A Supervised Machine-Learning Prediction of Textile’s Antimicrobial Capacity Coated with Nanomaterials
Abstract
:1. Introduction
1.1. Nanotechnology for Functional Textiles
- (1)
- production of reactive oxygen species (ROS) and oxygen-free radicals in the microbial cells [36];
- (2)
- (3)
- (4)
- (a)
- There are several methods used for deposition of NMs on textiles, depending on the type of NM and fibre. The NMs are either incorporated into the fibres during extrusion or attached to the surface during finishing. Physical methods such as plasma pre-treatment, irradiation, or ultrasound, and chemical methods, for example, chemical reduction in aqueous media, electrochemical reduction, sonochemistry synthesis, and chemically assisted radiation, are the most widely employed techniques for nano-textile production [45,46]. The finishing methods can be dip-pad technique [47], pad-dry-cure processes [48], spraying [49], foam finishing [50], grafting [51], layer by layer assembly [52], dip coating [53], impregnation process [54], exhaustion method [55], microwave-assisted deposition [56], ultrasonic agitation [57], ultrasound irradiation [58], vapor deposition [59], chemical reduction deposition [60], drop-coating [61], sputtering [62], sol-gel [63], and electroless deposition [64].
- (b)
- Textiles are exposed to a range of circumstances during their lifespan, including washing, heat, and dry cleaning; in some instances, it is important to know how well the textile can preserve its antimicrobial capacities. Therefore, the effect of washing conditions on the antimicrobial capacity of nano-textiles can be determined by (1) various durability tests (laboratory scale, industrial and domestic washing machines), (2) type of detergent where applicable, (3) amount of water used (tap or distilled), (4) number of washing cycles, and (5) temperature [65].
1.2. Machine Learning
2. Materials and Methods
2.1. Approach
2.2. Data Collection
2.3. Data Extraction
2.4. Data Pre-Processing
2.5. Regression Models
2.6. Model Validation
2.7. Important Attribute Analysis
3. Results
3.1. Data Pre-Processing
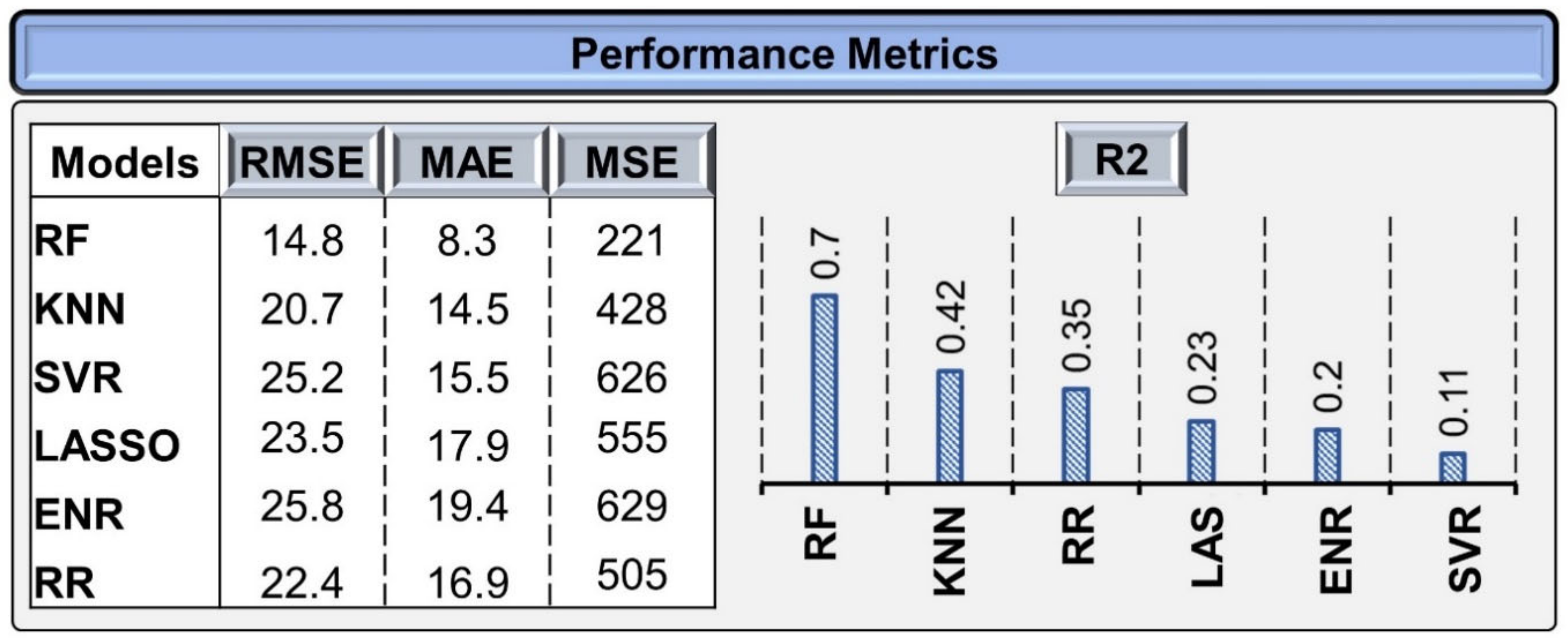
3.1.1. Validation of the Models
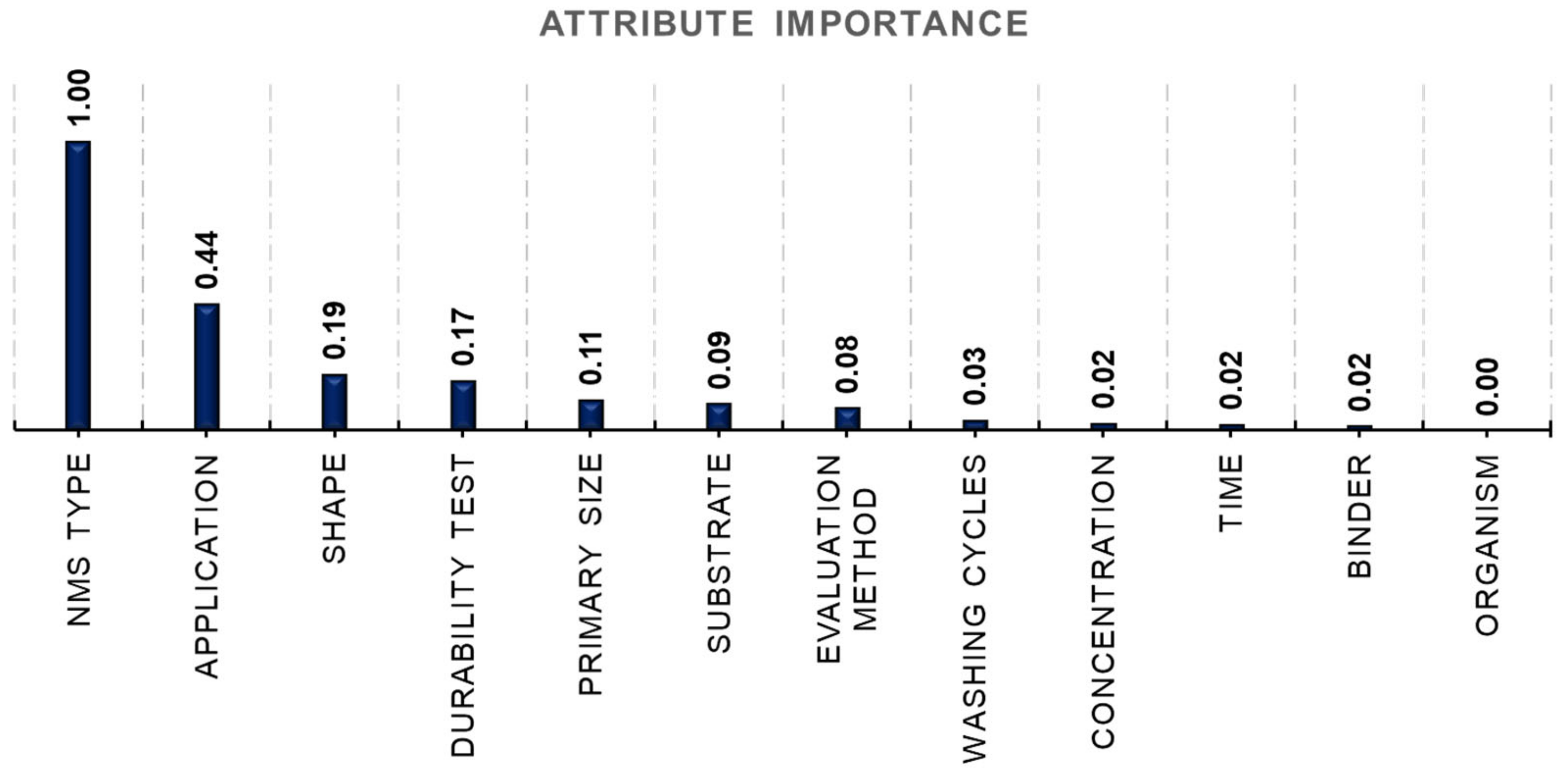
3.1.2. Important Attribute Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMs | Nanomaterials |
| P-chem | Physicochemical |
| MO | Metal oxide |
| M | Metal |
| ROS | Reactive oxygen species |
| ML | Machine learning |
| AI | Artificial intelligence |
| MAE | Mean absolute error |
| MSE | Mean square error |
| RMSE | Root mean square error |
| R2 | R-squared |
| ZOI | Zone of inhibition |
| LASSO | Least absolute shrinkage and selection operator |
| RR | Ridge regression |
| ENR | Elastic net regression |
| RF | Random forest |
| KNN | k-nearest neighbours |
| SVR | Supervised vector regression |
| nm | Nanometer |
| µg | Microgram |
| ml | Mililiter |
| PET | Polyethylene terephthalate |
| MIC | Minimum inhibitory concentration |
| Microb-Eff | Microb effect |
| MBC | Minimum bactericidal concentration |
References
- Bagherzadeh, R.; Montazer, M.; Latifi, M.; Sheikhzadeh, M.; Sattari, M. Evaluation of comfort properties of polyester knitted spacer fabrics finished with water repellent and antimicrobial agents. Fibers Polym. 2007, 8, 386–392. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial textile: Recent developments and functional perspective. Polym. Bull. 2021, 1–25. [Google Scholar] [CrossRef]
- Van Herreweghen, F.; Amberg, C.; Marques, R.; Callewaert, C. Biological and Chemical Processes that Lead to Textile Malodour Development. Microorganisms 2020, 8, 1709. [Google Scholar] [CrossRef]
