Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Extract Production
2.2. Antioxidant Capacity Assays
2.2.1. DPPH Radical Scavenging Assay
2.2.2. β-Carotene Bleaching Assay
2.3. Total Phenolic Compounds (TPC)
2.4. Whey Protein Film Production
2.5. Film Mechanical Properties and Thickness
2.6. Water Vapor Permeability (WVP)
2.7. Film Optical Properties
2.8. Evaluation of the Lipid Oxidation
Thiobarbituric Reactive Substances Assay (TBARS)
2.9. Statistical Analysis
3. Results
3.1. Antioxidant Assays
3.2. Active Whey Protein Film
3.2.1. Thickness, Mechanical Properties, Optical Properties and WVP
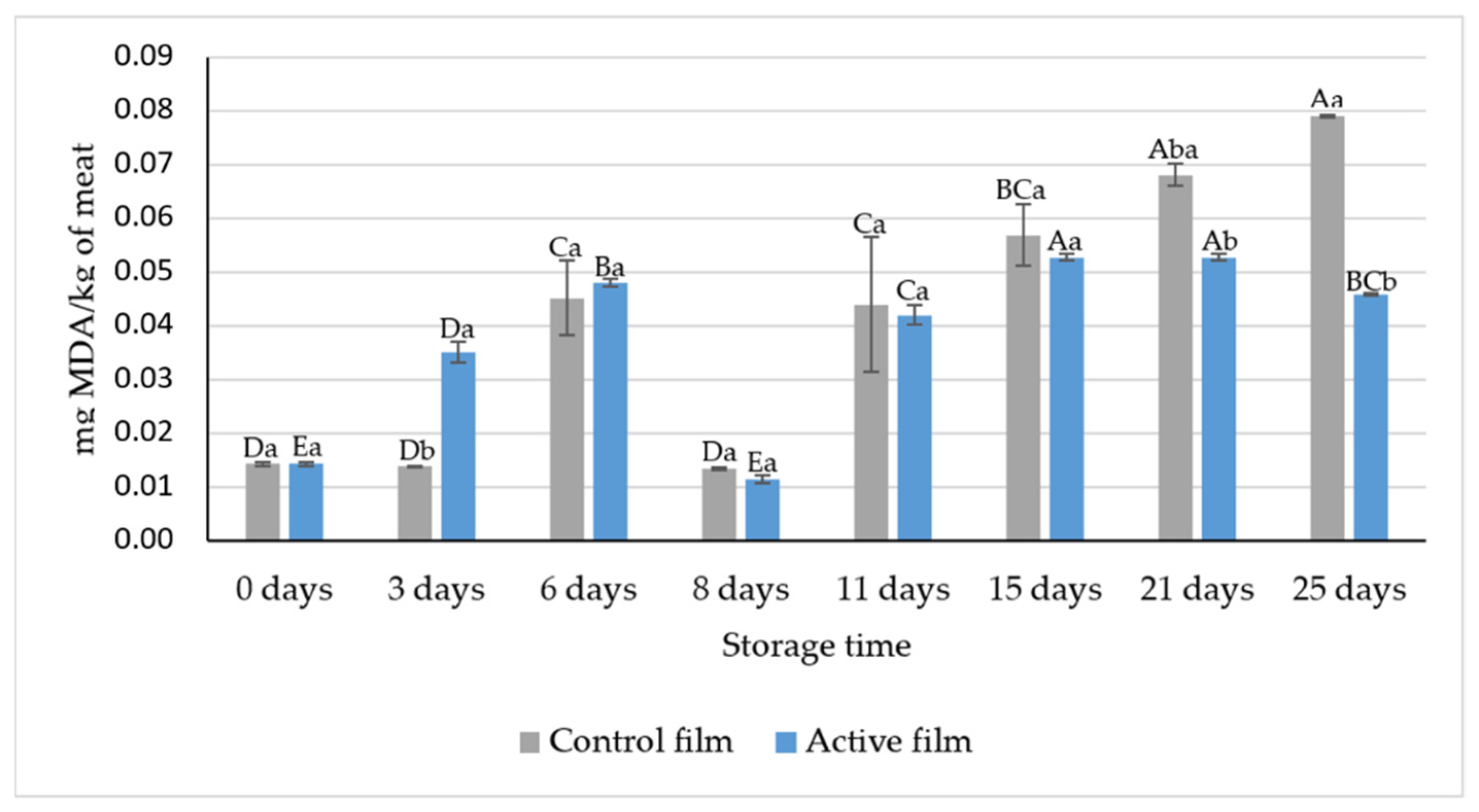
3.2.2. TBARS Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balina, K.; Romagnoli, F.; Blumberga, D. Chemical Composition and Potential Use of Fucus Vesiculosus from Gulf of Riga. Energy Procedia 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Chapman, V.J.; Chapman, D.J. Sea Vegetables (Algae as Food for Man). In Seaweeds and Their Uses; Springer: Dordrecht, The Netherlands, 1980; pp. 62–97. [Google Scholar]
- Agregán, R.; Munekata, P.E.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Nisizawa, K.; Noda, H.; Kikuchi, R.; Watanabe, T. The main seaweed foods in Japan. Hydrobiologia 1987, 5–29. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Chan, C.-C.J.; Cheung, C.-K.P.; Ang, P.O. Comparative Studies on the Effect of Three Drying Methods on the Nutritional Composition of Seaweed Sargassum hemiphyllum (Turn.) C. Ag. J. Agric. Food Chem. 1997, 45, 3056–3059. [Google Scholar]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free. Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Spáčil, Z.; Nováková, L.; Solich, P. Analysis of phenolic compounds by high performance liquid chromatography and ultra performance liquid chromatography. Talanta 2008, 76, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Winter, F.C.; Estes, J.A. A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. J. Chem. Ecol. 1996, 22, 1273–1293. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Giovanelli, E.; Deans, S.; Eaglesham, E. Antibacterial and antioxidant properties of Mediterranean aromatic plants. Ind. Crop. Prod. 1993, 2, 47–50. [Google Scholar] [CrossRef]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimi-crobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
- Russell, G. Spatial and environmental components of evolutionary change: Interactive effects of salinity and temperature onFucus vesiculosus as an example. Helgol. Mar. Res. 1987, 41, 371–376. [Google Scholar] [CrossRef][Green Version]
- Saha, M.; Rempt, M.; Grosser, K.; Pohnert, G.; Weinberger, F. Surface-associated fucoxanthin mediates settlement of bacterial epiphytes on the rockweedFucus vesiculosus. Biofouling 2011, 27, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary fiber and antioxidant capacity in Fucus vesiculosus products. Int. J. Food Sci. Nutr. 2009, 60, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Tuccilli, C.; Baldini, E.; Truppa, E.; D’Auria, B.; De Quattro, D.; Cacciola, G.; Aceti, T.; Cirillo, G.; Faiola, A.; Indigeno, P.; et al. Iodine deficiency in pregnancy: Still a health issue for the women of Cassino city, Italy. Nutrition 2018, 50, 60–65. [Google Scholar] [CrossRef]
- World Health Organizatio. Towards the Elimination of Iodine Deficiency by 2020. Available online: http://www.who.int/nutrition/events/2017-elimination-idd-2020-side-events-23May/en/ (accessed on 15 September 2020).
