Investigation of the Long-Term Antibacterial Properties of Titanium by Two-Step Micro-Arc Oxidation Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
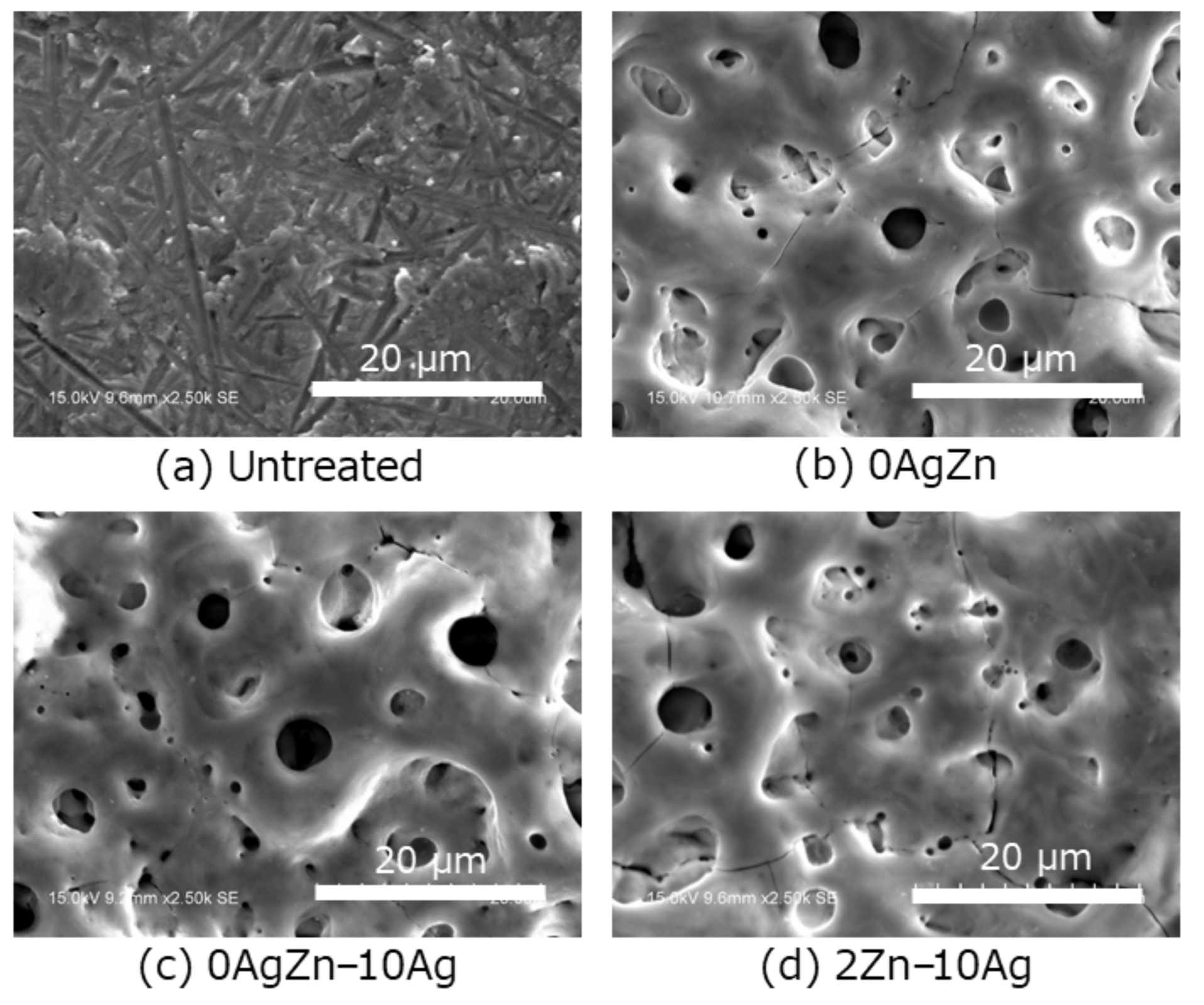
2.2. Surface Characterization
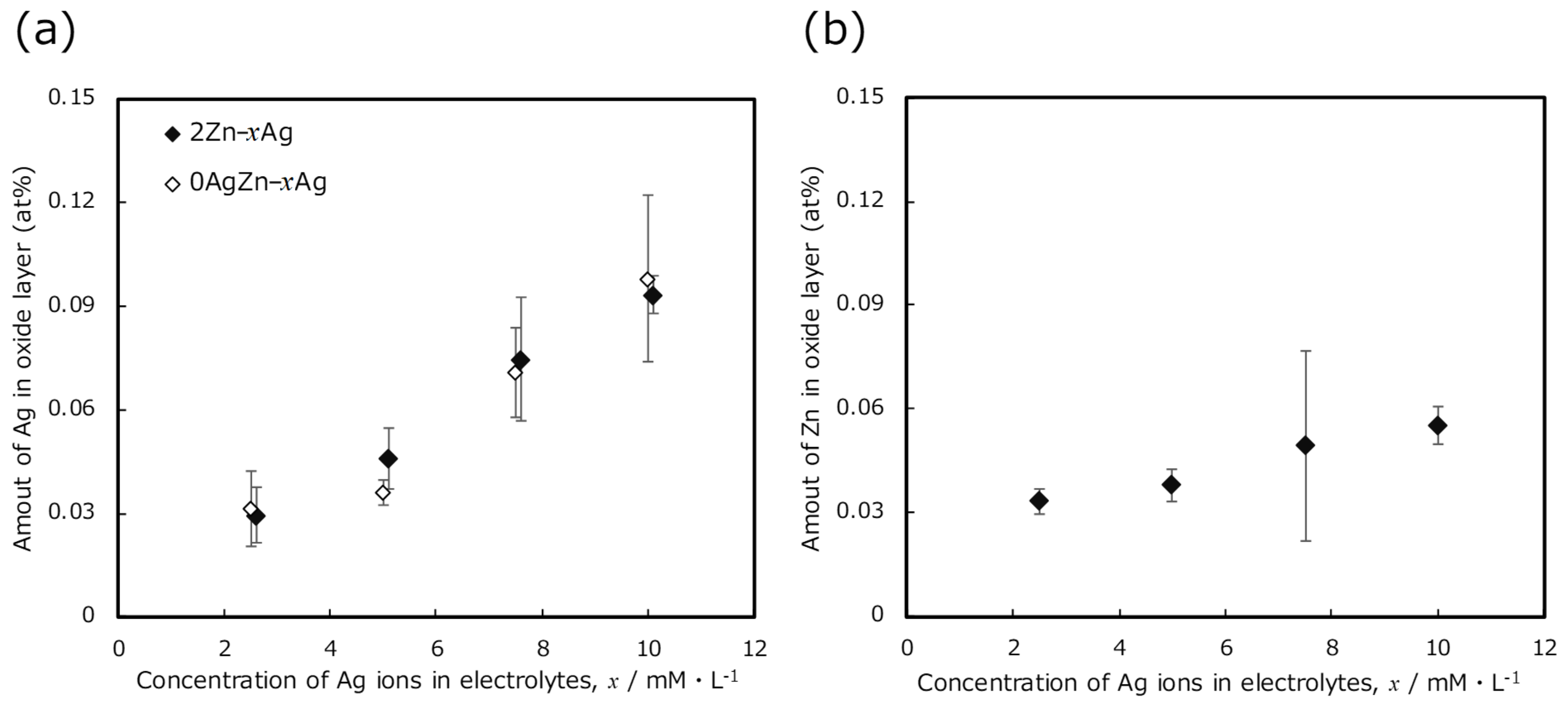
2.3. Metal Ion Release Evaluation
2.4. Evaluation of Antibacterial Properties
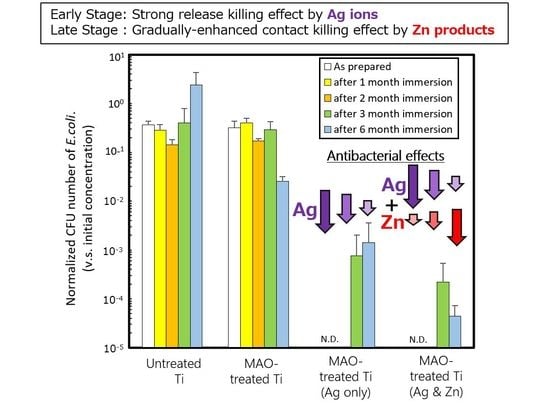
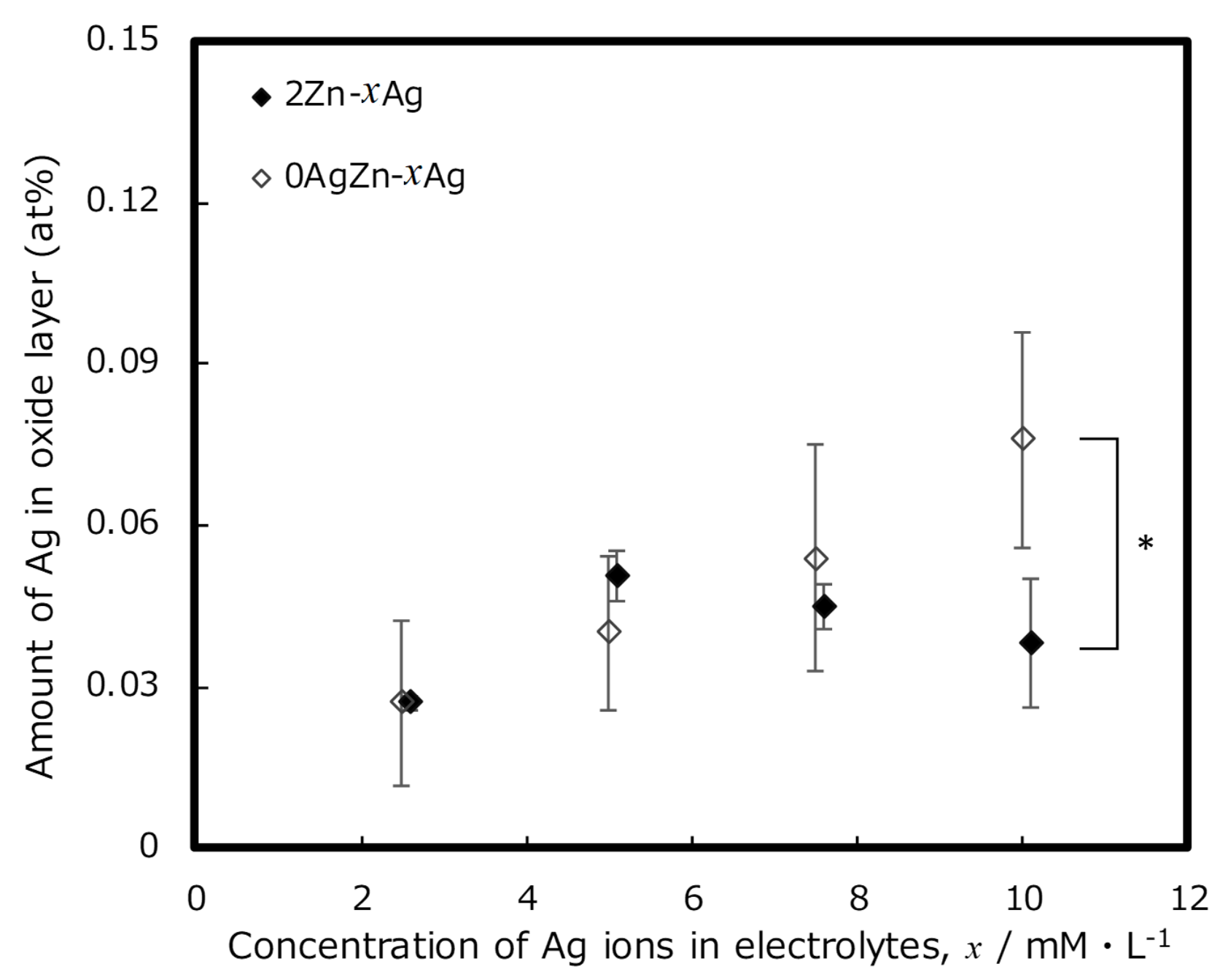
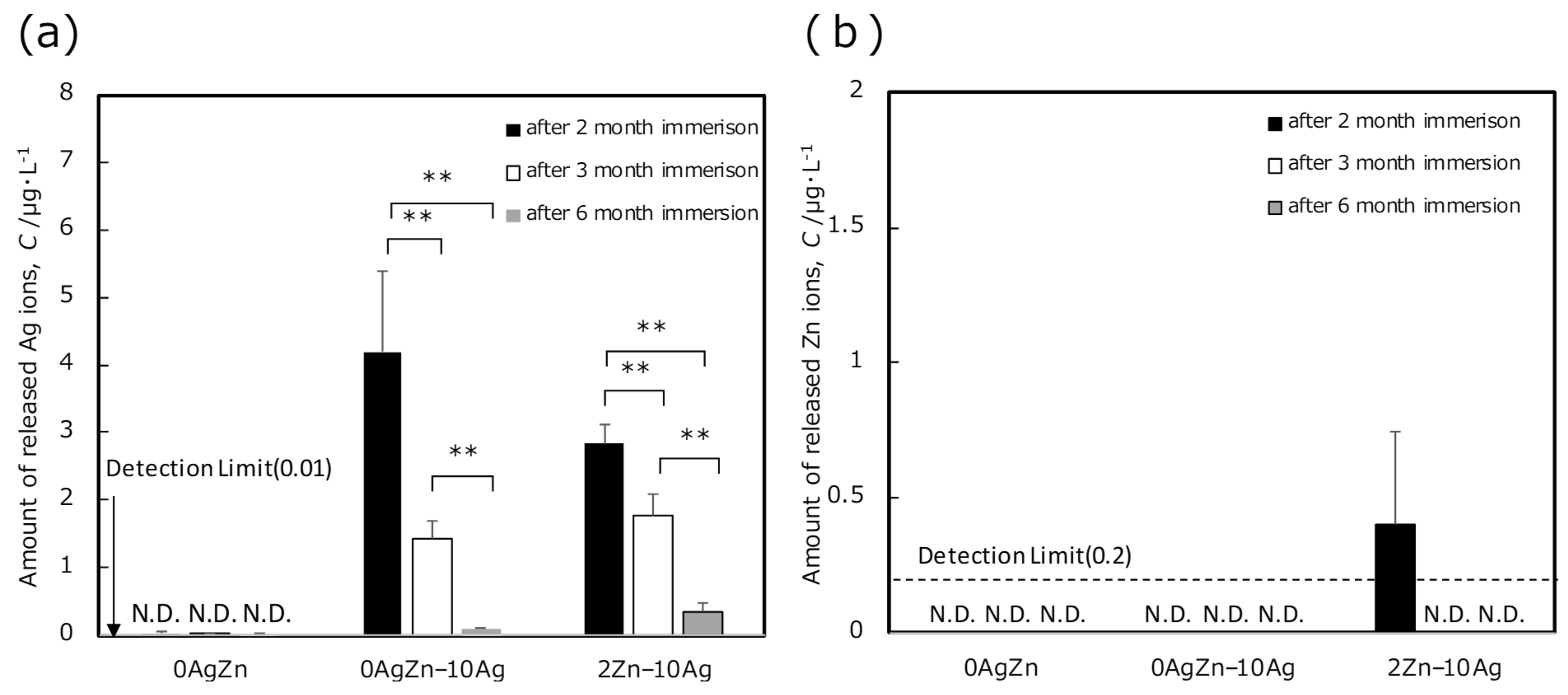
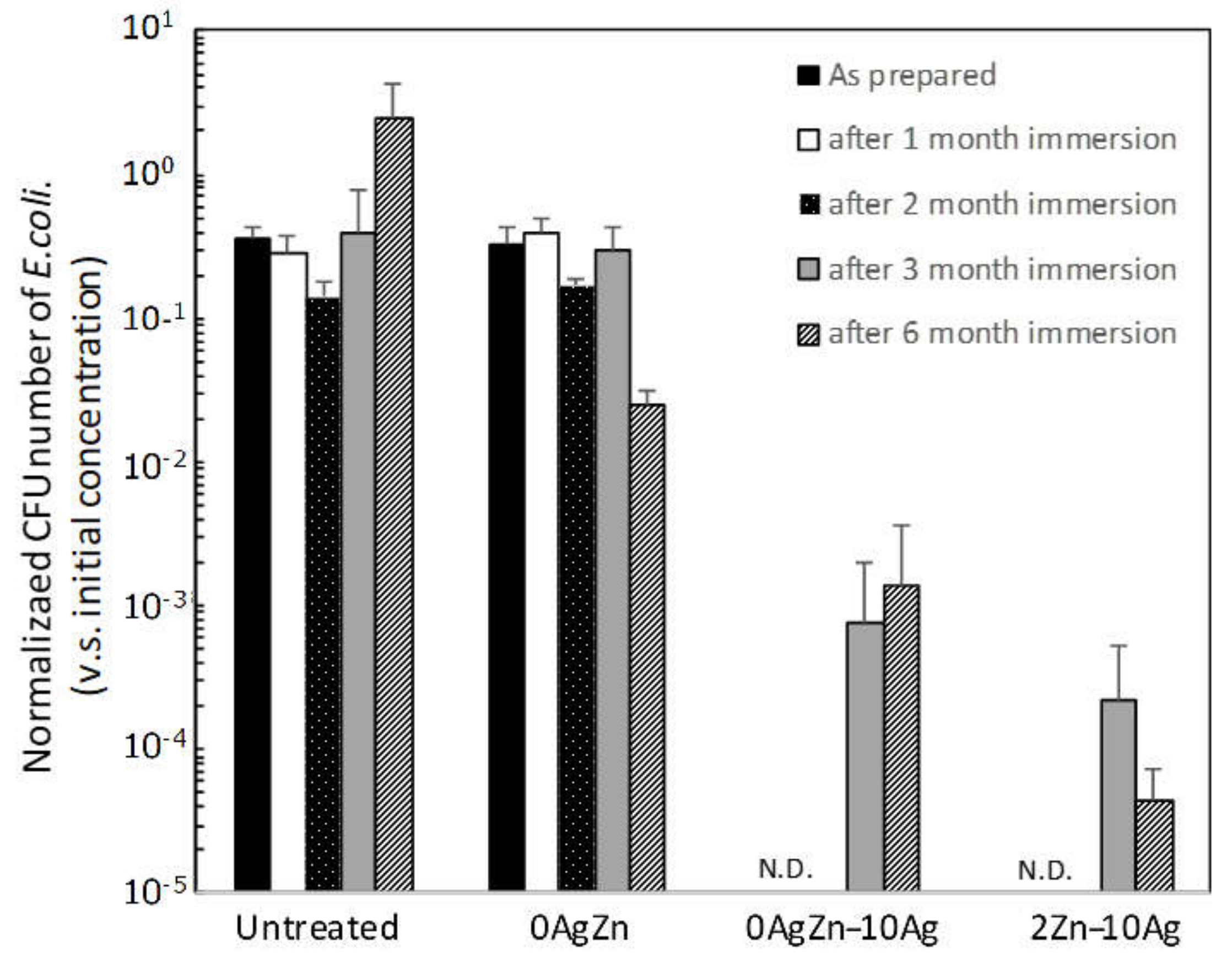
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zubery, Y.; Bichacho, N.; Moses, O.; Tal, H. Immediate loading of modular transitional implants: A histologic and histomorphometric study in dogs. Int. J. Periodontics Restor. Dent. 1999, 19, 343–353. [Google Scholar]
- Hui, E.; Chow, J.; Li, D.; Liu, J.; Wat, P.; Law, H. Immediate provisional for single-tooth implant replacement with Brånemark system: Preliminary report. Clin. Implant Dent. Relat. Res. 2001, 3, 79–86. [Google Scholar] [CrossRef]
- Cochran, D.L.; Buser, D.; Ten Bruggenkate, C.M.; Weingart, D.; Taylor, T.M.; Bernard, J.P.; Peters, F.; Simpson, J.M. The use of reduced healing times on ITI® implants with a