Simple and Rapid Preparation of MIL-121 with Small Particles for Lithium Adsorption from Brine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Device
2.2. Investigation of the Crystallization Process
2.3. Synthesis of MIL-121 by Low-Temperature Hydrothermal Method
2.4. The Influence of Synthesis Temperature on Particle Size and Stability of MOF Particle
2.5. The Influence of NaOH on the Cooling Crystallization Process of MIL-121
2.6. Cooling Crystallization Experiment
2.7. Adsorption Performance Test
3. Results
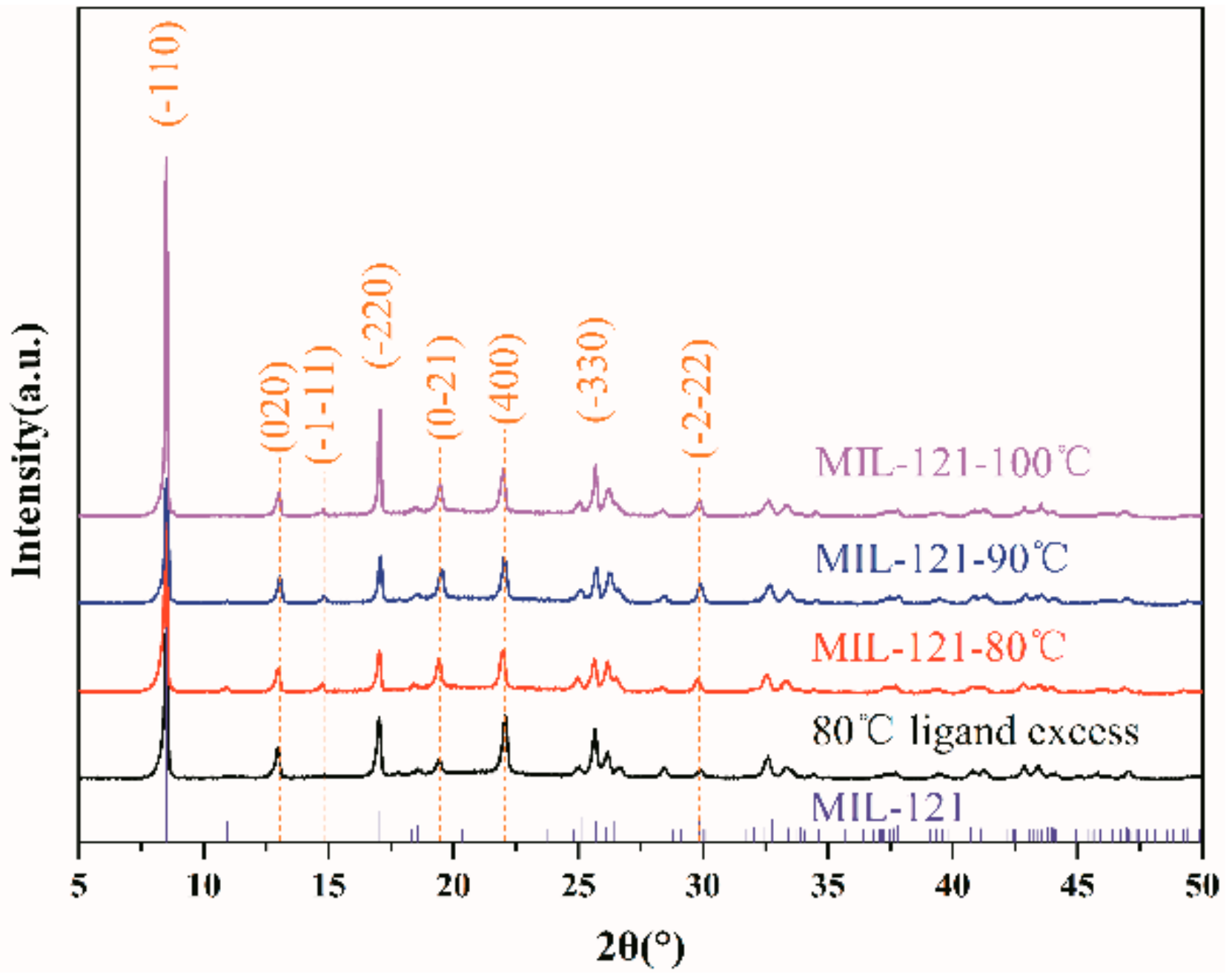
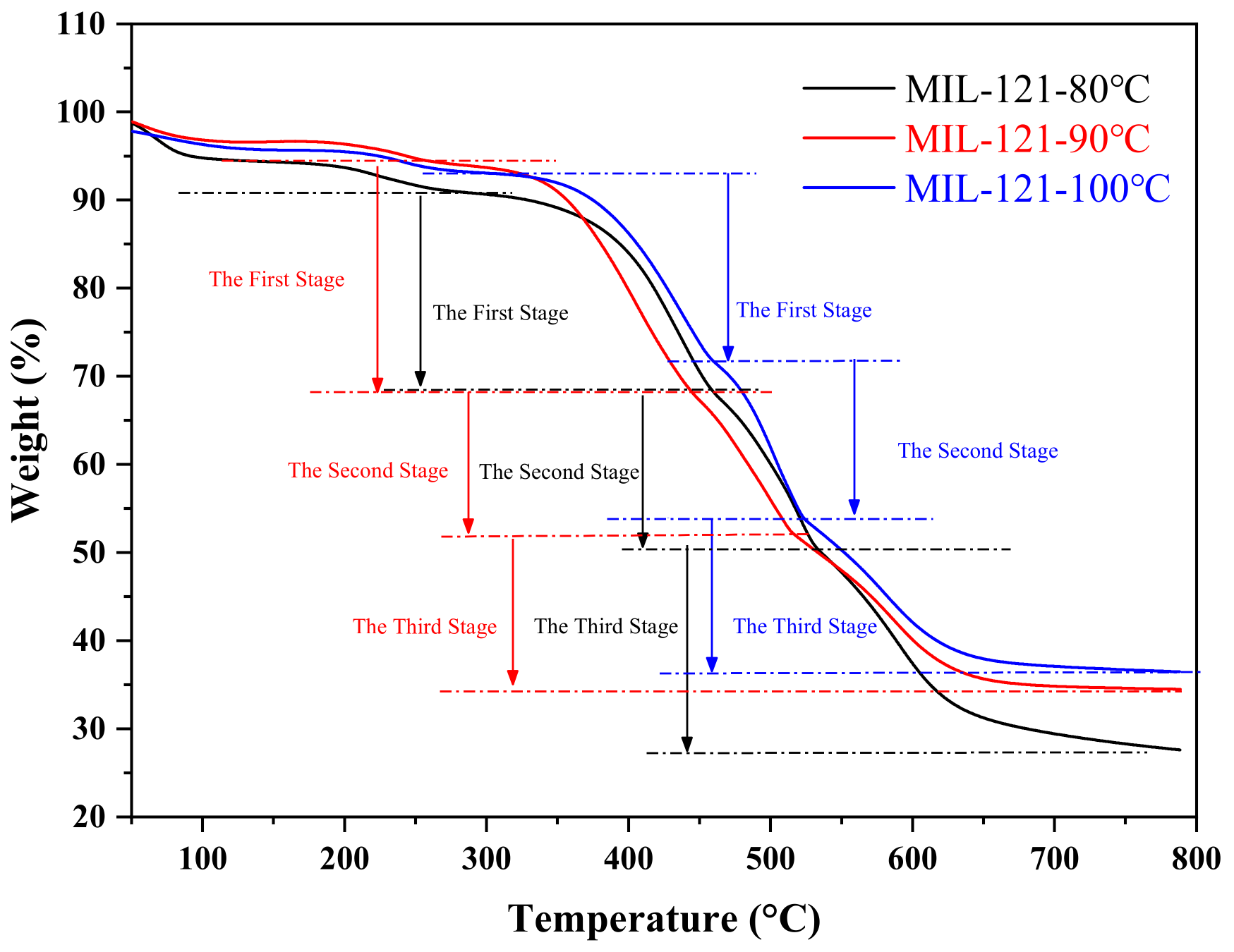
3.1. Morphology, Size, and Stability of Small Particle Size MIL-121
3.2. Prediction of Crystal Morphology
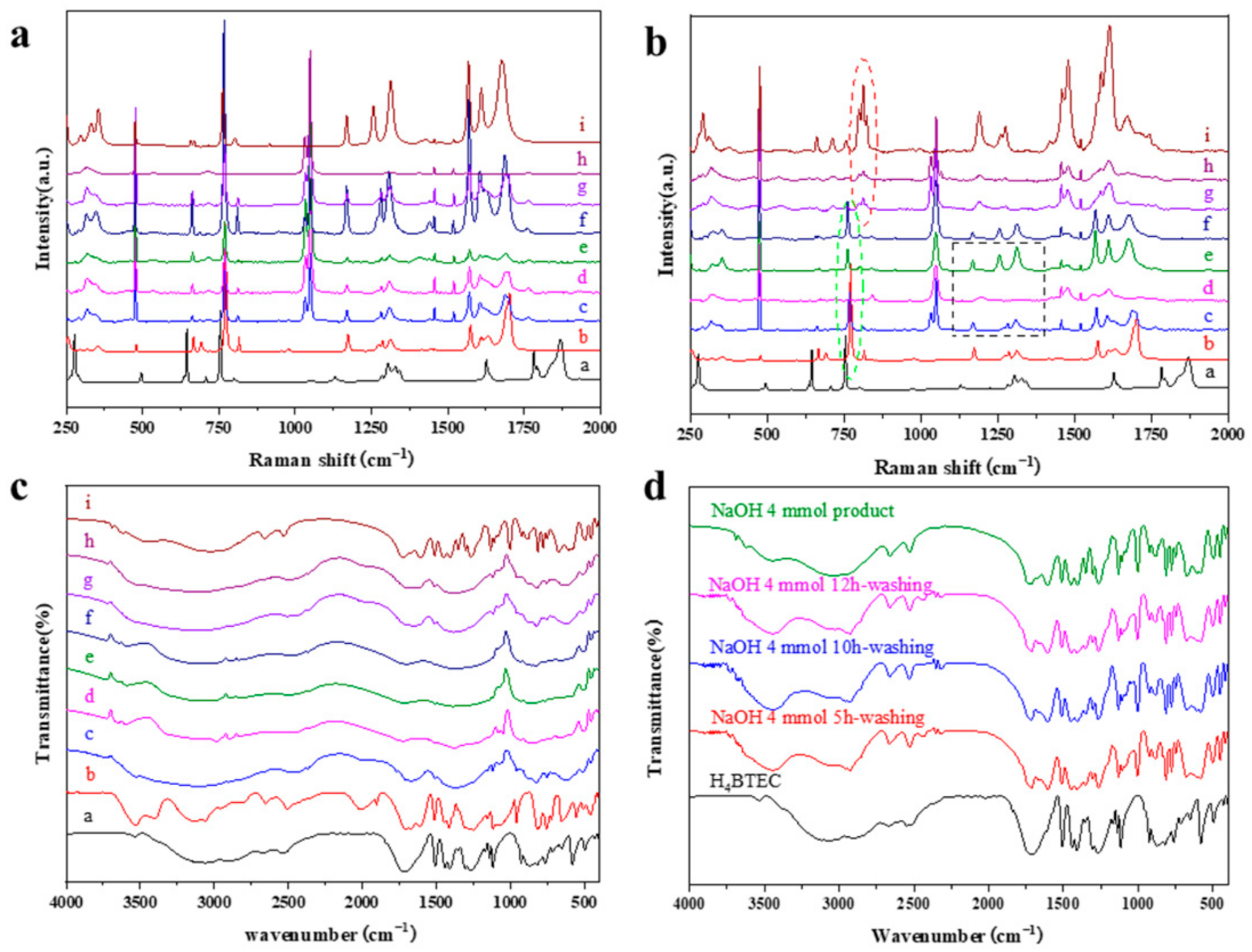
3.3. Crystallization Process Promoted by NaOH
3.4. Optimization of Synthesis Parameters
3.5. Lithium Ion Adsorption Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Sun, S.; Yu, J. Adsorption of lithium ions on lithium-aluminum hydroxides: Equilibrium and kinetics. Can. J. Chem. Eng. 2020, 98, 544–555. [Google Scholar] [CrossRef]
- Chung, K.; Lee, J.; Kim, W.; Kim, S.; Cho, K. Inorganic adsorbent containing polymeric membrane reservoir for the recovery of lithium from seawater. J. Membr. Sci. 2008, 325, 503–508. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, L.; Sun, W. Systematic review of lithium extraction from salt-lake brines via precipitation approaches. Miner. Eng. 2019, 139, 105868. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Wang, Y.; Sha, Z. Review on the electrochemical extraction of lithium from seawater/brine. J. Electroanal. Chem. 2019, 850, 113389. [Google Scholar] [CrossRef]