- Ren, X.; Kou, L.; Kocer, H.B.; Zhu, C.; Worley, S.; Broughton, R.; Huang, T. Antimicrobial coating of an N-halamine biocidal monomer on cotton fibers via admicellar polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 711–716. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M.; Shahsavan, S. A new method to stabilize nanoparticles on textile surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2009, 345, 202–210. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses 2008, 70, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Ramadhan, A.; Hegedus, E. Survivability of vancomycin resistant enterococci and fitness cost of vancomycin resistance acquisition. J. Clin. Pathol. 2005, 58, 744–746. [Google Scholar] [CrossRef] [Green Version]
- Arena, M.P.; Capozzi, V.; Spano, G.; Fiocco, D. The potential of lactic acid bacteria to colonize biotic and abiotic surfaces and the investigation of their interactions and mechanisms. Appl. Microbiol. Biotechnol. 2017, 101, 2641–2657. [Google Scholar] [CrossRef]
- Yadav, S.; Kapley, A. Antibiotic resistance: Global health crisis and metagenomics. Biotechnol. Rep. 2021, 29, e00604. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Bartels, V. Handbook of Medical Textiles; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Windler, L.; Height, M.; Nowack, B. Comparative evaluation of antimicrobials for textile applications. Environ. Int. 2013, 53, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Padhye, R. Antimicrobial Finishes for Textiles; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Höfer, D. Antimicrobial textiles–evaluation of their effectiveness and safety. Biofunct. Text. Ski. 2006, 33, 42–50. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645. [Google Scholar] [CrossRef] [Green Version]
- Mohanraj, V.; Chen, Y. Nanoparticles–A review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Andra, S.; Balu, S.k.; Jeevanandam, J.; Muthalagu, M. Emerging nanomaterials for antibacterial textile fabrication. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1355–1382. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [Green Version]
- Radetić, M. Functionalization of textile materials with silver nanoparticles. J. Mater. Sci. 2013, 48, 95–107. [Google Scholar] [CrossRef]
- Radetić, M. Functionalization of textile materials with TiO2 nanoparticles. J. Photochem. Photobiol. C Photochem. Rev. 2013, 16, 62–76. [Google Scholar] [CrossRef]
- Singh, G.; Joyce, E.M.; Beddow, J.; Mason, T.J. Evaluation of antibacterial activity of ZnO nanoparticles coated sonochemically onto textile fabrics. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 106–120. [Google Scholar]
- Shahidi, S.; Jamali, A.; Dalal Sharifi, S.; Ghomi, H. In-situ synthesis of CuO nanoparticles on cotton fabrics using spark discharge method to fabricate antibacterial textile. J. Nat. Fibers 2018, 15, 870–881. [Google Scholar] [CrossRef]
- El-Gabry, L.K.; Allam, O.G.; Hakeim, O.A. Surface functionalization of viscose and polyester fabrics toward antibacterial and coloration properties. Carbohydr. Polym. 2013, 92, 353–359. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Gouda, M. Nano-zirconium oxide and nano-silver oxide/cotton gauze fabrics for antimicrobial and wound healing acceleration. J. Ind. Text. 2012, 41, 222–240. [Google Scholar] [CrossRef]
- Ganesan, R.; Prabu, H.G. Synthesis of gold nanoparticles using herbal Acorus calamus rhizome extract and coating on cotton fabric for antibacterial and UV blocking applications. Arab. J. Chem. 2019, 12, 2166–2174. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-S.; Woo, J.-Y.; Ahn, C.-B.; Je, J.-Y. Chitosan–hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef]
