wang, P.; Miao, D.; Ning, X. Enhanced dyeability and wash fastness through a salt-free plasma-induced grafting of cationic monomers on cotton fabrics. Fiber. Polym. 2021, 22, 3378–3384. [Google Scholar] [CrossRef]
- Dehabadi, V.A.; Buschmann, H.-J.; Gutmann, J.S. Pretreatment of cotton fabrics with polyamino carboxylic acids for salt-free dyeing of cotton with reactive dyes. Color. Technol. 2013, 129, 155–158. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Yu, C.; Chang, Y.; Xi, Z.; Lu, Y. Salt-free dyeing of cotton fabric using 3-chloro-2-hydroxypropyltrimethyl ammonium chloride by pad-irradiate-pad-steam process, and prediction of its k/s value by ls-svm. J. Nat. Fibers 2019, 18, 674–684. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, L. Research progress of supercritical fluid dyeing and finishing technology. J. Text. Inst. 2015, 36, 141–148. [Google Scholar]
- Wang, M.; Wang, W.; Qu, Y.; Liu, Y.; Xu, Z. Supercritical Fluid Spray Dyeing: A New Method of Dyeing and Simulations of its Flow Field. Adv. Mater. 2011, 396–398, 2038–2043. [Google Scholar] [CrossRef]
- Banchero, M. Recent advances in supercritical fluid dyeing. Color. Technol. 2020, 136, 317–335. [Google Scholar] [CrossRef]
- Bai, T.; Kobayashi, K.; Tamura, K.; Jun, Y.; Zheng, L. Supercritical CO2 dyeing for nylon, acrylic, polyester, and casein buttons and their optimum dyeing conditions by design of experiments. J. CO2 Util. 2019, 33, 253–261. [Google Scholar] [CrossRef]
- Sawada, K.; Ueda, M. Adsorption and fixation of a reactive dye on cotton in non-aqueous systems. Color. Technol. 2003, 3, 182–186. [Google Scholar] [CrossRef]
- Yi, S.; Deng, Y.; Sun, S. Adsorption and dyeing characteristics of reactive dyes onto cotton fiber in nonionic Triton X-100 reverse micelles. Fibers Polym. 2014, 10, 2131–2138. [Google Scholar] [CrossRef]
- An, Y.; Ma, J.; Zhu, Z.; Hu, M.; Shao, J. Study on a water-saving and salt-free reactive dyeing of cotton fabrics in non-aqueous medium of liquid paraffin system. J. Text. Inst. 2020, 111, 1538–1545. [Google Scholar] [CrossRef]
- Fan, J.; Shao, M.; Miao, J.; Ma, J.; Hu, M.; An, Y.; Shao, J. Thermodynamic properties of cotton dyeing with indigo dyes in non-aqueous media of liquid paraffin and D5. Text. Res. J. 2021, 91, 2692–2704. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, H.; Chen, J.; Yang, W.; Ou, X. Application of liquid paraffin as lubricant in sputum suction. Jpn. J. Nurs. Sci. 2010, 25, 54–55. [Google Scholar]
- Fu, C.; Wang, J.; Shao, J.; Pu, D.; Chen, J.; Liu, J. A non-aqueous dyeing process of reactive dye on cotton. J. Text. Inst. P Abstr. 2015, 106, 152–161. [Google Scholar] [CrossRef]
- Pu, D.; Zhong, Q.; Wang, J. Adsorption Kinetics of Amide Softeners on Cotton Fabrics in Decamethyl Cyclopentasiloxane Medium. AATCC J. Res. 2016, 3, 45–51. [Google Scholar] [CrossRef]
- Alebeid, O.K.; Pei, L.; Zhou, W.; Wang, J. Sustainable wool fibers dyeing using henna extract in non-aqueous medium. Environ. Chem. Lett. 2020, 2, 489–494. [Google Scholar] [CrossRef]
- Pei, L.; Li, H.; Shen, J.; Zhang, H.; Wang, J. Salt-free dyeing of cotton fabric and adsorption of reactive dyes in non-aqueous dyeing system: Equilibrium, kinetics, and thermodynamics. Cellulose 2022, 29, 4753–4765. [Google Scholar]