- Zhao, X.; Li, G.; Feng, M.; Wang, Y. Semi-continuous electrochemical extraction of lithium from brine using CF-NMMO/AC asymmetric hybrid capacitors. Electrochim. Acta 2019, 331, 135285. [Google Scholar] [CrossRef]
- Zhao, X.; Jiao, Y.; Xue, P.; Feng, M.; Sha, Z. Efficiently lithium extraction from brine by using three-dimensional (3D) nanostructured hybrid inorganic-gel framework electrode. ACS Sustain. Chem. Eng. 2020, 8, 4827–4837. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, M.; Jiao, Y.; Zhang, Y.; Wang, Y.; Sha, Z. Lithium extraction from brine in an ionic selective desalination battery. Desalination 2020, 481, 114360. [Google Scholar] [CrossRef]
- Hu, S.; Sun, Y.; Pu, M.; Yun, R.; Xiang, X. Determination of boundary conditions for highly efficient separation of magnesium and lithium from salt lake brine by reaction-coupled separation technology. Sep. Purif. Technol. 2019, 229, 115813. [Google Scholar] [CrossRef]
- Liu, Y.T.; Chen, T.-Y.; Wang, M.K.; Huang, P.M.; Chiang, P.-N.; Lee, J.F. Mechanistic study of arsenate adsorption on lithium/aluminum layered double hydroxide. Appl. Clay Sci. 2010, 48, 485–491. [Google Scholar] [CrossRef]
