Can a-C:H-Sputtered Coatings Be Extended to Orthodontics?
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of a-C:H Coatings
2.2. Coatings Characterization
2.3. Saliva Immersion Test
2.4. In Vitro Cytotoxicity
2.4.1. Cell Cultures
2.4.2. Extract Testing
2.4.3. MTT Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. As-Deposited Characterization
3.2. As-Immersed Characterization
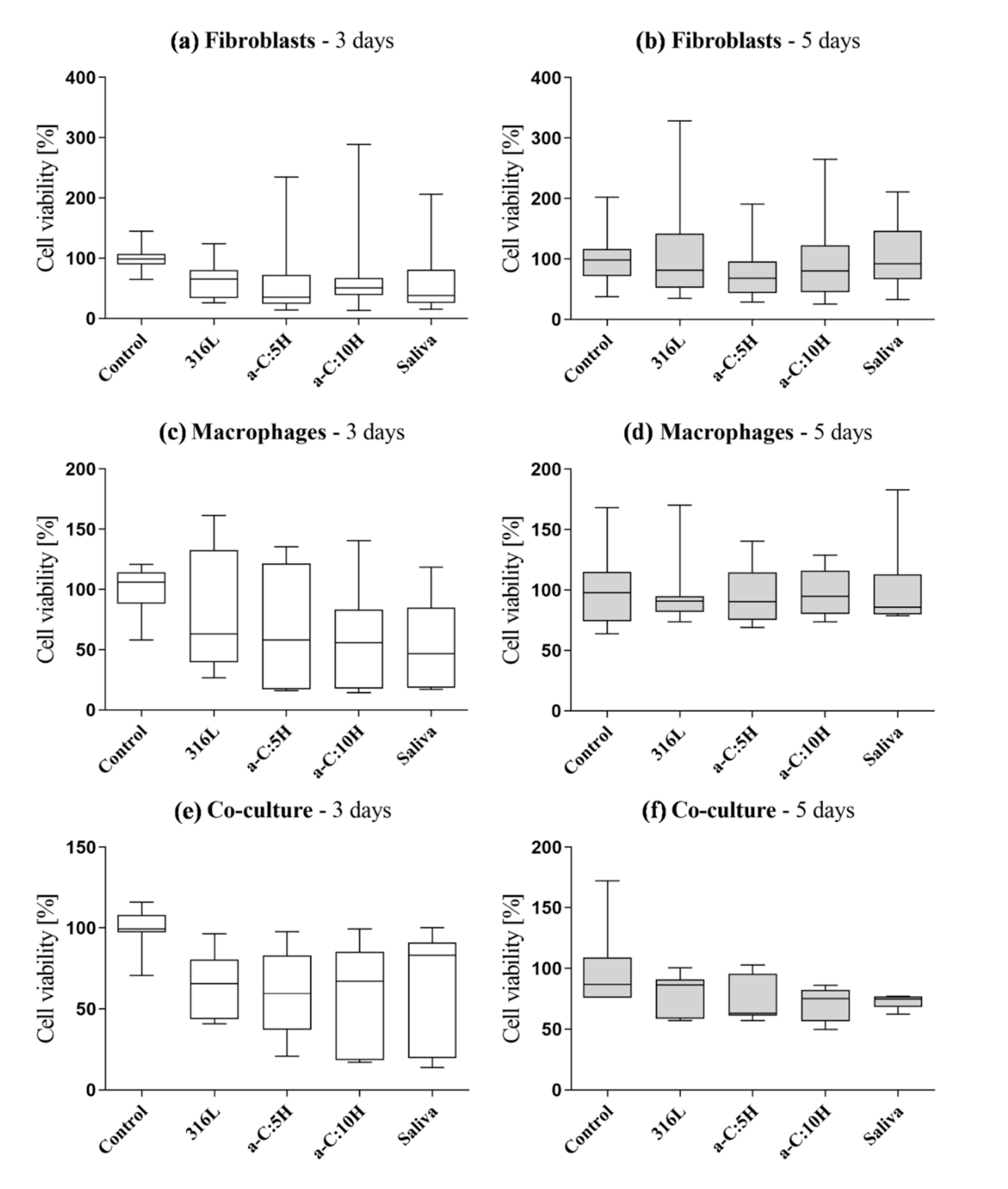
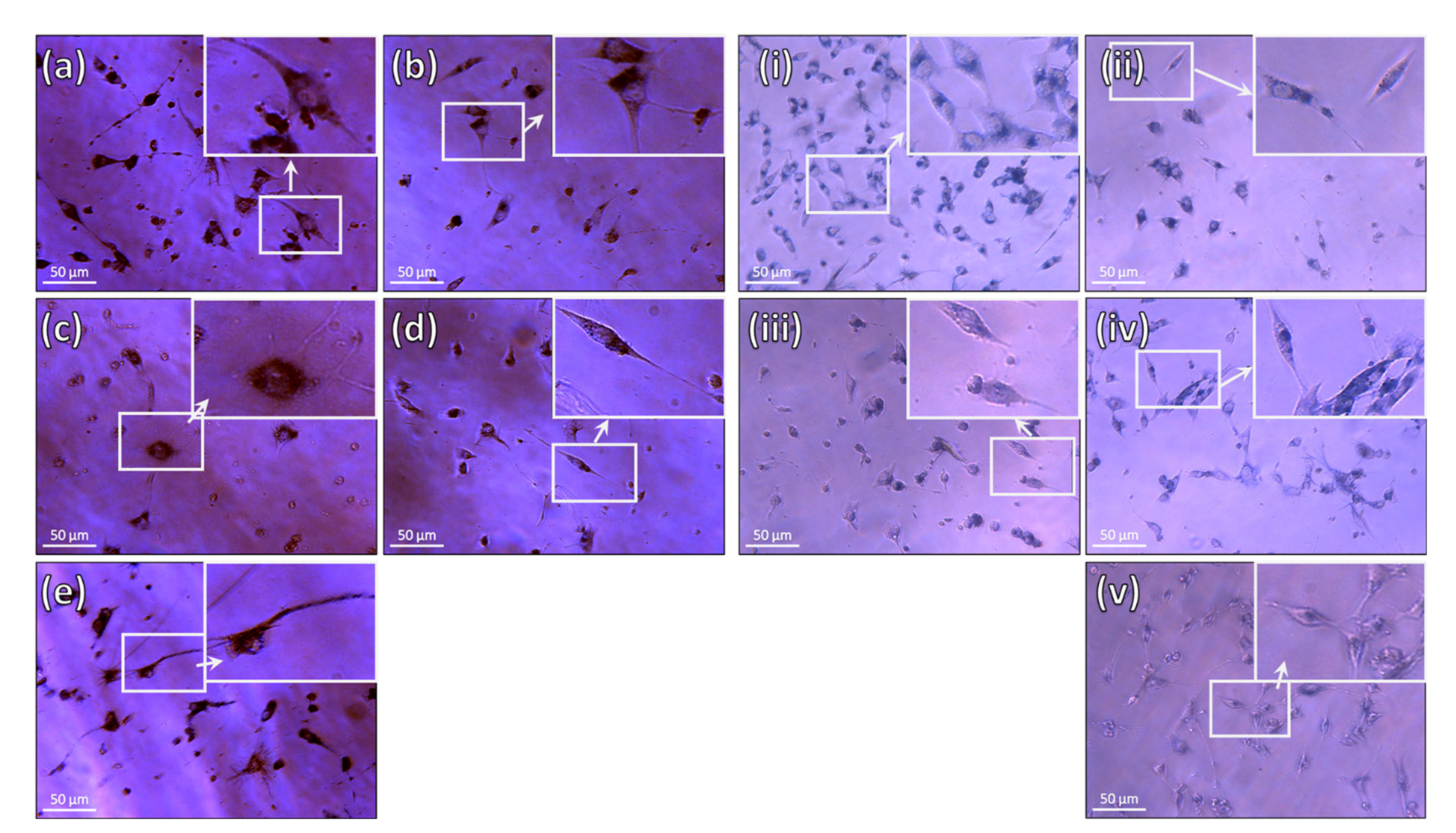
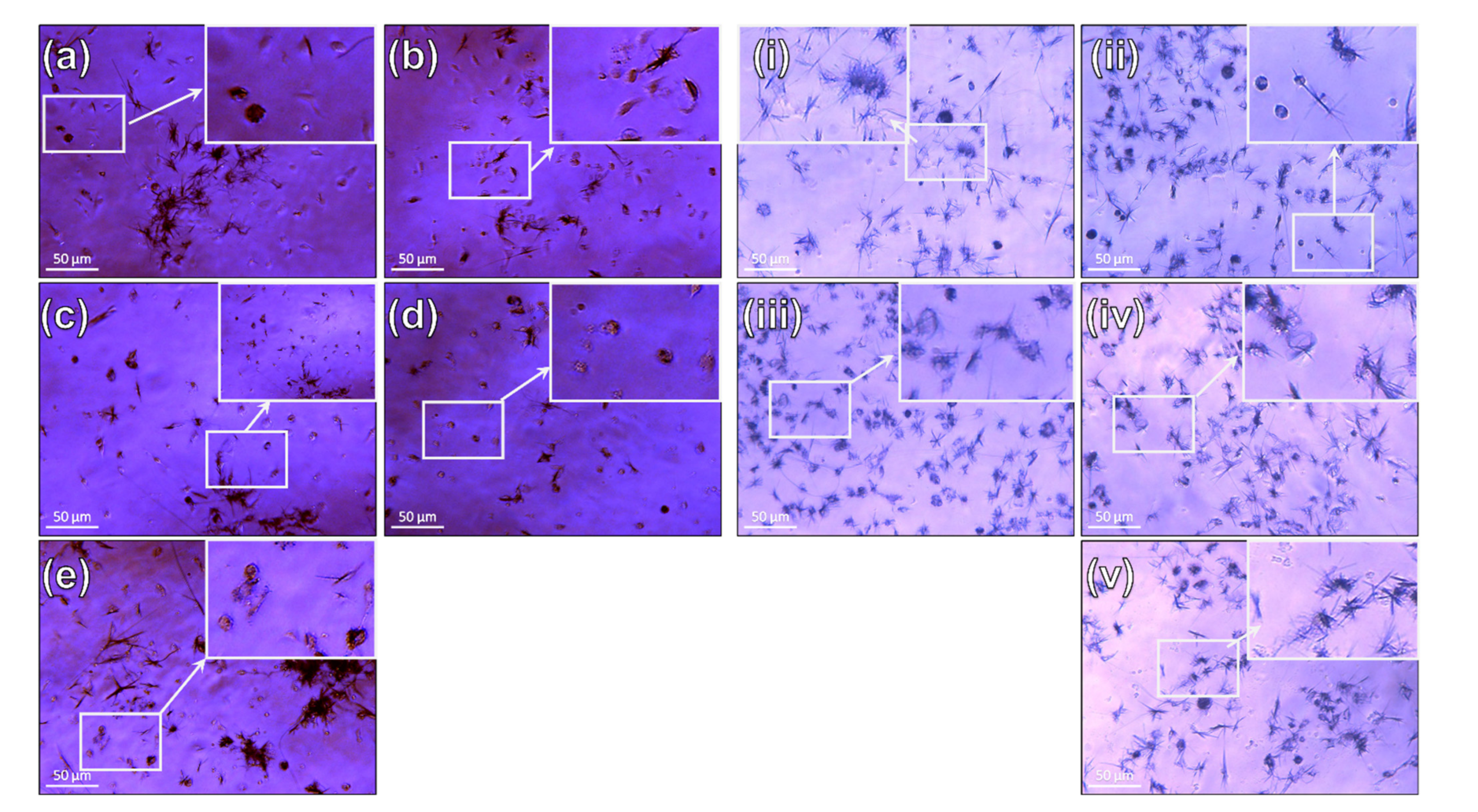
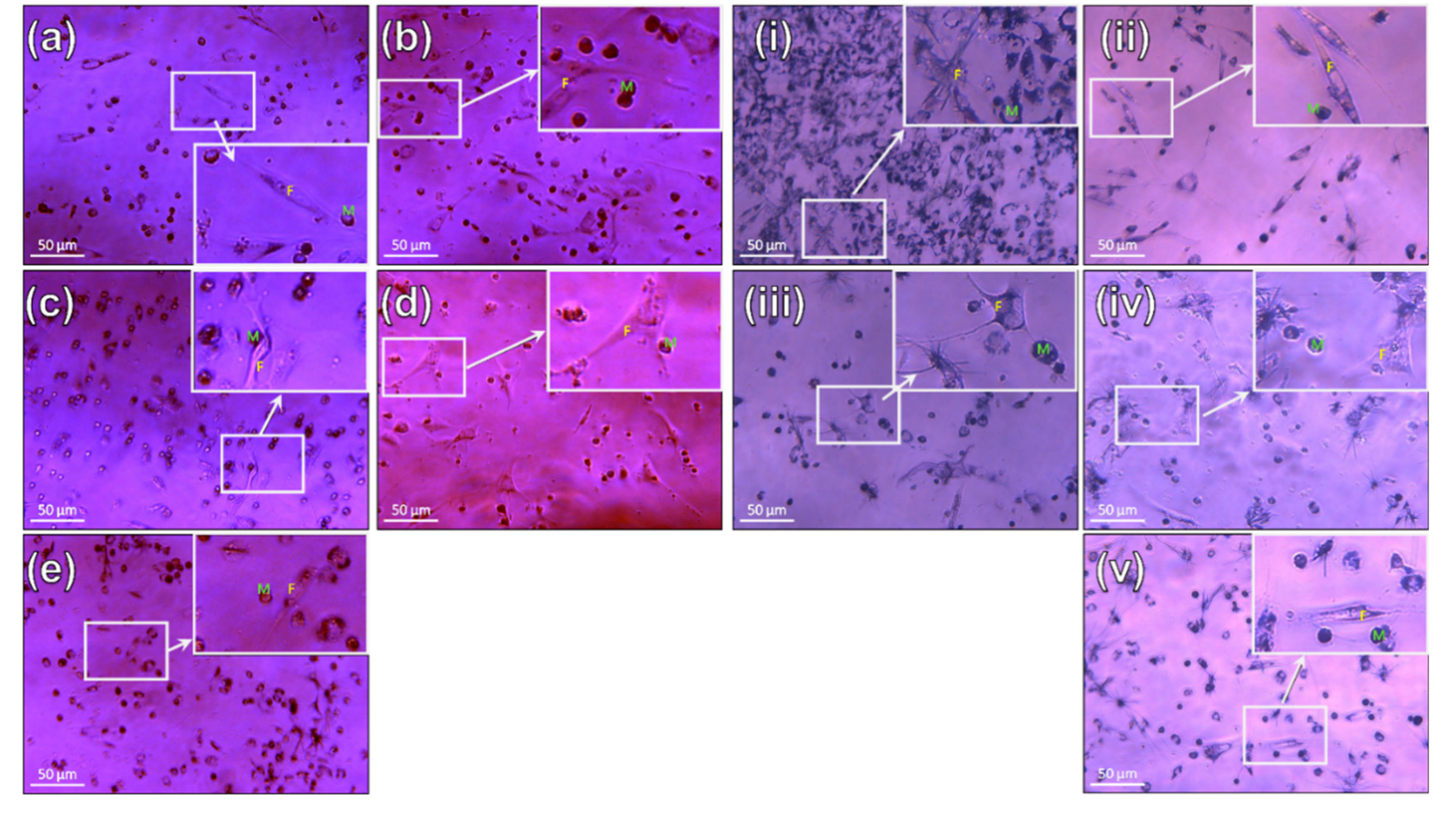
3.3. Biological Characterization: In Vitro Citotoxicity
3.3.1. Fibroblasts
3.3.2. Macrophages
3.3.3. Co-Culture (Fibroblasts and Macrophages)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Proffit, W.R.; Fields, H.W.; Sarver, D.M.; Ackerman, J.L. Contemporary orthodontic appliances. In Contemporary Orthodontics; Mosby, Elsevier: Amsterdam, The Netherlands, 2012; pp. 347–389. ISBN 978032308317. [Google Scholar]
- Abdallah, M.-N.; Lou, T.; Retrouvey, J.-M.; Suri, S. Biomaterials used in orthodontics: Brackets, archwires, and clear aligners. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 541–579. ISBN 978-0-08-102476-8. [Google Scholar]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The role of oral cavity biofilm on metallic biomaterial surface destruction–corrosion and friction aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef]
- Mosca, A.C.; Chen, J. Food-saliva interactions: Mechanisms and implications. Trends Food Sci. Technol. 2017, 66, 125–134. [Google Scholar] [CrossRef]
- Kwak, D.Y.; Kim, N.Y.; Kim, H.J.; Yang, S.Y.; Yoon, J.E.; Hyun, I.A.; Nam, S.H. Changes in the oral environment after tooth brushing and oral gargling. Biomed. Res. 2017, 28, 7093–7097. [Google Scholar]
- Moore, R.J.; Watts, J.T.F.; Hood, J.A.A.; Burritt, D.J. Intra-oral temperature variation over 24 hours. Eur. J. Orthod. 1999, 21, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Head, D.A.; Devine, D.A. Dental plaque as a biofilm and a microbial community—Implications for treatment. J. Oral Biosci. 2015, 57, 185–191. [Google Scholar] [CrossRef]