sandblasted and acid-etched (SLA) surface: Early results from clinical trials on ITI® SLA implants. Clin. Oral Implant. Res. 2002, 13, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, M.; Pertl, C.; Zhang, K.; Wimmer, G.; Wegscheider, W.A. Immediate loading of single-tooth implants in the anterior maxilla. Preliminary results after one year. Clin. Oral Implant. Res. 2003, 14, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Paquette, D.W.; Brodala, N.; Williams, R.C. Risk factors for endosseous dental implant failure. Dent. Clin. N. Am. 2006, 50, 361–374. [Google Scholar] [CrossRef]
- Dibart, S.; Warbington, M.; Su, M.F.; Skobe, Z. In vitro evaluation of the implant-abutment bacterial seal: The locking taper system. Int. J. Oral Maxillofac. Implant. 2005, 20, 732–737. [Google Scholar]
- Glauser, R.; Schupbach, P.; Gottlow, J.; Hammerle, C.H.F. Periimplant soft tissue barrier at experimental one-piece mini-implants with different surface topography in humans: A light-microscopic overview and histometric analysis. Clin. Implant Dent. Relat. Res. 2005, 7, s44–s51. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, M.; Wallet, S.; Koutouzis, T.; Lundgren, T. Bacterial colonization of the dental implant fixture–abutment interface: An in vitro study. J. Periodontol. 