- Shi, C.; Jing, Y.; Jia, Y. Solvent extraction of lithium ions by tri-n-butyl phosphate using a room temperature ionic liquid. J. Mol. Liq. 2016, 215, 640–646. [Google Scholar] [CrossRef]
- Wei, X.; Liang, S.; Zhou, Z.; Qin, W.; Fei, W. Extraction of lithium from salt lake brine containing borate anion and high concentration of magnesium. Hydrometallurgy 2016, 166, 9–15. [Google Scholar]
- Tian, L.; Wei, M.; Mei, H. Adsorption behavior of Li+ onto nano-lithium ion sieve from hybrid magnesium/lithium manganese oxide-sciencedirect. CEJ 2010, 156, 134–140. [Google Scholar]
- Kotsupalo, N.; Ryabtsev, A. Effect of structure on the sorption properties of chlorine-containing form of double aluminum lithium hydroxide. Russ. J. Appl. Chem. 2013, 86, 482–487. [Google Scholar] [CrossRef]
- Nisola, G.M.; Limjuco, L.A.; Vivas, E.L.; Lawagon, C.P.; Park, M.J.; Shon, H.K.; Mittala, N.; Nah, I.W.; Kim, H.; Chung, W. Macroporous flexible polyvinyl alcohol lithium adsorbent foam composite prepared via surfactant blending and cryo-desiccation. CEJ 2015, 280, 536–548. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Magnesium-doped manganese oxide with lithium ion-sieve property: Lithium adsorption from salt lake brine. Bull. Chem. Soc. Jpn. 2013, 86, 850–855. [Google Scholar] [CrossRef]
- Bi, Q.; Zhang, Z.; Zhao, C.; Tao, Z. Study on the recovery of lithium from high Mg2+/Li+ ratio brine by nanofiltration. Water Sci. Technol. 2014, 70, 1690–1694. [Google Scholar] [CrossRef]