- Chaturvedi, T.P.; Upadhayay, S.N. An overview of orthodontic material degradation in oral cavity. Indian J. Dent. Res. 2010, 21, 275–284. [Google Scholar] [CrossRef]
- Eliades, T.; Athanasiou, A.E. In Vivo Aging of orthodontic alloys: Implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod. 2002, 72, 222–237. [Google Scholar]
- Frois, A.; Cunha, L.; Louro, C.S. Functionalization of Orthodontic Alloys with DLC Coatings. In Proceedings of the 2019 IEEE 6th Portuguese Meeting on Bioengineering (ENBENG), Lisbon, Portugal, 22–23 February 2019; IEEE: Lisbon, Portugal, 2019; pp. 1–4. [Google Scholar]
- Kao, C.-T.; Huang, T.-H. Variations in surface characteristics and corrosion behaviour of metal brackets and wires in different electrolyte solutions. Eur. J. Orthod. 2010, 32, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Han, E.; Ke, W. Influence of fluoride and chloride on corrosion behavior of NiTi orthodontic wires. Acta Biomater. 2007, 3, 807–815. [Google Scholar] [CrossRef]
- Daems, J.; Celis, J.-P.; Willems, G. Morphological characterization of as-received and in vivo orthodontic stainless steel archwires. Eur. J. Orthod. 2009, 31, 260–265. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Martín-Cameán, A.; Jos, Á.; Mellado-García, P.; Iglesias-Linares, A.; Solano, E.; Cameán, A.M. In vitro and in vivo evidence of the cytotoxic and genotoxic effects of metal ions released by orthodontic appliances: A review. Environ. Toxicol. Pharmacol. 2015, 40, 86–113. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Nickel and nickel compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2011, 100C, 169–218. [Google Scholar] [CrossRef]
- Saito, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Molecular mechanisms of nickel allergy. Int. J. Mol. Sci. 2016, 17, 202. [Google Scholar] [CrossRef]