- Mohamed, A.L.; El-Naggar, M.E.; Hassabo, A.G. Preparation of hybrid nanoparticles to enhance the electrical conductivity and performance properties of cotton fabrics. J. Mater. Res. Technol. 2021, 12, 542–554. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Ren, N.; Wang, Y.; Wang, Y.; Yu, F. Biosynthesis of silver oxide nanoparticles and their photocatalytic and antimicrobial activity evaluation for wound healing applications in nursing care. J. Photochem. Photobiol. B Biol. 2019, 199, 111593. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Popescu, T.; Matei, C.O.; Vlaicu, I.D.; Tivig, I.; Kuncser, A.C.; Stefan, M.; Ghica, D.; Miclea, L.C.; Savopol, T.; Culita, D.C. Influence of surfactant-tailored Mn-doped ZnO nanoparticles on ROS production and DNA damage induced in murine fibroblast cells. Sci. Rep. 2020, 10, 18062. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Hovhannisyan, A.; Gevorgyan, V.; Ananyan, M.; Trchounian, A. Antibacterial effects of iron oxide (Fe3O4) nanoparticles: Distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl. Microbiol. Biotechnol. 2019, 103, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Baylan, S.; Park, B.-W.; Richter, G.; Sitti, M. Hydrophobic pinning with copper nanowhiskers leads to bactericidal properties. PLoS ONE 2017, 12, e0175428. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Front. Microbiol. 2021, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Javed, B.; Ikram, M.; Farooq, F.; Sultana, T.; Mashwani, Z.-U.-R.; Raja, N.I. Biogenesis of silver nanoparticles to treat cancer, diabetes, and microbial infections: A mechanistic overview. Appl. Microbiol. Biotechnol. 2021, 105, 2261–2275. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Wang, G.; Hou, H.; Wang, S.; Yan, C.; Liu, Y. Exploring the interaction of silver nanoparticles with lysozyme: Binding behaviors and kinetics. Colloids Surf. B Biointerfaces 2017, 157, 138–145. [Google Scholar] [CrossRef]
- Baek, Y.-W.; An, Y.-J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total. Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Silver nanoparticles: Synthesis and therapeutic applications. J. Biomed. Nanotechnol. 2007, 3, 301–316. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, A.; Perkas, N.; Amiryan, G.; Meyer, M.; Zimmermann, W.; Gedanken, A. Coating silver nanoparticles on poly (methyl methacrylate) chips and spheres via ultrasound irradiation. J. Appl. Polym. Sci. 2007, 104, 2868–2876. [Google Scholar] [CrossRef]
- Montazer, M.; Shamei, A.; Alimohammadi, F. Synthesizing and stabilizing silver nanoparticles on polyamide fabric using silver-ammonia/PVP/UVC. Prog. Org. Coat. 2012, 75, 379–385. [Google Scholar] [CrossRef]
- Hady, A.; Farouk, A.; Sharaf, S. Flame retardancy and UV protection of cotton based fabrics using nano ZnO and polycarboxylic acid. Carbohydr. Polym. 2013, 92, 400–406. [Google Scholar] [CrossRef]
- Sasaki, K.; Tenjimbayashi, M.; Manabe, K.; Shiratori, S. Asymmetric superhydrophobic/superhydrophilic cotton fabrics designed by spraying polymer and nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 651–659. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, J.H.; Choi, C.-H. Cotton Fabrics with Single-Faced Superhydrophobicity. Langmuir 2012, 28, 17426–17434. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G. Durable and regenerable antimicrobial textile materials prepared by a continuous grafting process. J. Appl. Polym. Sci. 2002, 84, 1592–1599. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Ruch, D. Layer-by-layer deposition of a TiO2-filled intumescent coating and its effect on the flame retardancy of polyamide and polyester fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 1–10. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Blanco, I.; Piccolella, S.; Pacifico, S. Use of the Sol–Gel Method for the Preparation of Coatings of Titanium Substrates with Hydroxyapatite for Biomedical Application. Coatings 2020, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Alongi, J.; Tata, J.; Carosio, F.; Rosace, G.; Frache, A.; Camino, G. A comparative analysis of nanoparticle adsorption as fire-protection approach for fabrics. Polymers 2015, 7, 47–68. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.; Jarrais, B.; Pereira, C.; Morgado, J.; Freire, C.; Pereira, M. Functionalization of textiles with multi-walled carbon nanotubes by a novel dyeing-like process. J. Mater. Sci. 2012, 47, 5263–5275. [Google Scholar] [CrossRef]