- Ou, R.; Zhang, H.; Wei, J.; Kim, S.; Wan, L.; Nguyen, N.S.; Hu, Y.; Zhang, X.; Simon, G.P. Thermoresponsive amphoteric metal-organic frameworks for efficient and reversible adsorption of multiple salts from water. Adv. Mater. 2018, 30, e1802767. [Google Scholar] [CrossRef]
- Ji, C.; Wu, D.; Lu, J.; Shan, C.; Ren, Y.; Li, T.; Lv, L.; Pan, B.; Zhang, W. Temperature regulated adsorption and desorption of heavy metals to a-Mil-121: Mechanisms and the role of exchangeable protons. Water Res. 2020, 189, 116599. [Google Scholar] [CrossRef]
- Yamamoto, D.; Maki, T.; Watanabe, S.; Tanaka, H.; Miyahara, M.T.; Mae, K. Synthesis and adsorption properties of zif-8 na-noparticles using a micromixer. CEJ 2013, 227, 145–150. [Google Scholar]
- Marshall, C.; Staudhammer, S.A.; Brozek, C.K. Size control over metal–organic framework porous nanocrystals. Chem. Sci. 2019, 10, 9396–9408. [Google Scholar] [CrossRef]
- Han, Y.-H.; Tian, C.-B.; Li, Q.-H.; Du, S.-W. Highly chemical and thermally stable luminescent EuxTb1−xMOF materials for broad-range pH and temperature sensors. J. Mater. Chem. C 2014, 2, 8065–8070. [Google Scholar] [CrossRef]
- Mendiratta, S.; Usman, M.; Chang, C.; Lee, Y.C.; Chen, J.W.; Wu, M.K. Zn(ii)-based metal–organic framework: An excep-tionally thermally stable, guest-free low dielectric material. J. Mater. Chem. 2017, 5, 1508–1513. [Google Scholar] [CrossRef]