- Dunlap, C.L.; Vincent, S.K.; Barker, B.F. Allergic reaction to orthodontic wire: Report of case. J. Am. Dent. Assoc. 1989, 118, 449–450. [Google Scholar] [CrossRef]
- Noble, J.; Ahing, S.I.; Karaiskos, N.E.; Wiltshire, W.A. Nickel allergy and orthodontics, a review and report of two cases. Br. Dent. J. 2008, 204, 297–300. [Google Scholar] [CrossRef]
- Kolokitha, O.E.; Chatzistavrou, E. A severe reaction to Ni-containing orthodontic appliances. Angle Orthod. 2009, 79, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.E.; Benson, P.E. Potential hazards of orthodontic treatment—What your patient should know. Dent. Update 2002, 29, 492–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- House, K.; Sernetz, F.; Dymock, D.; Sandy, J.R.; Ireland, A.J. Corrosion of orthodontic appliances-should we care? Am. J. Orthod. Dentofac. Orthop. 2008, 133, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.S. Current products and practice: Aesthetic orthodontic brackets. J. Orthod. 2005, 32, 146–163. [Google Scholar] [CrossRef]
- Arango, S.; Peláez-Vargas, A.; García, C. Coating and surface treatments on orthodontic metallic materials. Coatings 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Vetter, J. 60years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Zahid, R.; Masjuki, H.H.; Varman, M.; Kalam, M.A.; Mufti, R.A.; Zulkifli, N.W.B.M.; Gulzar, M.; Azman, S.S.B.N. Influence of intrinsic and extrinsic conditions on the tribological characteristics of diamond-like carbon coatings: A review. J. Mater. Res. 2016, 31, 1814–1836. [Google Scholar] [CrossRef]
- Ohgoe, Y.; Hirakuri, K.K.; Saitoh, H.; Nakahigashi, T.; Ohtake, N.; Hirata, A.; Kanda, K.; Hiratsuka, M.; Fukui, Y. Classification of DLC films in terms of biological response. Surf. Coat. Technol. 2012, 207, 350–354. [Google Scholar] [CrossRef]
- Casiraghi, C.; Ferrari, A.C.; Robertson, J. Raman spectroscopy of hydrogenated amorphous carbons. Phys. Rev. B 2005, 72, 085401. [Google Scholar] [CrossRef]