- Wang, C.; Guo, R.; Lan, J.; Jiang, S.; Zhang, Z. Microwave-assisted synthesis of silver/reduced graphene oxide on cotton fabric. Cellulose 2017, 24, 4045–4055. [Google Scholar] [CrossRef]
- Petkova, P.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Simultaneous sonochemical-enzymatic coating of medical textiles with antibacterial ZnO nanoparticles. Ultrasonics Sonochem. 2016, 29, 244–250. [Google Scholar] [CrossRef]
- Sun, G.; Hong, K.H. Photo-induced antimicrobial and decontaminating agents: Recent progresses in polymer and textile applications. Text. Res. J. 2013, 83, 532–542. [Google Scholar] [CrossRef]
- Aminayi, P.; Abidi, N. Imparting super hydro/oleophobic properties to cotton fabric by means of molecular and nanoparticles vapor deposition methods. Appl. Surf. Sci. 2013, 287, 223–231. [Google Scholar] [CrossRef]
- Kazemi, N.M.; Sandalnia, M. In situ production and deposition of bismuth oxide nanoparticles on cotton fabric. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 1217–1223. [Google Scholar] [CrossRef]
- Acuautla, M.; Bernardini, S.; Gallais, L.; Fiorido, T.; Patout, L.; Bendahan, M. Ozone flexible sensors fabricated by photolithography and laser ablation processes based on ZnO nanoparticles. Sens. Actuators B Chem. 2014, 203, 602–611. [Google Scholar] [CrossRef]
- Irfan, M.; Perero, S.; Miola, M.; Maina, G.; Ferri, A.; Ferraris, M.; Balagna, C. Antimicrobial functionalization of cotton fabric with silver nanoclusters/silica composite coating via RF co-sputtering technique. Cellulose 2017, 24, 2331–2345. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in sol-gel technology for the coatings of fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef] [Green Version]
- Frunza, L.; Preda, N.; Matei, E.; Frunza, S.; Ganea, C.P.; Vlaicu, A.M.; Diamandescu, L.; Dorogan, A. Synthetic fabrics coated with zinc oxide nanoparticles by electroless deposition: Structural characterization and wetting properties. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1427–1437. [Google Scholar] [CrossRef]
- Salat, M.; Petkova, P.; Hoyo, J.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Durable antimicrobial cotton textiles coated sonochemically with ZnO nanoparticles embedded in an in-situ enzymatically generated bioadhesive. Carbohydr. Polym. 2018, 189, 198–203. [Google Scholar] [PubMed]
- Joiner, B.G. Determining Antimicrobial Efficacy and Biocompatibility of Treated Articles Using Standard Test Methods; ACS Publications: Washington, DC, USA, 2001. [Google Scholar]
- Zille, A.; Almeida, L.; Amorim, T.; Carneiro, N.; Esteves, M.F.; Silva, C.J.; Souto, A.P. Application of nanotechnology in antimicrobial finishing of biomedical textiles. Mater. Res. Express 2014, 1, 032003. [Google Scholar] [CrossRef] [Green Version]
- Sacha, G.M.; Varona, P. Artificial intelligence in nanotechnology. Nanotechnology 2013, 24, 452002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furxhi, I.; Murphy, F.; Mullins, M.; Arvanitis, A.; Poland, C.A. Practices and Trends of Machine Learning Application in Nanotoxicology. Nanomaterials 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.V.; Ansari, M.H.D.; Rosenkranz, D.; Maharjan, R.S.; Kriegel, F.L.; Gandhi, K.; Kanase, A.; Singh, R.; Laux, P.; Luch, A. Artificial Intelligence and Machine Learning in Computational Nanotoxicology: Unlocking and Empowering Nanomedicine. Adv. Healthc. Mater. 2020, 9, 1901862. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.C.; Zidar, J.; Ho, J.; Wang, Y.; Lee, K.K.; Zheng, J.; Sullivan, M.B.; You, X.; Kriegel, R. Assessment of several machine learning methods towards reliable prediction of hormone receptor binding affinity. Chem. Data Collect. 2017, 9, 114–124. [Google Scholar] [CrossRef]
- Mirzaei, M.; Furxhi, I.; Murphy, F.; Mullins, M. A Machine Learning Tool to Predict the Antibacterial Capacity of Nanoparticles. Nanomaterials 2021, 11, 1774. [Google Scholar] [CrossRef]