- Vanderpas, J.B.; Moreno-Reyes, R. Historical aspects of iodine deficiency control. Minerva Med. 2017, 108, 124–135. [Google Scholar] [PubMed]
- Moro, C.; Basile, G. Obesity and medicinal plants. Fitoterapia 2000, 71, S73–S82. [Google Scholar] [CrossRef]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Beuken, E.Z.-V.D.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Food Packaging Technology; Coles, R., McDowell, D., Kirwan, M.J., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2003; ISBN 9780520072817. [Google Scholar]
- Sebti, I.; Delves-Broughton, J.; Coma, V. Physicochemical Properties and Bioactivity of Nisin-Containing Cross-Linked Hy-droxypropylmethylcellulose Films. J. Agric. Food Chem. 2003, 51, 6468–6474. [Google Scholar] [CrossRef]
- Galstyan, V.; Bhandari, M.P.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; De Melo, N.R.; Andrade, M.; Azevedo, G.; Machado, A.V.; Carvalho-Costa, D.; Sanches-Silva, A. Whey protein active films incorporated with a blend of essential oils: Characterization and effectiveness. Packag. Technol. Sci. 2018, 31, 27–40. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; Moura, L.D.A.G.; De Melo, N.R.; Sanches-Silva, A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Gutiérrez, J.B. Ciencia Bromatológica: Principios Generales de los Alimentos; Ediciones Díaz de Santos: Madrid, Spain, 2000. [Google Scholar]
- Márquez-Ruiz, G.; García-Martínez, M.; Holgado, F. Changes and Effects of Dietary Oxidized Lipids in the Gastrointestinal Tract. Lipid Insights 2008, 2, LPI.S904. [Google Scholar] [CrossRef]
- Mariutti, L.R.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ribeiro-Santos, R.; Guerra, M.; Sanches-Silva, A. Evaluation of the Oxidative Status of Salami Packaged with an Active Whey Protein Film. Foods 2019, 8, 387. [Google Scholar] [CrossRef]
- Castro, F.V.R.; Andrade, M.A.; Sanches Silva, A.; Vaz, M.F.; Vilarinho, F. The Contribution of a Whey Protein Film Incorpo-rated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo salar L.). Foods 2019, 8, 327. [Google Scholar] [CrossRef]
- Vilarinho, F.; Andrade, M.; Buonocore, G.G.; Stanzione, M.; Vaz, M.F.; Silva, A.S. Monitoring lipid oxidation in a processed meat product packaged with nanocomposite poly(lactic acid) film. Eur. Polym. J. 2018, 98, 362–367. [Google Scholar] [CrossRef]
- Brody, A.L.; Strupinsky, E.R.; Kline, L.R. Ative Packaging for Food Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Silva, A.S.; Freire, J.M.C.; Franz, R.; Losada, P.P. Mass transport studies of model migrants within dry foodstuffs. J. Cereal Sci. 2008, 48, 662–669. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.; Verma, A.K.; Barba, F.J.; Singh, V.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ribeiro-Santos, R.; Bonito, M.C.C.; Saraiva, M.; Sanches-Silva, A. Characterization of rosemary and thyme extracts for incorporation into a whey protein based film. LWT 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Miller, H.E. A simplified method for the evaluation of antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant Capacities of Phlorotan-nins Extracted from the Brown Algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef] [PubMed]