- Hauert, R.; Thorwarth, K.; Thorwarth, G. An overview on diamond-like carbon coatings in medical applications. Surf. Coat. Technol. 2013, 233, 119–130. [Google Scholar] [CrossRef]
- Love, C.A.; Cook, R.B.; Harvey, T.J.; Dearnley, P.A.; Wood, R.J.K. Diamond like carbon coatings for potential application in biological implants—A review. Tribol. Int. 2013, 63, 141–150. [Google Scholar] [CrossRef]
- Kang, T.; Huang, S.-Y.; Huang, J.-J.; Li, Q.-H.; Diao, D.-F.; Duan, Y.-Z. The effects of diamond-like carbon films on fretting wear behavior of orthodontic archwire-bracket contacts. J. Nanosci. Nanotechnol. 2015, 15, 4641–4647. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, T.; Iijima, M.; Kawaguchi, M.; Mizoguchi, I. Effects of sp2/sp3 ratio and hydrogen content on in vitro bending and frictional performance of dlc-coated orthodontic stainless steels. Coatings 2018, 8, 199. [Google Scholar] [CrossRef]
- Akaike, S.; Kobayashi, D.; Aono, Y.; Hiratsuka, M.; Hirata, A.; Hayakawa, T.; Nakamura, Y. Relationship between static friction and surface wettability of orthodontic brackets coated with diamond-like carbon (DLC), fluorine- or silicone-doped DLC coatings. Diam. Relat. Mater. 2016, 61, 109–114. [Google Scholar] [CrossRef]
- Ferrari, A.C. Non-destructive characterisation of carbon films. In Tribology of Diamond-Like Carbon Films: Fundamentals and Applications; Donnet, C., Erdemir, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 25–82. ISBN 9780387302645. [Google Scholar]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Singha, A.; Ghosh, A.; Roy, A.; Ray, N.R. Quantitative analysis of hydrogenated diamondlike carbon films by visible raman spectroscopy. J. Appl. Phys. 2006, 100, 044910. [Google Scholar] [CrossRef]
- ISO 10271:2001—Dental Metallic Materials—Corrosion Test Methods; ISO (International Organization for Standardization): Geneva, Switzerland, 2001.
- ATCC (American Type Culture Collection). NIH/3T3 (ATCC CRL-1658). Available online: https://www.atcc.org/products/crl-1658 (accessed on 21 November 2020).