2009, 80, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, E.E.; Patel, J.D.; Marchant, R.E.; Anderson, J.M. Effects of biomaterial surface chemistry on the adhesion and biofilm formation of staphylococcus epidermidis in vitro. J. Biomed. Mater. Res. A 2006, 78, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Nikaido, T.; Ikeda, M.; Imai, S.; Hanada, N.; Tagami, J.; Matin, K. Surface properties of resin composite materials relative to biofilm formation. Dent. Mater. J. 2007, 26, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Meredith, D.O.; Eschbach, L.; Wood, M.A.; Riehle, M.O.; Curtis, A.S.G.; Richards, R.G. Human fibroblast reactions to standard and electropolished titanium and Ti-6Al-7Nb, and electropolished stainless steel. J. Biomed. Mater. Res. A 2005, 75, 541–555. [Google Scholar] [CrossRef]
- Koerner, R.J.; Butterworth, L.A.; Mayer, I.V.; Dasbach, R.; Busscher, H.J. Bacterial adhesion to titanium-oxy-nitride (TiNOX) coatings with different resistivities: A novel approach for the development of biomaterials. Biomaterials 2002, 23, 2835–2840. [Google Scholar] [CrossRef]
- Simonetti, N.; Simonetti, G.; Bougnol, F.; Scalzo, M. Electrochemical Ag+ for preservative use. Appl. Environ. Microbiol. 1992, 58, 3834–3836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassall, M.A.; Santin, M.; Isalberti, C.; Cannas, M.; Denyer, S.P. Adhesion of bacteria to stainless steel and silver-coated orthopedic external fixation pins. J. Biomed. Mater. Res. 1997, 36, 325–330. [Google Scholar] [CrossRef]
- Massè, A.; Bruno, A.; Bosetti, M.; Biasibetti, A.; Cannas, M.; Gallinaro, P. Prevention of pin track infection in external fixation with silver coated pins: Clinical and microbiological results. J. Biomed. Mater. Res. 2000, 53, 600–604. [Google Scholar] [CrossRef]
- Samani, S.; Hossainalipour, S.M.; Tamizifar, M.; Rezaie, H.R. In vitro antibacterial evaluation of sol-gel-derived Zn-, Ag-, and (Zn + Ag)-doped hydroxyapatite coatings against methicillin resistant Staphylococcus aureus. J. Biomed. Mater. Res. A 2013, 101, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Storrie, H.; Stupp, S.I. Cellular response to zinc-containing organoapatite: An in vitro study of proliferation, alkaline phosphatase activity and biomineralization. Biomaterials 2005, 26, 5492–5499. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Suh, J.Y.; Jang, B.C.; Zhu, X.; Ong, J.L.; Kim, K. Effect of hydrothermally treated anodic oxide films on osteoblast attachment and proliferation. Biomaterials 2003, 24, 347–355. [Google Scholar] [CrossRef]
- Son, W.W.; Zhu, X.; Shin, H.I.; Ong, J.L.; Kim, K.H. In vivo histological response to anodized and anodized/hydrothermally treated titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66, 520–525. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.; Kim, H.E.; Koh, Y.H.; Kim, H.W.; Jang, J.H. Formation of hydroxyapatite within porous TiO2 layer by micro-arc oxidation coupled with electrophoretic deposition. Acta Biomater. 2009, 5, 2196–2205. [Google Scholar] [CrossRef]
- Li, Y.; Lee, I.S.; Cui, F.Z.; Choi, S.H. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium. Biomaterials 2008, 29, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.Y.; Tsutsumi, Y.; Doi, Y.; Nomura, N.; Kim, K.H.; Hanawa, T. Enhancement of calcium phosphate formation on zirconium by micro-arc oxidation and chemical treatments. Surf. Coat. Technol. 2011, 205, 4948–4955. [Google Scholar] [CrossRef]
- Song, W.H.; Ryu, H.S.; Hong, S.H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. A 2009, 88, 246–254. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Yamada, R.; Ashida, M.; Chen, P.; Doi, H.; Nozaki, K.; Nagai, A.; Hanawa, T. Investigation of realizing both antibacterial property and osteogenic cell compatibility on titanium surface by simple electrochemical treatment. ACS Biomater. Sci. Eng. 2019, 5, 5623–5630. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M. Antibacterial property and biocompatibility of silver, copper, and zinc in titanium dioxide layers incorporated by one-step micro-arc oxidation: A review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Yamada, R.; Ashida, M.; Doi, H.; Nagai, A.; Hanawa, T. Chemical and biological roles of zinc in a porous titanium dioxide layer formed by micro-arc oxidation. Coatings 2019, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Shimabukuro, M.; Hiji, A.; Manaka, T.; Nozaki, K.; Chen, P.; Ashida, M.; Tsutsumi, Y.; Nagai, A.; Hanawa, T. Time-transient effects of silver and copper in the porous titanium dioxide layer on antibacterial properties. J. Funct. Biomater. 2020, 11, 44. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, H.; Tsutsumi, Y.; Manaka, T.; Chen, P.; Ashida, M.; Ishikawa, K.; Katayama, H.; Hanawa, T. Enhancement of antibacterial property of titanium by two-step micro arc oxidation treatment. Dent. Mater. J 2021, 40, 592–598. [Google Scholar]
- Coventry, M.B. Treatment of infections occurring in total hip surgery. Orthop. Clin. N. Am. 1975, 6, 991–1003. [Google Scholar] [CrossRef]
- Rees, R.T. Infections associated with dental procedures in total hip arthroplasty. J. Bone Jt. Surg. Br. 2000, 82, 307. [Google Scholar] [CrossRef]
- Rubin, R.; Salvati, E.A.; Lewis, R. Infected total hip replacement after dental procedures. Oral. Surg. Oral Med. Oral Pathol. 1976, 41, 18–23. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kobayashi, E.; Hiromoto, S.; Asami, K.; Imai, H.; Hanawa, T. Calcium phosphate formation on titanium by low voltage electrolytic treatments. J. Mater. Sci. Mater. Med. 2007, 18, 797–806. [Google Scholar] [CrossRef] [PubMed]
- JIS Z 2801:2012 Antimicrobial Products—Test for Antimicrobial Activity and Efficacy; Japanese Standards Association: Tokyo, Japan. 2012. Available online: https://www.jisc.go.jp/eng/index.html (accessed on 30 June 2021).