- Gal, M.S.; Rubinfeld, D.L. Data standardization. NYUL Rev. 2019, 94, 737. [Google Scholar] [CrossRef]
- Potdar, K.; Pardawala, T.S.; Pai, C. A Comparative Study of Categorical Variable Encoding Techniques for Neural Network Classifiers. Int. J. Comput. Appl. 2017, 175, 7–9. [Google Scholar] [CrossRef]
- Jakulin, A. Machine Learning Based on Attribute Interactions; Univerza v Ljubljani: Ljubljana, Slovenija, 2005. [Google Scholar]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Nanomaterials for Functional Textiles and Fibers. Nanoscale Res. Lett. 2015, 10, 501. [Google Scholar] [CrossRef] [Green Version]
- Djurišić, A.B.; Leung, Y.H.; Ng, A.M.C.; Xu, X.Y.; Lee, P.K.H.; Degger, N.; Wu, R.S.S. Toxicity of Metal Oxide Nanoparticles: Mechanisms, Characterization, and Avoiding Experimental Artefacts. Small 2015, 11, 26–44. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M. Recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug-resistant (MDR) bacteria. Expert Rev. Anti-Infect. Ther. 2019, 17, 419–428. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Perkas, N.; Nitzan, Y.; Gedanken, A.; Banin, E. ZnO nanoparticle-coated surfaces inhibit bacterial biofilm formation and increase antibiotic susceptibility. Rsc Adv. 2012, 2, 2314–2321. [Google Scholar] [CrossRef]
- Meier, L.; Van De Geer, S.; Bühlmann, P. The group lasso for logistic regression. J. R. Stat. Soc. Ser. B Stat. Methodol. 2008, 70, 53–71. [Google Scholar] [CrossRef] [Green Version]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- McDonald, G.C. Ridge regression. WIREs Comput. Stat. 2009, 1, 93–100. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Menze, B.H.; Kelm, B.M.; Masuch, R.; Himmelreich, U.; Bachert, P.; Petrich, W.; Hamprecht, F.A. A comparison of random forest and its Gini importance with standard chemometric methods for the feature selection and classification of spectral data. BMC Bioinform. 2009, 10, 213. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.V.; Rosenkranz, D.; Ansari, M.H.D.; Singh, R.; Kanase, A.; Singh, S.P.; Johnston, B.; Tentschert, J.; Laux, P.; Luch, A. Artificial Intelligence and Machine Learning Empower Advanced Biomedical Material Design to Toxicity Prediction. Adv. Intell. Syst. 2020, 2, 2000084. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Dehuri, S.; Jagadev, A.K.; Mishra, S. Chapter 1—Introduction. In EEG Brain Signal Classification for Epileptic Seizure Disorder Detection; Satapathy, S.K., Dehuri, S., Jagadev, A.K., Mishra, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Kohli, S.; Godwin, G.T.; Urolagin, S. Sales Prediction Using Linear and KNN Regression. In Advances in Machine Learning and Computational Intelligence; Springer: Berlin/Heidelberg, Germany, 2021; pp. 321–329. [Google Scholar]
- Refaeilzadeh, P.; Tang, L.; Liu, H. Cross-validation. Encycl. Database Syst. 2009, 5, 532–538. [Google Scholar]
- Picard, R.R.; Cook, R.D. Cross-validation of regression models. J. Am. Stat. Assoc. 1984, 79, 575–583. [Google Scholar] [CrossRef]
- Geisser, S. The predictive sample reuse method with applications. J. Am. Stat. Assoc. 1975, 70, 320–328. [Google Scholar] [CrossRef]
- Jung, Y.; Hu, J. A K-fold Averaging Cross-validation Procedure. J. Nonparametr. Stat. 2015, 27, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobl, C.; Boulesteix, A.-L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinform. 2007, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.; Burger, K.; Kaliyaperumal, R.; Roos, M.; Santos, L.O.B.D.S. Making FAIR Easy with FAIR Tools: From Creolization to Convergence. Data Intell. 2020, 2, 87–95. [Google Scholar] [CrossRef]
- OECD. OECD Series on Testing and Assessment, Guidance Document on the Validation of (Quantitative) Structure-Activity Relationship [(Q)SAR] Models. Available online: https://www.oecd-ilibrary.org/environment/guidance-document-on-the-validation-of-quantitative-structure-activity-relationship-q-sar-models_9789264085442-en (accessed on 8 November 2021).