- Rejmontová, P.; Capáková, Z.; Mikušová, N.; Maráková, N.; Kašpárková, V.; Lehocký, M.; Humpolíček, P. Adhesion, proliferation and migration of NIH/3T3 cells on modified polyaniline surfaces. Int. J. Mol. Sci. 2016, 17, 1439. [Google Scholar] [CrossRef]
- Xiao, W.; Su, Y.; Zhou, S.; Yi, C.; He, G.; Liu, Y.; Qi, Y. Rasgrp2 regulates the permissiveness of NIH3T3 cells to a herpes simplex virus 1 mutant with inactivated ICP34.5 gene. Acta Virol. 2013, 57, 41–49. [Google Scholar] [CrossRef][Green Version]
- Santos, A.C.A. Água Trocável do Pulmão: Contribuição para o Desenvolvimento de uma Metodologia para a sua Avaliação; Universidade de Coimbra: Coimbra, Portugal, 2001. [Google Scholar]
- ISO 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO (International Organization for Standardization): Geneva, Switzerland, 2009.
- Costa, M.T.; Lenza, M.A.; Gosch, C.S.; Costa, I.; Ribeiro-Dias, F. In vitro evaluation of corrosion and cytotoxicity of orthodontic brackets. J. Dent. Res. 2007, 86, 441–445. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Bejcek, B., Caaveiro, J.M.M., Chung, T.D.Y., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013; pp. 295–320. [Google Scholar]
- Louro, C.; Moura, C.W.; Carvalho, N.; Stueber, M.; Cavaleiro, A. Thermal stability in oxidative and protective environments of a-C:H cap layer on a functional gradient coating. Diam. Relat. Mater. 2011, 20, 57–63. [Google Scholar] [CrossRef]
- Chowdhury, S.; Laugier, M.T.; Rahman, I.Z. Characterization of DLC coatings deposited by rf magnetron sputtering. J. Mater. Process. Technol. 2004, 153–154, 804–810. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; Lee, P.; Wei, R. Thick diamond like carbon coatings deposited by deep oscillation magnetron sputtering. Surf. Coat. Technol. 2017, 315, 294–302. [Google Scholar] [CrossRef]
- Hatem, A.; Lin, J.; Wei, R.; Torres, R.D.; Laurindo, C.; Soares, P. Tribocorrosion behavior of DLC-coated Ti-6Al-4V alloy deposited by PIID and PEMS + PIID techniques for biomedical applications. Surf. Coat. Technol. 2017, 332, 223–232. [Google Scholar] [CrossRef]
- Keunecke, M.; Weigel, K.; Bewilogua, K.; Cremer, R.; Fuss, H.-G. Preparation and comparison of a-C:H coatings using reactive sputter techniques. Thin Solid Films 2009, 518, 1465–1469. [Google Scholar] [CrossRef]
- Drescher, D.; Koskinen, J.; Scheibe, H.J.; Mensch, A. A model for particle growth in arc deposited amorphous carbon films. Diam. Relat. Mater. 1998, 7, 1375–1380. [Google Scholar] [CrossRef]