- Kamal, S.; Chiou, K.R.; Sainbileg, B.; Inamdar, A.I.; Usman, M.; Pathak, A.; Luo, T.-T.; Chen, J.-W.; Hayashi, M.; Hung, C.-H.; et al. Thermally stable indium based metal–organic frameworks with high dielectric permittivity. J. Mater. Chem. C 2020, 8, 9724–9733. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Yin, J.E.; Abou-Hamad, X.; Liu, W.; Chen, T. Highly stable phosphonate-based mofs with engineered bandgaps for efficient photocatalytic hydrogen production. Adv. Mater. 2020, 32, 1906368. [Google Scholar] [CrossRef]
- Ricco, R.; Liang, W.; Li, S.; Gassensmith, J.J.; Caruso, F.; Doonan, C.; Falcaro, P. Metal-organic frameworks for cell and virus biology: A perspective. ACS Nano 2018, 12, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lv, N.; Li, X.; Liu, B.; Feng, J.; Ren, X.; Guo, T.; Chen, D.; Stoddart, J.F.; Gref, R.; et al. Composite CD-MOF nanocrystals-containing microspheres for sustained drug delivery. Nanoscale 2017, 9, 7454–7463. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huo, F. Metal-organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef]
- Liu, M.; Xie, K.; Nothling, M.D.; Gurr, P.A.; Tan, S.S.L.; Fu, Q.; Webley, P.A.; Qiao, G.G. Ultrathin metal-organic frame-work nanosheets as a gutter layer for flexible composite gas separation membranes. ACS Nano 2018, 12, 11591–11599. [Google Scholar] [CrossRef]
- Stavila, V.; Bhakta, R.K.; Alam, T.M.; Majzoub, E.H.; Allendorf, M.D. Reversible hydrogen storage by NaAlH4 confined within a titanium-functionalized MOF-74(Mg) nanoreactor. ACS Nano 2012, 6, 9807–9817. [Google Scholar] [CrossRef]