- Speck-Planche, A.; Kleandrova, V.V.; Luan, F.; Cordeiro, M.N.D. Computational modeling in nanomedicine: Prediction of multiple antibacterial profiles of nanoparticles using a quantitative structure–activity relationship perturbation model. Nanomedicine 2015, 10, 193–204. [Google Scholar] [CrossRef]
- Daly, C.A.; Hernandez, R. Learning from the Machine: Uncovering Sustainable Nanoparticle Design Rules. J. Phys. Chem. C 2020, 124, 13409–13420. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Limpiteeprakan, P.; Babel, S.; Nowack, B. Durability of nano-enhanced textiles through the life cycle: Releases from landfilling after washing. Environ. Sci. Nano 2016, 3, 375–387. [Google Scholar] [CrossRef]
- Nadi, A.; Jamoudi Sbai, S.; Bentiss, A.; Belaiche, M.; Briche, S.; Gmouh, S. Application of Fe3O4 nanoparticles on cotton fabrics by the Pad-Dry-Cure process for the elaboration of magnetic and conductive textiles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 827, 012021. [Google Scholar] [CrossRef]
- Inam, M.; Foster, J.C.; Gao, J.; Hong, Y.; Du, J.; Dove, A.P.; O’Reilly, R.K. Size and shape affects the antimicrobial activity of quaternized nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Simon-Deckers, A.; Loo, S.; Mayne-L’hermite, M.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Carrière, M. Size-, Composition- and Shape-Dependent Toxicological Impact of Metal Oxide Nanoparticles and Carbon Nanotubes toward Bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Gouda, S. Application of nano technology in textile engineering: An overview. J. Eng. Technol. Res. 2013, 5, 104–111. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. Fabrication of cotton fabrics with durable antibacterial activities finishing by Ag nanoparticles. Text. Res. J. 2019, 89, 867–880. [Google Scholar] [CrossRef]
- Zille, A.; Fernandes, M.M.; Francesko, A.; Tzanov, T.; Fernandes, M.; Oliveira, F.R.; Almeida, L.; Amorim, T.; Carneiro, N.; Esteves, M.F.; et al. Size and Aging Effects on Antimicrobial Efficiency of Silver Nanoparticles Coated on Polyamide Fabrics Activated by Atmospheric DBD Plasma. ACS Appl. Mater. Interfaces 2015, 7, 13731–13744. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.j.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Periolatto, M.; Ferrero, F.; Vineis, C.; Varesano, A.; Gozzelino, G. Novel antimicrobial agents and processes for textile applications. In Antibacterial Agents; InTechOpen: Rijeka, Croatia, 2017; p. 17. [Google Scholar] [CrossRef] [Green Version]
- Klemenčič, D.; Tomšič, B.; Kovač, F.; Žerjav, M.; Simončič, A.; Simončič, B. Preparation of novel fibre–silica–Ag composites: The influence of fibre structure on sorption capacity and antimicrobial activity. J. Mater. Sci. 2014, 49, 3785–3794. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Sustainable Use of Nanomaterials in Textiles and Their Environmental Impact. Materials 2020, 13, 5134. [Google Scholar] [CrossRef]