- Dalibón, E.L.; Escalada, L.; Simison, S.; Forsich, C.; Heim, D.; Brühl, S.P. Mechanical and corrosion behavior of thick and soft DLC coatings. Surf. Coat. Technol. 2017, 312, 101–109. [Google Scholar] [CrossRef]
- Marchon, B.; Jing, G.; Grannen, K.; Rauch, G.C.; Ager, J.W.; Silva, S.R.P.; Robertson, J. Photoluminescence and Raman spectroscopy in hydrogenated carbon films. IEEE Trans. Magn. 1997, 33, 3148–3150. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X.; Lin, Y.; Wang, F. A ternary phase diagram for amorphous carbon. Carbon N. Y. 2015, 94, 202–213. [Google Scholar] [CrossRef]
- Sun, L.; Guo, P.; Li, X.; Wang, A. Comparative study on structure and wetting properties of diamond-like carbon films by W and Cu doping. Diam. Relat. Mater. 2017, 73, 278–284. [Google Scholar] [CrossRef]
- Gotzmann, G.; Beckmann, J.; Wetzel, C.; Scholz, B.; Herrmann, U.; Neunzehn, J. Electron-beam modification of DLC coatings for biomedical applications. Surf. Coat. Technol. 2017, 311, 248–256. [Google Scholar] [CrossRef]
- Ishige, H.; Akaike, S.; Hayakawa, T.; Hiratsuka, M.; Nakamura, Y. Evaluation of protein adsorption to diamond-like carbon (DLC) and fluorinedoped DLC films using the quartz crystal microbalance method. Dent. Mater. J. 2019, 38, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Lau, S.P.; Sun, Z.; Chen, G.Y.; Li, Y.J.; Tay, B.K.; Chai, J.W. Metal-containing amorphous carbon films for hydrophobic application. Thin Solid Films 2001, 398–399, 110–115. [Google Scholar] [CrossRef]
- Escudeiro, A.; Polcar, T.; Cavaleiro, A. Adsorption of bovine serum albumin on Zr co-sputtered a-C(:H) films: Implication on wear behaviour. J. Mech. Behav. Biomed. Mater. 2014, 39, 316–327. [Google Scholar] [CrossRef][Green Version]
- Berg, J.M.; Eriksson, L.G.T.; Claesson, P.M.; Borve, K.G.N. Three-component langmuir-blodgett films with a controllable degree of polarity. Langmuir 1994, 10, 1225–1234. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Ryan, B.J.; Poduska, K.M. Roughness effects on contact angle measurements. Am. J. Phys. 2008, 76, 1074–1077. [Google Scholar] [CrossRef]
- Hans, R.; Thomas, S.; Garla, B.; Dagli, R.J.; Hans, M.K. Effect of various sugary beverages on salivary ph, flow rate, and oral clearance rate amongst adults. Scientifica 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Ilic, E.; Pardo, A.; Suter, T.; Mischler, S.; Schmutz, P.; Hauert, R. A methodology for characterizing the electrochemical stability of DLC coated interlayers and interfaces. Surf. Coat. Technol. 2019, 375, 402–413. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ohgoe, Y.; Ozeki, K.; Hirakuri, K.; Aoki, H. Dissolution effect and cytotoxicity of diamond-like carbon coatings on orthodontic archwires. J. Mater. Sci. Mater. Med. 2007, 18, 2263–2268. [Google Scholar] [CrossRef]