- Babal, A.S.; Tan, J.-C. Influence of mechanical, thermal, and electrical perturbations on the dielectric behaviour of guest-encapsulated HKUST-1 crystals. J. Mater. Chem. C 2020, 8, 12886–12892. [Google Scholar] [CrossRef]
- Bi, S.; Banda, H.; Chen, M.; Niu, L.; Chen, M.; Wu, T.; Wang, J.; Wang, R.; Feng, J.; Chen, T.; et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with Mof electrodes and ionic liquid electrolytes. Nat. Mater. 2019, 19, 552–558. [Google Scholar] [CrossRef]
- Van Vleet, M.; Weng, T.; Li, X.; Schmidt, J. In situ, time-resolved, and mechanistic studies of metal–organic framework nucleation and growth. Chem. Rev. 2018, 118, 3681–3721. [Google Scholar] [CrossRef]
- Chen, J.; Shen, K.; Li, Y. Greening the processes of metal-organic framework synthesis and their use in sustainable catalysis. ChemSusChem 2017, 10, 3165–3187. [Google Scholar] [CrossRef]
- Ibarra, I.A.; Bayliss, P.A.; Yang, S.; Nowell, H.; Poliakoff, M.; Pérez, E.; Blake, A.J.; Allan, D.R.; Schröder, M. Near-critical water, a cleaner solvent for the synthesis of a metal–organic framework. Green Chem. 2012, 14, 117–122. [Google Scholar] [CrossRef]
- Guo, W.; Sun, W.; Lv, L.P.; Kong, S.; Wang, Y. Microwave-assisted morphology evolution of Fe-based metal-organic frameworks and their derived Fe2O3 nanostructures for Li-ion storage. ACS Nano 2017, 11, 4198–4205. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Qiu, L.G.; Tao, X.; Yun, W.; Wei, W.; Wu, Z.Y. Ultrasonic synthesis of the microporous metal–organic framework cu 3 (btc) 2 at ambient temperature and pressure: An efficient and environmentally friendly method. Mater. Lett. 2019, 63, 78–80. [Google Scholar] [CrossRef]
- Yan, D.; Gao, R.; Wei, M.; Li, S.; Lu, J.; Evans, D.G.; Duan, X. Mechanochemical synthesis of a fluorenone-based metal organic framework with polarized fluorescence: An experimental and computational study. J. Mater. Chem. C 2013, 1, 997–1004. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Štrukil, V.; Eckert-Maksić, M.; Dinnebier, R.E. Clean and efficient synthesis using mechanochemistry: Coordination polymers, metal-organic frameworks and metallodrugs. Croat. Chem. Acta 2012, 85, 367–378. [Google Scholar] [CrossRef]
- Gaab, M.; Trukhan, N.; Maurer, S.; Gummaraju, R.; Müller, U. The progression of Al-based metal-organic frameworks—From academic research to industrial production and applications. Microporous Mesoporous Mater. 2012, 157, 131–136. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, L.; Wei, Q.Y.; Wang, Y.F. Research on the effects of hydrothermal synthesis conditions on the crystal Hab-it of Mil-121. R. Soc. Open Sci. 2020, 7, 201212. [Google Scholar] [CrossRef] [PubMed]
- Volkringer, C.; Loiseau, T.; Guillou, N.; Férey, G.; Haouas, M.; Taulelle, F.; Elkaim, E.; Stock, N. High-throughput aided synthesis of the porous metal−organic framework-type aluminum pyromellitate, MIL-121, with extra carboxylic acid functionalization. Inorg. Chem. 2010, 49, 9852–9862. [Google Scholar] [CrossRef] [PubMed]