- Top Market Reports. Antimicrobial Textile Market. Available online: https://www.marketsandmarkets.com/Market-Reports/antimicrobial-textile-market-254286152.html (accessed on 8 November 2021).
- Jeon, H.-Y. Woven Fabrics; BoD—Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Hebeish, A.; El-Naggar, M.; Fouda, M.M.; Ramadan, M.; Al-Deyab, S.S.; El-Rafie, M. Highly effective antibacterial textiles containing green synthesized silver nanoparticles. Carbohydr. Polym. 2011, 86, 936–940. [Google Scholar] [CrossRef]
- Van der Heijden, I.M.; Levin, A.S.; De Pedri, E.H.; Fung, L.; Rossi, F.; Duboc, G.; Barone, A.A.; Costa, S.F. Comparison of disc diffusion, Etest and broth microdilution for testing susceptibility of carbapenem-resistant P. aeruginosa to polymyxins. Ann. Clin. Microbiol. Antimicrob. 2007, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Joyce, M.; Woods, C.W. Antibacterial susceptibility testing in the clinical laboratory. Infect. Dis. Clin. N. Am. 2004, 18, 401–434. [Google Scholar] [CrossRef]
- Limpiteeprakan, P.; Babel, S.; Lohwacharin, J.; Takizawa, S. Release of silver nanoparticles from fabrics during the course of sequential washing. Environ. Sci. Pollut. Res. 2016, 23, 22810–22818. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Rimmele, E.; Wichser, A.; Erni, R.; Height, M.; Nowack, B. Presence of nanoparticles in wash water from conventional silver and nano-silver textiles. ACS Nano 2014, 8, 7208–7219. [Google Scholar] [CrossRef]
- Bajpai, V.; Dey, A.; Ghosh, S.; Bajpai, S.; Jha, M.K. Quantification of bacterial adherence on different textile fabrics. Int. Biodeterior. Biodegrad. 2011, 65, 1169–1174. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Timm, D.A.; Merry, J. Bacterial adherence on fabrics by a radioisotope labeling method. Text. Res. J. 1987, 57, 20–28. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Furxhi, I.; Varesano, A.; Salman, H.; Mirzaei, M.; Battistello, V.; Tomasoni, I.T.; Blosi, M. Data Shepherding in Nanotechnology: An Antimicrobial Functionality Data Capture Template. Coatings 2021, 11, 1486. [Google Scholar] [CrossRef]

| Dataset I | Dataset II | ||||
|---|---|---|---|---|---|
| Variables | Type | Min-Max, Mean, or Label | Data Transformation | Min-Max, Mean, or Label | |
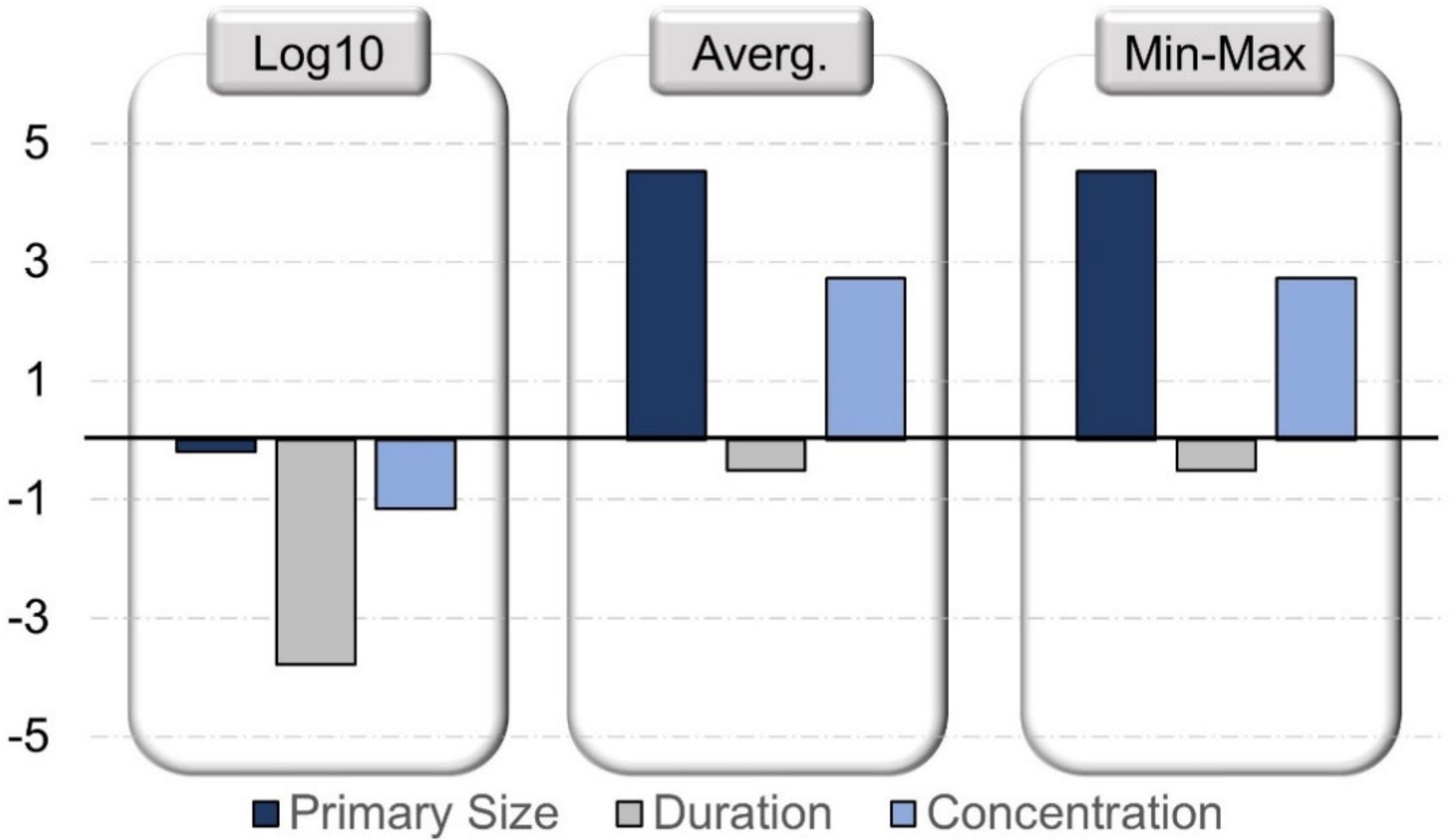
| P-chem properties of NM | Primary size | Numeric | 0.65–500, 42.95 (nm), NaN | Selected, normalized | 0.187–2.69, 1.45 |
| NM type | Nominal | CuO, Ag, ZnO, Au, Ce-ZnO, ZrO2, Fe3O4, Mn, Co, CuO-TiO2, TiO2, SA-TSA, ZnO-Cs, Cs, SiO2-Ag-Cu, Ce, Fe3o4-ZnO | Selected and simplified | Ag, Au, Ce, Ce-ZnO, CS, CuO, CuO-TiO2, SiO2-Ag-Cu, TiO2, ZnO, others | |
| Shape | Ellipsoidal, spherical, crystalline, rod, wire, irregular, rectangular, hexagonal, others | Hexagonal, spherical, rod, others | |||
| Binder | Binary | Yes or no | Selected | Yes or no | |
| Exposure conditions Experimental study design | Concentration | Numeric | 0–33.25, 2.92 (µg/mL), NaN | Selected, Normalized | −4.6–1.5, 2.9 |
| Duration | 0–52, 22.79 (h), NaN | −3.6–4.62, 2.86 | |||