- Wang, J.; Zhang, Y.; Dou, H.; Pei, L. Influence of Ethylene Oxide Content in Nonionic Surfactant to the Hydrolysis of Reactive Dye in Silicone Non-Aqueous Dyeing System. Polymers 2018, 10, 1158. [Google Scholar] [CrossRef]
- Pei, L.; Luo, Y.; Gu, X.; Dou, H.; Wang, J. Diffusion Mechanism of Aqueous Solutions and Swelling of Cellulosic Fibers in Silicone Non-Aqueous Dyeing System. Polymers 2019, 3, 411. [Google Scholar] [CrossRef]
- Pei, L.; Wu, P.; Liu, J.; Wang, J. Effect of Nonionic Surfactant on the Micro-Emulsifying Water in Silicone Media. J. Surfactants Deterg. 2016, 20, 247–254. [Google Scholar] [CrossRef]
- Wang, D.; Steer, H.; Tait, T. Concentrations of cyclic volatile methylsiloxanes in biosolid amended soil, influent, effluent, receiving water, and sediment of wastewater treatment plants in Canada. Chemosphere 2013, 93, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J.; Stickney, V.C. Hydrolysis kinetics of Reactive Blue 19-Vinyl Sulfone. Water Res. 1993, 27, 63–67. [Google Scholar] [CrossRef]
- Pei, L.; Liu, J.; Wang, J. Study of dichlorotriazine reactive dye hydrolysis in siloxane reverse micro-emulsion. J. Clean. Prod. 2017, 165, 994–1004. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, G.; Tao, D.; Wang, Z. Reverse microemulsion-directed synthesis of hydroxyapatite nanoparticles under hydrothermal conditions. J. Phys. Chem. Solids 2007, 68, 373–377. [Google Scholar] [CrossRef]
- Luo, Y.; Pei, L.; Zhang, H.; Zhong, Q.; Wang, J. Improvement of the Rubbing Fastness of Cotton Fiber in Indigo/Silicon Non-Aqueous Dyeing Systems. Polymers 2019, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Islam, G.M.N.; Ke, G. Ultrasonic Effects on the Kinetics and Thermodynamics of Dyeing Wool Fiber with Reactive Dye. Fibers Polym. 2020, 5, 1071–1077. [Google Scholar] [CrossRef]
- Kim, S.D.; Choi, Y.J.; Lee, H.Y.; Lee, J.L. Dyeing properties of nylon, PET, and N/P mixture fabric with reactive-disperse dyes having a sulfatoethylsulfone group. Fibers Polym. 2012, 13, 199–205. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Petrolekas, P.D.; Maggenakis, G. Kinetic studies of the liquid-phase adsorption of a reactive dye onto activated lignite. Ind. Eng. Chem. Res. 2007, 46, 1326–1332. [Google Scholar] [CrossRef]
- Morris, K.F.; Lewis, D.M.; Broadbent, P.J. Design and application of a multifunctional reactive dye capable of high fixation efficiency on cellulose. Color. Technol. 2008, 124, 186–194. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Chiou, M.S.; Li, H.Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef]
- Blanco, S.P.D.M.; Scheufele, F.B.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Marin, P.; Kroumov, A.D.; Borba, C.E. Kinetic, equilibrium and thermodynamic phenomenological modeling of reactive dye adsorption onto polymeric adsorbent. Chem. Eng. J. 2017, 307, 466–475. [Google Scholar] [CrossRef]