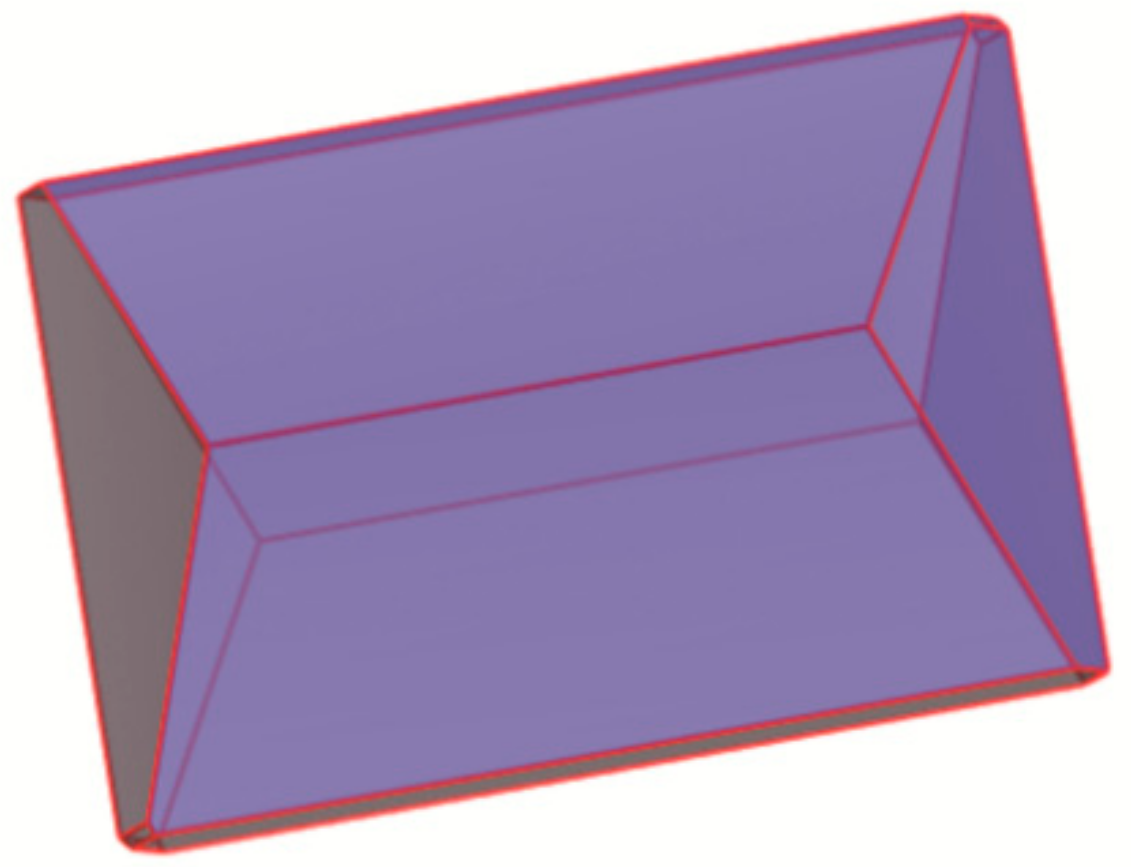
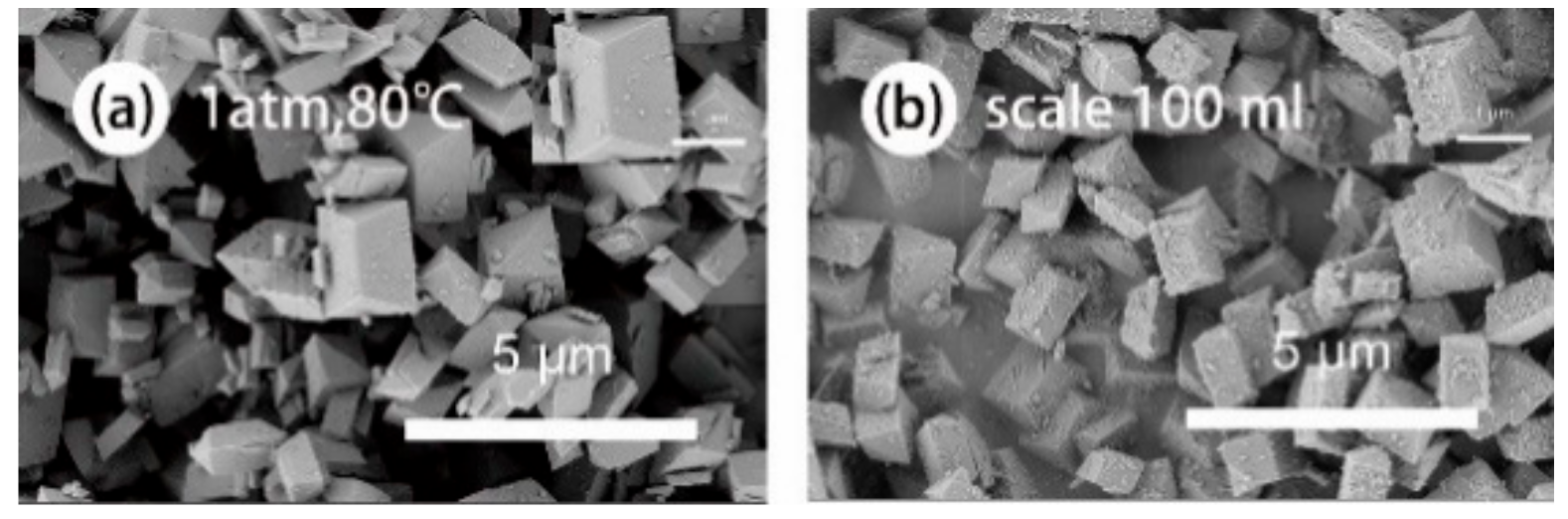
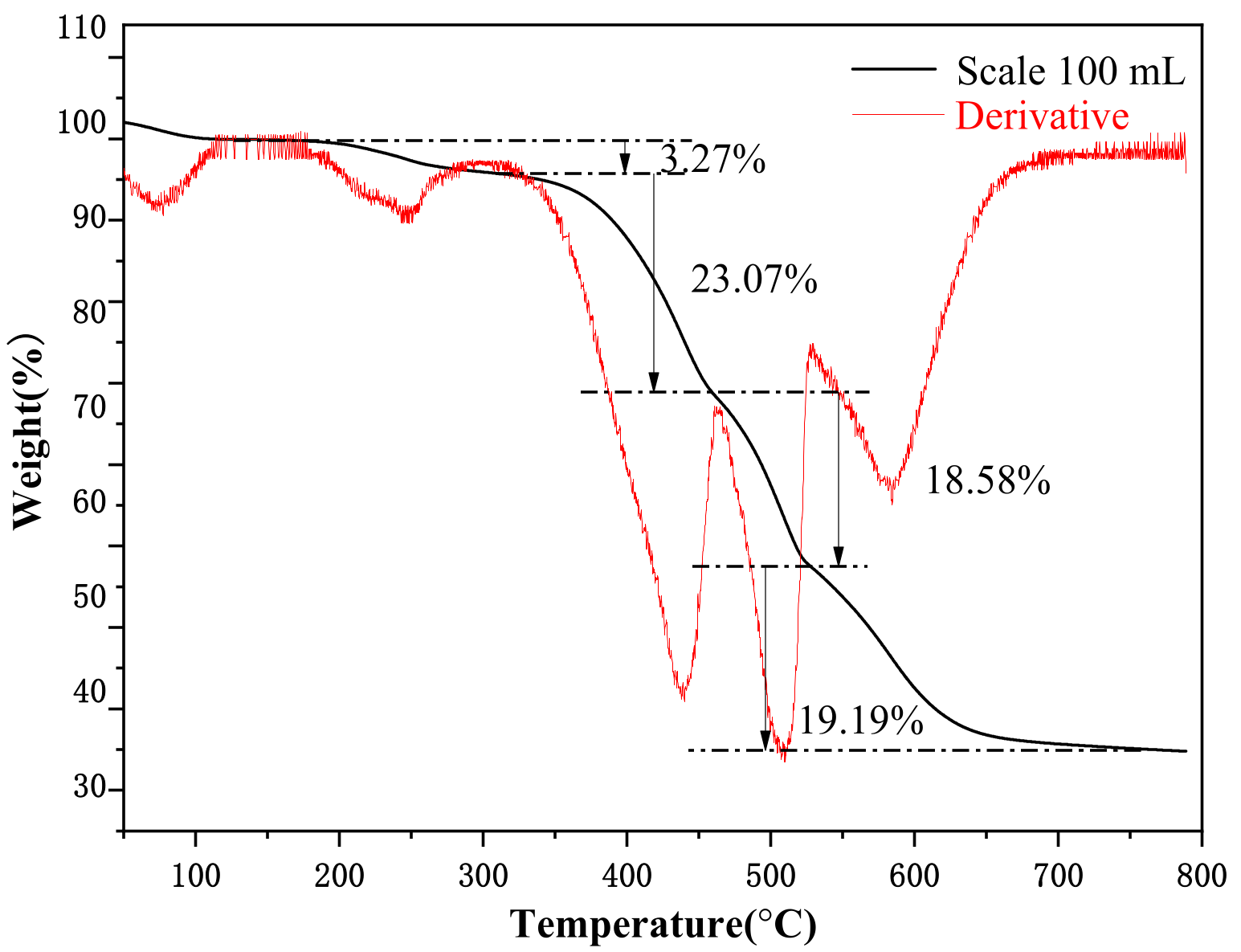
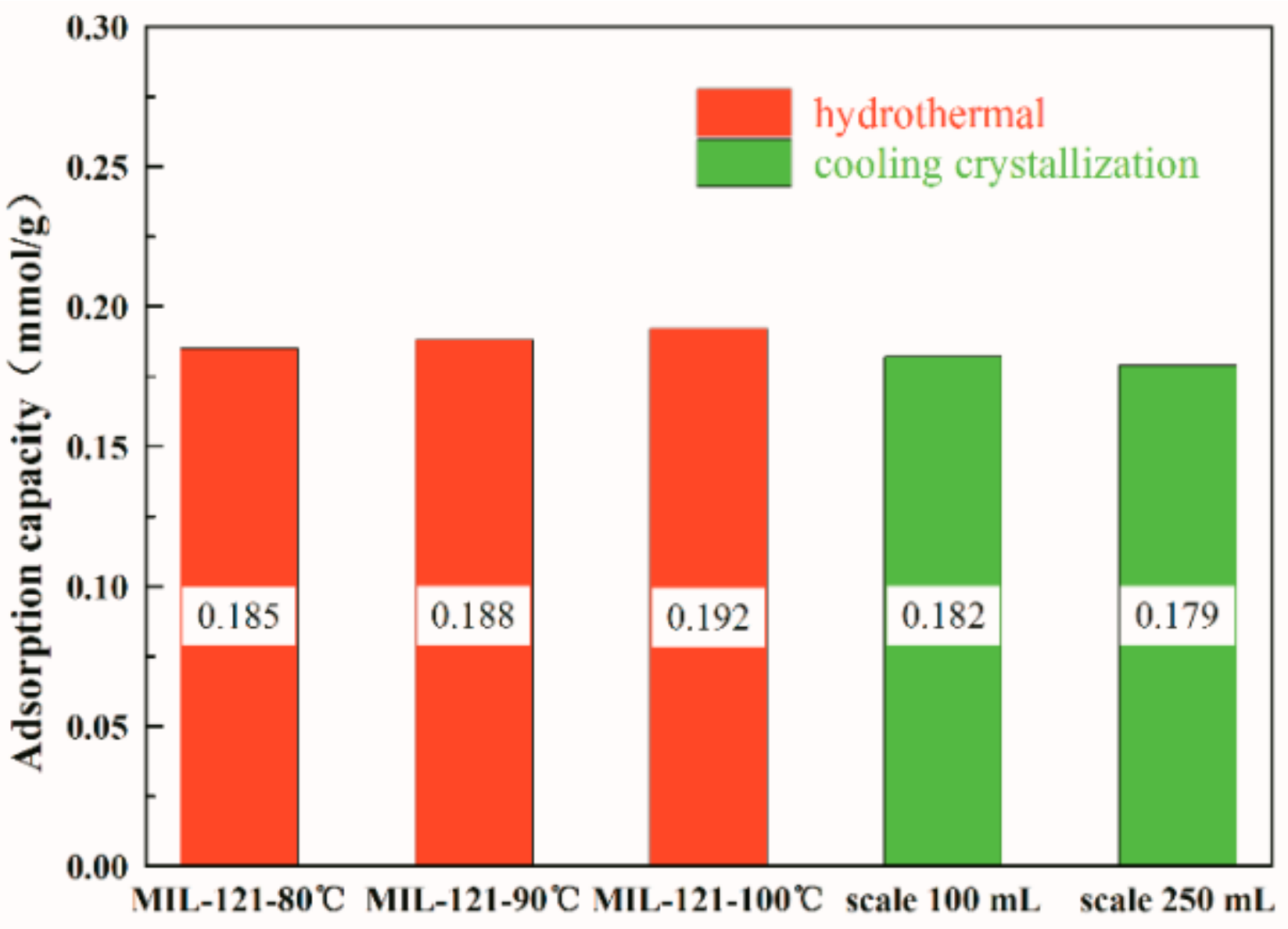
| Hkl | Dhkl/Å | Distance/Å | % Total Facet Area |
|---|---|---|---|
| {110} | 10.39 | 9.63 | 63.43 |
| {200} | 8.06 | 12.40 | 10.01 |
| {11} | 5.98 | 16.72 | 26.37 |
| {31} | 4.84 | 20.68 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Shi, B.; Wang, F.; Shao, S.; Zhu, L.; Zhao, X. Simple and Rapid Preparation of MIL-121 with Small Particles for Lithium Adsorption from Brine. Coatings 2021, 11, 854. https://doi.org/10.3390/coatings11070854
Wei Q, Shi B, Wang F, Shao S, Zhu L, Zhao X. Simple and Rapid Preparation of MIL-121 with Small Particles for Lithium Adsorption from Brine. Coatings. 2021; 11(7):854. https://doi.org/10.3390/coatings11070854
Chicago/Turabian StyleWei, Qinyan, Bingqian Shi, Fei Wang, Shuoshuo Shao, Liang Zhu, and Xiaoyu Zhao. 2021. "Simple and Rapid Preparation of MIL-121 with Small Particles for Lithium Adsorption from Brine" Coatings 11, no. 7: 854. https://doi.org/10.3390/coatings11070854
APA StyleWei, Q., Shi, B., Wang, F., Shao, S., Zhu, L., & Zhao, X. (2021). Simple and Rapid Preparation of MIL-121 with Small Particles for Lithium Adsorption from Brine. Coatings, 11(7), 854. https://doi.org/10.3390/coatings11070854