- Ohgoe, Y.; Kobayashi, S.; Ozeki, K.; Aoki, H.; Nakamori, H.; Hirakuri, K.K.; Miyashita, O. Reduction effect of nickel ion release on a diamond-like carbon film coated onto an orthodontic archwire. Thin Solid Films 2006, 497, 218–222. [Google Scholar] [CrossRef]
- Cempel, M.; Nikel, G. Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- De Souza, R.M.; De Menezes, L.M. Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod. 2008, 78, 345–350. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Terpiłowska, S.; Siwicka-Gieroba, D.; Krzysztof Siwicki, A. Cell viability in normal fibroblasts and liver cancer cells after treatment with iron (III), nickel (II), and their mixture. J. Vet. Res. 2018, 62, 535–542. [Google Scholar] [CrossRef]
- Taira, M.; Toguchi, M.S.; Hamada, Y.; Takahashi, J.; Itou, R.; Toyosawa, S.; Ijyuin, N.; Okazaki, M. Studies on cytotoxic effect of nickel ions on three cultured fibroblasts. J. Mater. Sci. Mater. Med. 2001, 12, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, H.; Gu, Y.; Song, X.; Zhao, J. Carcinogenicity of chromium and chemoprevention: A brief update. OncoTargets Ther. 2017, 10, 4065–4079. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, H.Y.; Ravikumar, T.; Shanmugasundaram, N.; Babu, M.; Unni Nair, B. Cytotoxicity studies of chromium(III) complexes on human dermal fibroblasts. Free Radic. Biol. Med. 2005, 38, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Borsi, L.; Allemanni, G.; Gaggero, B.; Zardi, L. Extracellular pH controls pre-mRNA alternative splicing of tenascin-C in normal, but not in malignantly transformed, cells. Int. J. Cancer 1996, 66, 632–635. [Google Scholar] [CrossRef]
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair Regen. 2017, 25, 260–269. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A.; Van Dyke, T.E. Oral Inflammatory Diseases and Systemic Inflammation: Role of the Macrophage. Front. Immunol. 2012, 3, 118. [Google Scholar] [CrossRef]
- Pereira, T.; Naik, S.; Tamgadge, A. Quantitative evaluation of macrophage expression using CD68 in oral submucous fibrosis: An immunohistochemical study. Ann. Med. Health Sci. Res. 2015, 5, 435. [Google Scholar] [CrossRef]