- ASTM—America Society Standard Testing and Materials. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; D882-12; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Sarantópoulos, C.; Oliveira, L.; Padula, M.; Coltro, L.; Alves, R.; Garcia, E. Embalagens Plásticas Flexíveis- Principais Polímeros e Avaliação de Propriedades; ITAL: Campinas, Brazil, 2002. [Google Scholar]
- Ferreira, A.R.; Torres, C.A.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.; Alves, V.D.; Coelhoso, I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016, 147, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Rosmini, M.R.; Perlo, F.; A Pérez-Alvarez, J.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA test by an extractive method applied to ’paté’. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Müller, J. Effect of convective-, vacuum- and freeze drying on sorption behaviour and bioactive compounds of lemon balm (Melissa officinalis L.). J. Appl. Res. Med. Aromat. Plants 2014, 1, 59–69. [Google Scholar] [CrossRef]
- Dos Santos, M.A.Z.; Alicieo, T.V.R.; Pereira, C.M.P.; Ramis-Ramos, G.; Mendonça, C.R.B.; Dos Santos, M.A.Z. Profile of Bioactive Compounds in Avocado Pulp Oil: Influence of the Drying Processes and Extraction Methods. J. Am. Oil Chem. Soc. 2013, 91, 19–27. [Google Scholar] [CrossRef]
- Fernandes, L.M.; Guimarães, J.T.; Silva, R.; Rocha, R.S.; Coutinho, N.M.; Balthazar, C.F.; Calvalcanti, R.N.; Piler, C.W.; Pimentel, T.C.; Neto, R.P.; et al. Whey protein films added with galactooligosaccharide and xylooligosaccharide. Food Hydrocoll. 2020, 104, 105755. [Google Scholar] [CrossRef]
- Gounga, M.E.; Xu, S.-Y.; Wang, Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. J. Food Eng. 2007, 83, 521–530. [Google Scholar] [CrossRef]
- Abdalrazeq, M.; Giosafatto, C.V.L.; Esposito, M.; Fenderico, M.; Di Pierro, P.; Porta, R. Glycerol-Plasticized Films Obtained from Whey Proteins Denatured at Alkaline pH. Coatings 2019, 9, 322. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crop. Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Rodrigues, P.F.; Lopes, A.; Silva, R.J.; Caldeira, J.; Duarte, M.P.; Fernandes, F.B.; Coelhoso, I.M.; et al. Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil. Coatings 2019, 9, 700. [Google Scholar] [CrossRef]
- Guerreiro, A.; Andrade, M.A.; Menezes, C.; Vilarinho, F.; Dias, E. Antioxidant and Cytoprotective Properties of Cyanobacteria: Potential for Biotechnological Applications. Toxins 2020, 12, 548. [Google Scholar] [CrossRef]
- Miller, D.D. Food Chemistry: A Laboratory Manual, 2nd ed.; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Osawa, C.C.; De Felício, P.E.; Gonçalves, L.A.G. Teste de TBA aplicado a carnes e derivados: Métodos tradicionais, modificados e alternativos. Química Nova 2005, 28, 655–663. [Google Scholar] [CrossRef]
- Ross, C.F.; Smith, D.M. Use of Volatiles as Indicators of Lipid Oxidation in Muscle Foods. Compr. Rev. Food Sci. Food Saf. 2006, 5, 18–25. [Google Scholar] [CrossRef]
- Dasgupta, A.; Klein, K. Methods for Measuring Oxidative Stress in the Laboratory. In Antioxidants in Food, Vitamins and Supplements; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 19–40. [Google Scholar]
- Shahbazi, Y.; Shavisi, N. Chitosan Coatings Containing Mentha spicata Essential Oil and Zinc Oxide Nanoparticle for Shelf Life Extension of Rainbow Trout Fillets. J. Aquat. Food Prod. Technol. 2018, 27, 1–12. [Google Scholar] [CrossRef]
