Oxidation Behavior and Microstructural Evolution of ZrB2–35MoSi2–10Al Composite Coating
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
- -
- The penetrative cracks and pores in the coating were not found before or after oxidation at 1400 °C in air;
- -
- The oxidized ZrB2–35MoSi2–10Al coating mainly consists of c-ZrO2, m-ZrO2, and small amounts of mullite and zircon;
- -
- The thermal degradation process of the ZrB2–35MoSi2–10Al coating can be divided into three temperature stages;
- -
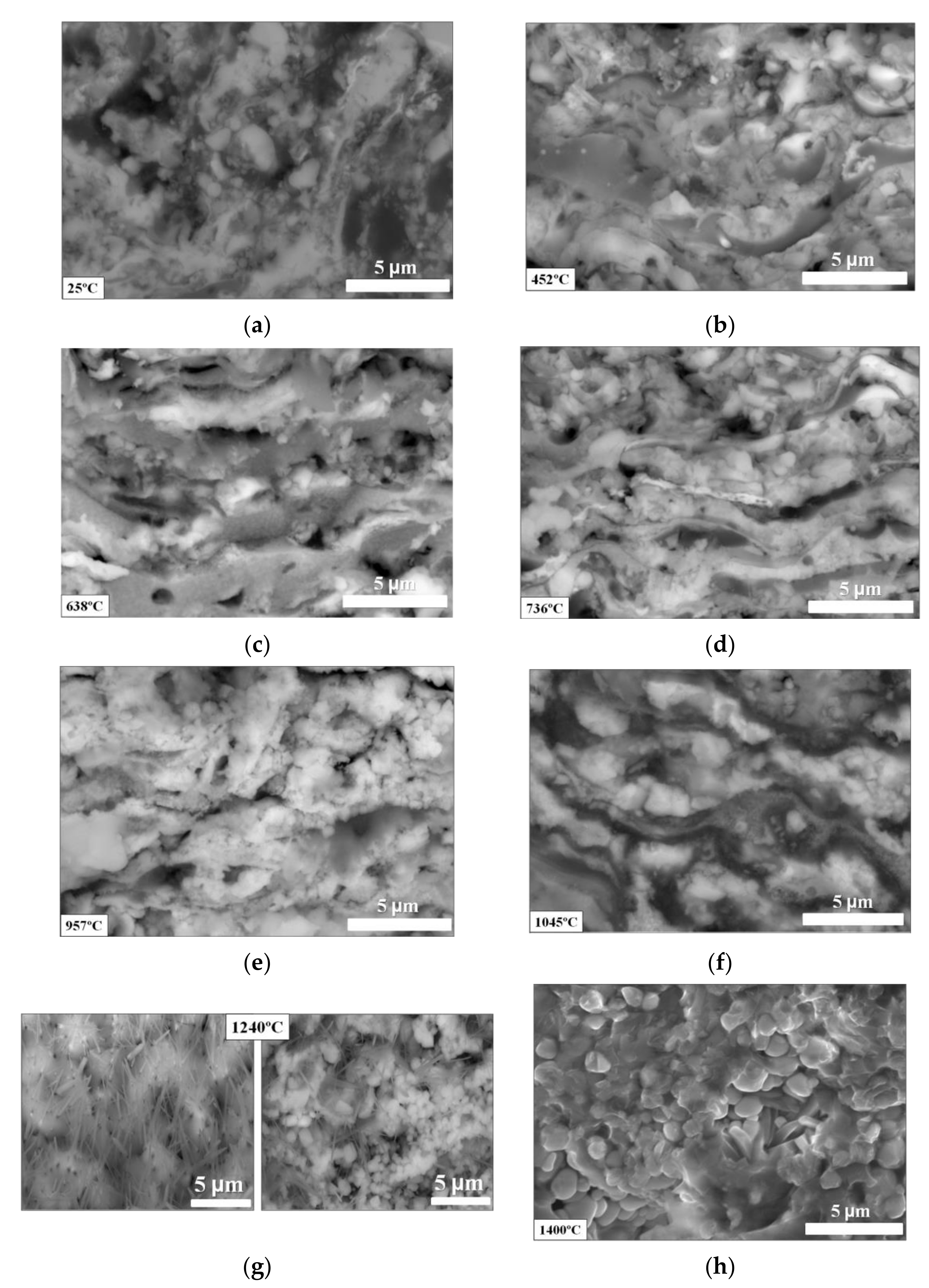
- The crystallization of the monoclinic phase of zirconium dioxide (m-ZrO2) takes place at 450 °C;
- -
- The actual onset of thermal degradation in the coating into other phases—such as c-Al12Mo, m-SiO2, and t-ZrO2—occurred from 640 to 960 °C;
- -
- The complete oxidation of the starting materials into the phases c-ZrO2, m-ZrO2, t-ZrSiO4, and mullite takes place with annealing temperature from 960 to 1400 °C;
- -
- The dense silicate glass layer containing ZrO2 and small amounts of mullite and zircon was formed during the oxidation. The ZrO2 and zircon particles (immiscible phases) were inlaid in SiO2 glass, so they could enhance its stability and prevent the propagation of cracks in the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krenkel, W.; Berndt, F. C/C–SiC Composites for Space Applications and Advanced Friction Systems. Mater. Sci. Eng. A 2005, 412, 177–181. [Google Scholar] [CrossRef]
- Devi, G.; Rao, K. Carbon-Carbon Composites—An Overview. Def. Sci. J. 1993, 43, 369–383. [Google Scholar] [CrossRef]
- Lalit, M.M. High Performance Carbon–Carbon Composites. Sadhana 2003, 28, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Windhorst, T.; Blount, G. Carbon-Carbon Composites: A Summary of Recent Developments and Applications. Mater. Des. 1997, 18, 11–15. [Google Scholar] [CrossRef]