| Substrate | Nominal | Cotton, polyethylene terephthalate (PET), viscose, cotton-polyester, polyamide, polyester, wool, silk, wool polyester, bamboo, denim | Selected and simplified | Bamboo, polyester, cotton, others | |
| Washing cycles | Numeric | 0–50, 9, NaN | Selected | 0–50, 9 | |
| Durability test | Nominal | Industrial, domestic, and commercial washing machines; agitation; boiling; bath; NaN | Selected and simplified | Agitation, domestic, domestic and commercial, industrial, others | |
| Detergent | Nonionic, standard, commercial, water, anionic, commercial, NaN | Eliminated due to high NaN | - | ||
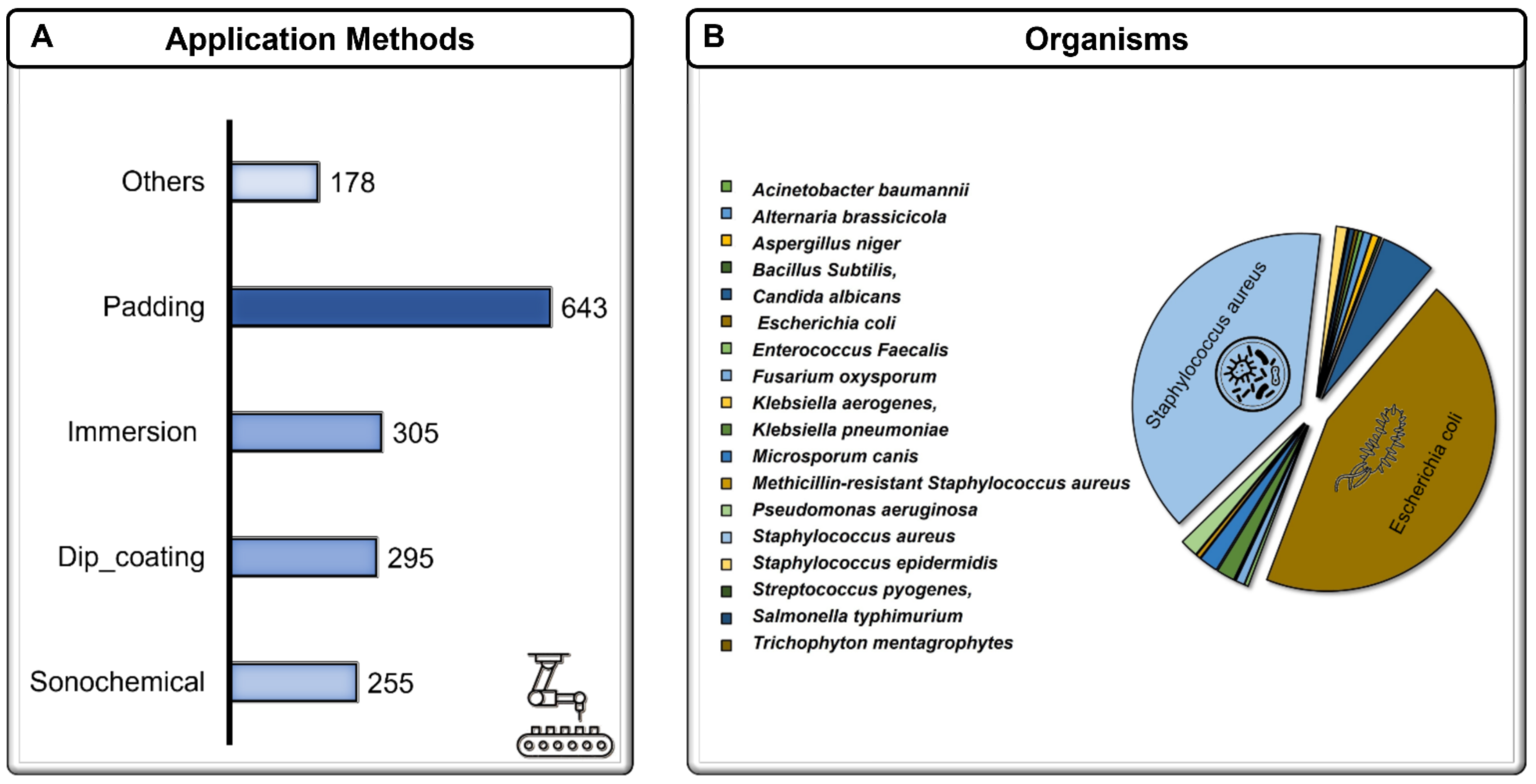
| Application method | Sonochemical, dip coating, exhaust, immersion, grafting, sorption, padding, spraying, blade coating | Selected and simplified | Dip coating, immersion, padding, sonochemical, others | ||
| Evaluation standard | ISO_20743, AATCC_100, AATCC_147, GB_T_20944_AATCC_61, AATCC_147_ISO_20645, ISO_20645, ASTME_2149, AATCC_30, ASTM_2180 | Eliminated | - | ||
| Evaluation method | Agar diffusion, dynamic shake flask | Selected | Agar diffusion, dynamic shake flask | ||
| Washing temperature | Numeric | 20–95, 40, NaN | Eliminated due to high NaN | - | |
| Method of synthesis of NM | Nominal | Biosynthesis, degradation, dip-coated temp-curated ultrasound, ex situ synthesis, in situ biosynthesis, in situ deposition (alkalization and deposition), in situ microwave irradiation, in situ reductions, in situ sol–gel immersion, in situ ultrasound irradiation, ionic gelation, photochemical reduction, reduction of cellulose in viscose, reverse micellar cores, sol–gel, sonochemical, ultrasound irradiation, wet chemical method | - | ||
| Bacteria | Organisms | Acinetobacter baumannii, Alternaria brassicicola, Aspergillus niger, Bacillus Subtilis, Candida albicans, Escherichia coli, Enterococcus Faecalis, Fusarium oxysporum, Klebsiella aerogenes, Klebsiella pneumoniae, Microsporum canis, Methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Salmonella typhimurium, Trichophyton mentagrophytes | Selected and simplified | Gram-negative, Gram-positive, fungus | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzaei, M.; Furxhi, I.; Murphy, F.; Mullins, M. A Supervised Machine-Learning Prediction of Textile’s Antimicrobial Capacity Coated with Nanomaterials. Coatings 2021, 11, 1532. https://doi.org/10.3390/coatings11121532
Mirzaei M, Furxhi I, Murphy F, Mullins M. A Supervised Machine-Learning Prediction of Textile’s Antimicrobial Capacity Coated with Nanomaterials. Coatings. 2021; 11(12):1532. https://doi.org/10.3390/coatings11121532
Chicago/Turabian StyleMirzaei, Mahsa, Irini Furxhi, Finbarr Murphy, and Martin Mullins. 2021. "A Supervised Machine-Learning Prediction of Textile’s Antimicrobial Capacity Coated with Nanomaterials" Coatings 11, no. 12: 1532. https://doi.org/10.3390/coatings11121532
APA StyleMirzaei, M., Furxhi, I., Murphy, F., & Mullins, M. (2021). A Supervised Machine-Learning Prediction of Textile’s Antimicrobial Capacity Coated with Nanomaterials. Coatings, 11(12), 1532. https://doi.org/10.3390/coatings11121532