- Khezrian, A.; Shahbazi, Y. Application of nanocompostie chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel’s meat. Int. J. Biol. Macromol. 2018, 106, 1146–1158. [Google Scholar] [CrossRef]
- Reboleira, J.; Adão, P.; Guerreiro, S.F.C.; Dias, J.R.; Ganhão, R.; Mendes, S.; Andrade, M.; Vilarinho, F.; Sanches-Silva, A.; Mateus, A.; et al. Poultry Shelf-Life Enhancing Potential of Nanofibers and Nanoparticles Containing Porphyra dioica Extracts. Coatings 2020, 10, 315. [Google Scholar] [CrossRef]
- Gonçalves, C.F.; Schmatz, D.A.; Uebel, L.D.S.; Kuntzler, S.G.; Costa, J.A.V.; Zimmer, K.R.; de Morais, M.G. Microalgae Biopeptides Applied in Nanofibers for the Development of Active Packaging. Polímeros 2017, 27, 290–297. [Google Scholar] [CrossRef]

| Solvent | Yield (%) | IP (%) | β-Carotene Assay (AAC) | TPC Assay (mg PGE/g of Extract) | ||||
|---|---|---|---|---|---|---|---|---|
| FD | D | FD | D | FD | F | FD | D | |
| 100% H20 | 18.63 | 26.66 | 62.51 ± 0.17 Ca | 0.00 Cb | 842.67 ± 5.55 Aa | 575.10 ± 10.64 Bb | 26.18 ± 0.11 Ea | 19.03 ± 0.10 Cb |
| 25% Ethanol | 20.02 | 21.38 | 66.69 ± 0.33 Ba | 17.88 ± 0.79 Bb | 356.07 ± 6.42 Da | 250.76 ± 5.31 Db | 27.05 ± 0.31 Da | 16.18 ± 0.09 Db |
| 50% Ethanol | 13.09 | 22.30 | 57.00 ± 0.47 Da | 16.33 ± 0.86 Bb | 564.59 ± 11.06 Ca | 383.24 ± 7.07 Cb | 31.12 ± 0.26 Ca | 12.42 ± 0.11 Eb |
| 75% Ethanol | 4.71 | 18.77 | 78.26 ± 0.21 Aa | 57.44 ± 0.99 Ab | 636.22 ± 5.16 Ba | 611.91 ± 8.09 Ab | 45.21 ± 0.21 Ba | 21.06 ± 0.12 Bb |
| 100% Ethanol | 2.71 | 3.86 | 44.65 ± 0.79 Ea | 0.86 ± 0.03 Cb | 237.82 ± 5.03 Ea | 125.36 ± 11.90 Eb | 194.65 ± 0.87 Aa | 70.97 ± 0.74 Ab |
| Parameter | Control Film | Active Film |
|---|---|---|
| Thickness (μm) | 248.8 ± 6.7 sig ** | 280.7 ± 7.5 sig |
| Tensile strength (MPa) | 0.184 ± 0.022 ns | 0.203 ± 0.055 ns |
| Elongation at break (%) | 11.7 ± 2.5 ns | 8.7 ± 1.9 ns |
| Elastic Modulus (MPa) | 1.468 ± 0.617 ns | 3.364 ± 1.300 ns |
| WVP (10−10 mol/m·s·Pa) | 1.178 ± 0.21 | Sample collapsed |
| L* (lightness) | 84.51 ± 0.35 sig | 60.72 ± 0.19 sig |
| a* [red (+a) or green (−a)] | −2.89 ± 0.02 sig | −1.33 ± 0.02 sig |
| b* [yellow (+b) or blue (−b)] | 13.14 ± 0.66 sig | 23.50 ± 0.03 sig |
| Hue* | 102.42 ± 0.67 sig | 93.25 ± 0.05 sig |
| Chromaticity | 13.46 ± 0.64 sig | 23.54 ± 0.03 sig |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhão, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. https://doi.org/10.3390/coatings11020229
Andrade MA, Barbosa CH, Souza VGL, Coelhoso IM, Reboleira J, Bernardino S, Ganhão R, Mendes S, Fernando AL, Vilarinho F, et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings. 2021; 11(2):229. https://doi.org/10.3390/coatings11020229
Chicago/Turabian StyleAndrade, Mariana A., Cássia H. Barbosa, Victor G. L. Souza, Isabel M. Coelhoso, João Reboleira, Susana Bernardino, Rui Ganhão, Susana Mendes, Ana Luísa Fernando, Fernanda Vilarinho, and et al. 2021. "Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat" Coatings 11, no. 2: 229. https://doi.org/10.3390/coatings11020229
APA StyleAndrade, M. A., Barbosa, C. H., Souza, V. G. L., Coelhoso, I. M., Reboleira, J., Bernardino, S., Ganhão, R., Mendes, S., Fernando, A. L., Vilarinho, F., Sanches Silva, A., & Ramos, F. (2021). Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings, 11(2), 229. https://doi.org/10.3390/coatings11020229