- Mendes, M. Métodos de Esterilização versus Adesão Celular para Scaffolds Numa Aplicação em Medicina Dentária; Universidade de Coimbra: Coimbra, Portugal, 2017. [Google Scholar]
- Johnson, K.E.; Wilgus, T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Long, G.; Liu, D.; He, X.; Shen, Y.; Zhao, Y.; Hou, X.; Chen, B.; OuYang, W.; Dai, J.; Li, X.; et al. A dual functional collagen scaffold coordinates angiogenesis and inflammation for diabetic wound healing. Biomater. Sci. 2020, 8, 6337–6349. [Google Scholar] [CrossRef]
- Guimarães, J. Princípios de sutura na cavidade oral. Maxillaris 2012, 43, 28–34. [Google Scholar]
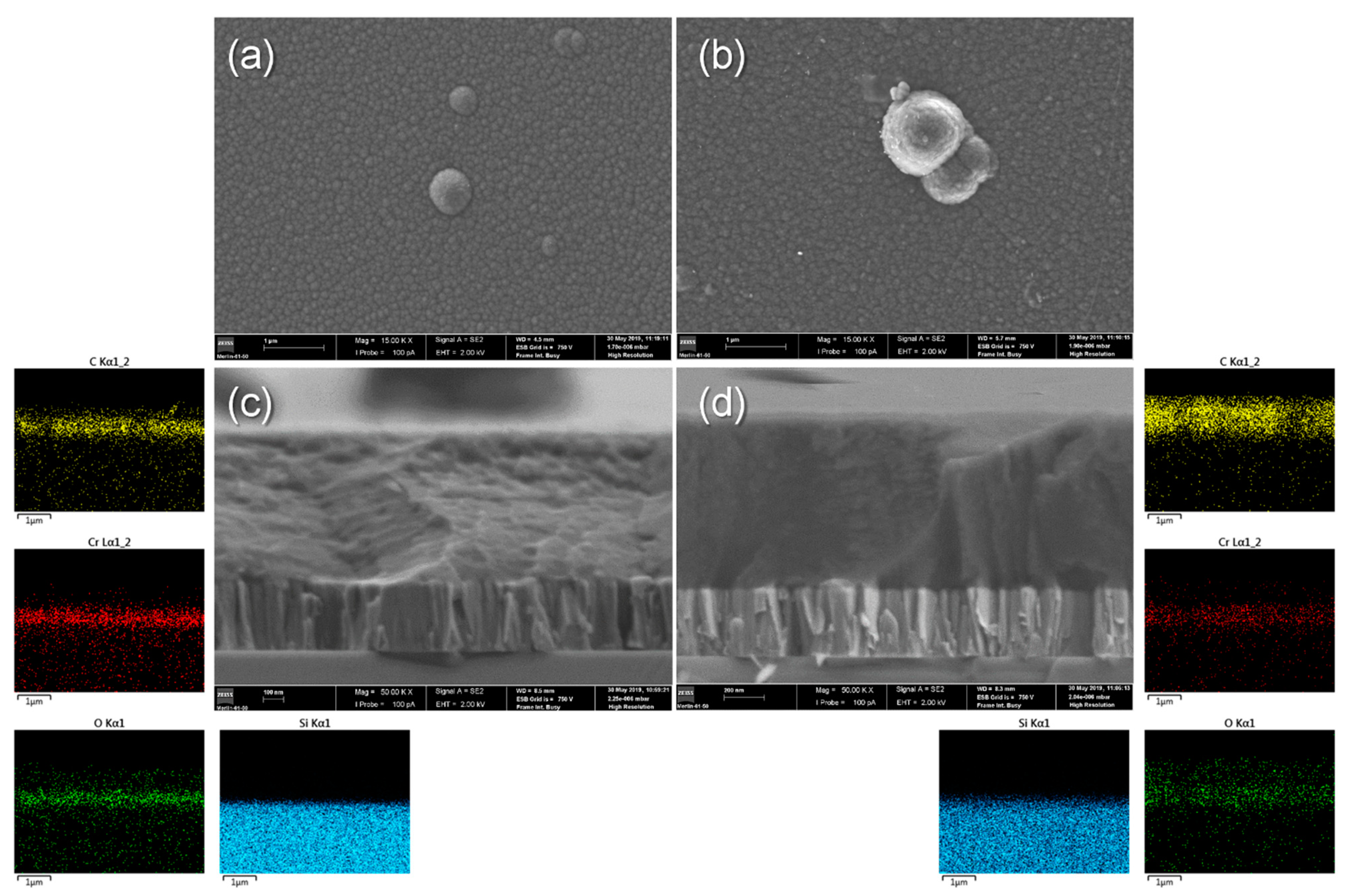
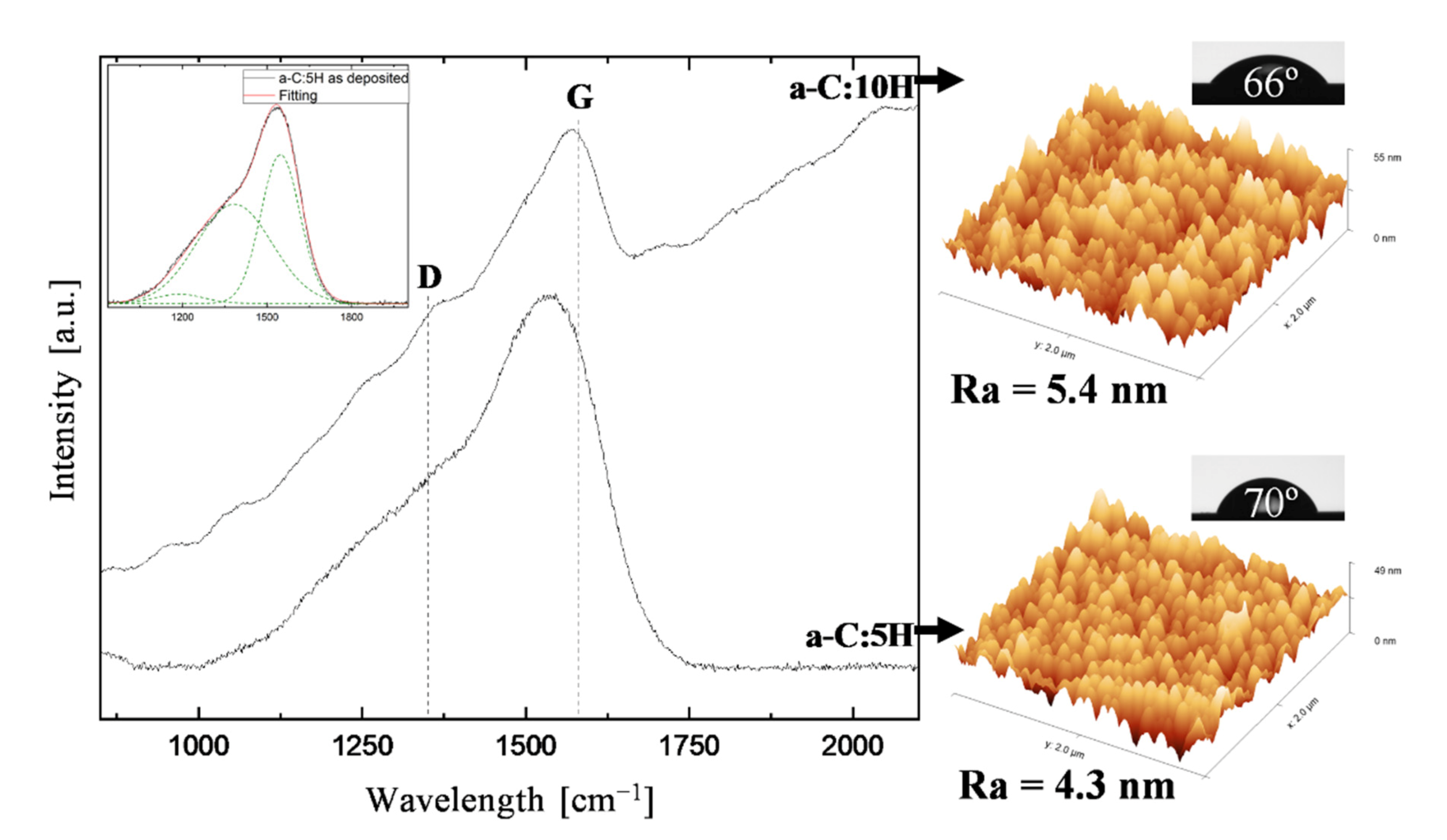
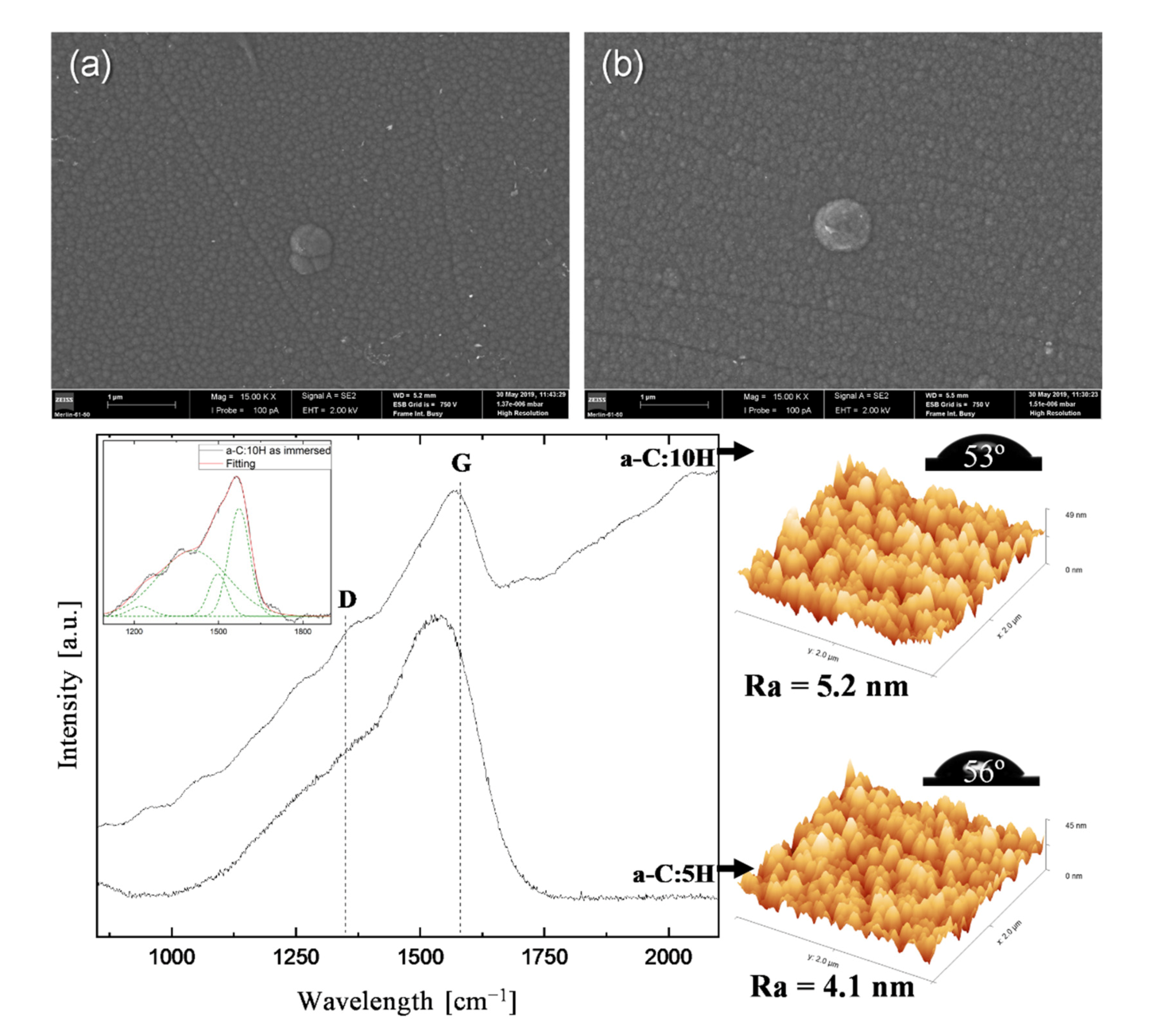

| Concentration [g/L] | ||||||
|---|---|---|---|---|---|---|
| NaCl | KCl | CaCl2·2H2O | NaH2PO4 | Na2S∙9H2O | CO(NH2)2 | HCl (1 M) |
| 0.4 | 0.4 | 0.795 | 0.78 | 0.005 | 1 | until pH = 2.3 |
| -- | - | a-C:5H | a-C:10H | ||
|---|---|---|---|---|---|
| As-dep. | As-imm. | As-dep. | As-imm. | ||
| Flow gas ratio | CH4:Ar | 1:9 | 1:5 | ||
| Thickness [nm] | Cr-based interlayera-C:H external layer | 343 743 | 313 849 | ||
| Elemental composition * [at.%] | C Cr O Ar | 95.2 2.8 0.4 1.6 | 94.6 3.1 0.5 1.8 | 91.3 2.0 6.7 0.0 | 91.5 2.1 6.4 0.0 |
| Roughness [nm] | Ra | 4.3 | 4.1 | 5.4 | 5.2 |
| Contac Angle [°] | 70 ± 1 | 56 ± 2 | 66 ± 1 | 53 ± 2 | |
| Nanohardness [GPa] | HB | 18.2 ± 1.8 | 18.1 ± 1.2 | 7.1 ± 0.3 | 7.0 ± 0.5 |
| Raman bonding configuration | G band [cm−1] ID/IG ratio sp3 bonds [%] | 1547 0.67 39.9 | 1547 0.65 40.2 | 1573 0.59 27.5 | 1573 0.61 27.3 |
| Empirical H content [at. %] | 28.0 ± 1.9 | 28.1 ± 1.3 | 39.9 ± 0.3 | 40.0 ± 0.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fróis, A.; Aleixo, A.S.; Evaristo, M.; Santos, A.C.; Louro, C.S. Can a-C:H-Sputtered Coatings Be Extended to Orthodontics? Coatings 2021, 11, 832. https://doi.org/10.3390/coatings11070832
Fróis A, Aleixo AS, Evaristo M, Santos AC, Louro CS. Can a-C:H-Sputtered Coatings Be Extended to Orthodontics? Coatings. 2021; 11(7):832. https://doi.org/10.3390/coatings11070832
Chicago/Turabian StyleFróis, António, Ana Sofia Aleixo, Manuel Evaristo, Ana Cristina Santos, and Cristina Santos Louro. 2021. "Can a-C:H-Sputtered Coatings Be Extended to Orthodontics?" Coatings 11, no. 7: 832. https://doi.org/10.3390/coatings11070832
APA StyleFróis, A., Aleixo, A. S., Evaristo, M., Santos, A. C., & Louro, C. S. (2021). Can a-C:H-Sputtered Coatings Be Extended to Orthodontics? Coatings, 11(7), 832. https://doi.org/10.3390/coatings11070832