- Ghiffary, M.R.; Prabowo, C.P.S.; Sharma, K.; Yan, Y.; Lee, S.Y.; Kim, H.U. High-level production of the natural blue pigment indigoidine from metabolically engineered Corynebacterium glutamicum for sustainable fabric dyes. ACS Sustain. Chem. Eng. 2021, 9, 6613–6622. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H.; Yoshida, T.; Sasaki, M.; Yasuda, H. Dye adsorption on mesoporous activated carbon fiber obtained from pitch containing yttrium complex. Carbon 1999, 37, 983–989. [Google Scholar] [CrossRef]
- Haque, A.M.A.; Sultana, N.; Sayem, A.M.; Smriti, S.A. Sustainable Adsorbents from Plant-Derived Agricultural Wastes for Anionic Dye Removal: A Review. Sustainability 2022, 14, 11098. [Google Scholar] [CrossRef]
- Hubbe, M.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. Bioresources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Alberti, G.; Degiorgi, M.R. Thermodynamic Features in Acrylic Fiber Dyeing with Basic Dyes. Text. Res. J. 1984, 54, 105–107. [Google Scholar] [CrossRef]
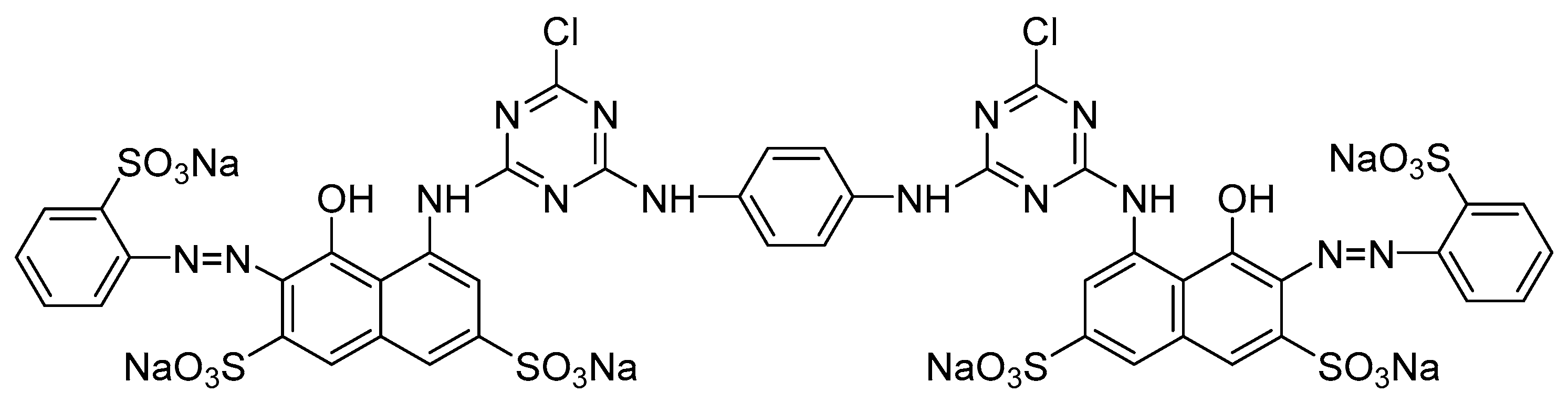

| Dye | Na2SO4 Concentration (o.w.f) | Rubbing | Washing | |||
|---|---|---|---|---|---|---|
| Dry | Wet | Color Change | Cotton | Polyester | ||
| Red 120 | 0 | 4 | 3~4 | 4~5 | 4~5 | 4~5 |
| 2.6 | 4 | 3~4 | 4~5 | 4~5 | 4~5 | |
| 5.2 | 4 | 3~4 | 4~5 | 4~5 | 4~5 | |
| 7.8 | 4~5 | 4 | 4~5 | 4~5 | 4~5 | |
| 13.0 | 4~5 | 4 | 4~5 | 4~5 | 4~5 | |
| Na2SO4 Concentration (o.w.f) | 0 | 2.6% | 7.8% | 13% |
|---|---|---|---|---|
| R2 | 0.9133 | 0.8604 | 0.9183 | 0.8147 |
| Na2SO4 Concentration | 0 | 2.6% | 7.8% | 13% |
|---|---|---|---|---|
| K2 × 10−2 (mg/g·min) | 3.8500 | 4.0036 | 4.7796 | 5.0419 |
| qe,exp (mg/g) | 19.8440 | 19.8330 | 19.8480 | 19.8870 |
| qe,cal (mg/g) | 20.2881 | 20.1857 | 20.1654 | 20.1979 |
| t0.5 (min) | 1.3089 | 1.2594 | 1.0541 | 0.9973 |
| R2 | 0.9994 | 0.9996 | 0.9998 | 0.9998 |
| Salt Concentration (o.w.f) | Temperature (°C) | Δµ° (KJ/mol) | ΔH° (KJ/mol) | ΔS° (KJ/K·mol−1) |
|---|---|---|---|---|
| 0 | 20 | 5.85 | −25.28 | −0.11 |
| 40 | 8.03 | |||
| 60 | 10.10 | |||
| 5.2% | 20 | 9.65 | −27.62 | −0.13 |
| 40 | 11.20 | |||
| 60 | 14.69 | |||
| 7.8% | 20 | 11.62 | −30.20 | −0.14 |
| 40 | 13.05 | |||
| 60 | 17.26 | |||
| 13% | 20 | 12.67 | −31.81 | −0.15 |
| 40 | 14.31 | |||
| 60 | 18.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Pei, L.; Chen, J.; Shen, J.; Alebeid, O.K.; Xu, J.; Luo, C.; Zhang, X.; Zhang, S.; Wang, J. Adsorption of Reactive Red 120 in Decamethyl-Cyclopentasiloxane Non-Aqueous Dyeing System. Coatings 2023, 13, 502. https://doi.org/10.3390/coatings13030502
Sun S, Pei L, Chen J, Shen J, Alebeid OK, Xu J, Luo C, Zhang X, Zhang S, Wang J. Adsorption of Reactive Red 120 in Decamethyl-Cyclopentasiloxane Non-Aqueous Dyeing System. Coatings. 2023; 13(3):502. https://doi.org/10.3390/coatings13030502
Chicago/Turabian StyleSun, Simin, Liujun Pei, Jingru Chen, Jifang Shen, Omer Kamal Alebeid, Jianchang Xu, Chaowen Luo, Xinjie Zhang, Suqing Zhang, and Jiping Wang. 2023. "Adsorption of Reactive Red 120 in Decamethyl-Cyclopentasiloxane Non-Aqueous Dyeing System" Coatings 13, no. 3: 502. https://doi.org/10.3390/coatings13030502
APA StyleSun, S., Pei, L., Chen, J., Shen, J., Alebeid, O. K., Xu, J., Luo, C., Zhang, X., Zhang, S., & Wang, J. (2023). Adsorption of Reactive Red 120 in Decamethyl-Cyclopentasiloxane Non-Aqueous Dyeing System. Coatings, 13(3), 502. https://doi.org/10.3390/coatings13030502