- ISO 22196:2011 Plastics-Measurement of Antibacterial Activity on Plastics Surfaces; International Organization of Standardization: Geneva, Switzerland. 2011. Available online: https://www.iso.org/standard/54431.html (accessed on 30 June 2021).
- Odnevall Wallinder, I.; Leygraf, C. A critical review on corrosion and runoff from zinc and zinc-based alloys in atmospheric environments. Corrosion 2017, 73, 1016–1077. [Google Scholar] [CrossRef]
- Poon, V.K.M.; Burd, A. In vitro cytotoxicity of silver: Implication for clinical wound care. Burns. 2004, 30, 140–147. [Google Scholar] [CrossRef]
- Wataha, J.C.; Lockwood, P.E.; Schedle, A. Effect of silver, copper, mercury, and nickel ions on cellular proliferation during extended, low-dose exposures. J. Biomed. Mater. Res. 2000, 52, 360–364. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honma, R.; Sumita, M. Cytotoxicity evaluation of 43 metal salts using murine fibroblasts and osteoblastic cells. J. Biomed. Mater. Res. 1998, 39, 331–340. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Rosário, F.; Hoet, P.; Santos, C.; Oliveira, H. Death and cell cycle progression are differently conditioned by the AgNP size in osteoblast-like cells. Toxicology 2016, 368–369, 103–115. [Google Scholar] [CrossRef]
- Contreras, R.G.; Vilchis, J.R.S.; Sakagami, H.; Nakamura, Y.; Nakamura, Y.; Hibino, Y.; Nakajima, H.; Shimada, J. Type of cell death induced by seven metals in cultured mouse osteoblastic cells. In Vivo 2010, 24, 507–512. [Google Scholar] [PubMed]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, characterization, and antibacterial activity of silver nanoparticle-decorated graphene oxide nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef] [PubMed]
- De Faria, A.F.; Perreault, F.; Shaulsky, E.; Arias Chavez, L.H.; Elimelech, M. Antimicrobial electrospun biopolymer nanofiber mats functionalized with graphene oxide-silver nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 12751–12759. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsutsumi, H.; Tsutsumi, Y.; Shimabukuro, M.; Manaka, T.; Chen, P.; Ashida, M.; Ishikawa, K.; Katayama, H.; Hanawa, T. Investigation of the Long-Term Antibacterial Properties of Titanium by Two-Step Micro-Arc Oxidation Treatment. Coatings 2021, 11, 798. https://doi.org/10.3390/coatings11070798
Tsutsumi H, Tsutsumi Y, Shimabukuro M, Manaka T, Chen P, Ashida M, Ishikawa K, Katayama H, Hanawa T. Investigation of the Long-Term Antibacterial Properties of Titanium by Two-Step Micro-Arc Oxidation Treatment. Coatings. 2021; 11(7):798. https://doi.org/10.3390/coatings11070798
Chicago/Turabian StyleTsutsumi, Harumi, Yusuke Tsutsumi, Masaya Shimabukuro, Tomoyo Manaka, Peng Chen, Maki Ashida, Kunio Ishikawa, Hideki Katayama, and Takao Hanawa. 2021. "Investigation of the Long-Term Antibacterial Properties of Titanium by Two-Step Micro-Arc Oxidation Treatment" Coatings 11, no. 7: 798. https://doi.org/10.3390/coatings11070798
APA StyleTsutsumi, H., Tsutsumi, Y., Shimabukuro, M., Manaka, T., Chen, P., Ashida, M., Ishikawa, K., Katayama, H., & Hanawa, T. (2021). Investigation of the Long-Term Antibacterial Properties of Titanium by Two-Step Micro-Arc Oxidation Treatment. Coatings, 11(7), 798. https://doi.org/10.3390/coatings11070798