- Zmij, V.I.; Rudenkyi, S.G.; Shepelev, A.G. Complex Protective Coatings for Graphite and Carbon-Carbon Composite Materials. Mater. Sci. Appl. 2015, 6, 879–888. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Qiu, X.; Zhang, B.; Xu, J.; Zhong, F.; Zhu, B.; Zhang, Y.; Ou-Yang, J.; Yang, X. Advances in Antioxidation Coating Materials for Carbon/Carbon. J. Alloys Compd. 2021, 886, 161143. [Google Scholar] [CrossRef]
- Morimoto, T.; Ogura, Y.; Kondo, M.; Ueda, T. Multilayer Coating for Carbon-Carbon Composites. Carbon 1995, 33, 351–357. [Google Scholar] [CrossRef]
- Wu, S.J.; Cheng, L.F.; Zhang, L.T.; Xu, Y. Oxidation Behavior of 2D C/SiC with a Multi-Layer CVD SiC Coating. Surf. Coat. Technol. 2006, 200, 4489–4492. [Google Scholar] [CrossRef]
- Fritze, H.; Jojic, J.; Witke, T.; Rüscher, C.; Weber, S.; Scherrer, S.; Schultrich, B.; Borchardt, G. Mullite Based Oxidation Protection for SiC–C/C Composites in Air at Temperatures up to 1900 K. J. Eur. Ceram. Soc. 1998, 18, 2351–2364. [Google Scholar] [CrossRef]
- Ren, C.; He, Y.D.; Wang, D.R. Cyclic Oxidation Behavior and Thermal Barrier Effect of YSZ–(Al2O3/YAG) Double-Layer TBCs Prepared by the Composite Sol-Gel Method. Surf. Coat. Technol. 2011, 206, 1461–1468. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiong, X.; Guo, S.; Zhang, W. SiC/SiC–YAG–YSZ Oxidation Protective Coatings for Carbon/Carbon Composites. Corros. Sci. 2013, 70, 68–73. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Xiong, X.; Xiao, P.; Chen, F.; Zhang, H.-B.; Huang, B.-Y. A Multilayer Coating of Dense SiC Alternated with Porous Si–Mo for the Oxidation Protection of Carbon/Carbon Silicon Carbide Composites. Carbon 2008, 46, 149–153. [Google Scholar] [CrossRef]
- Huang, J.F.; Zeng, X.R.; Li, H.J.; Xiong, X.-B.; Fu, Y. Influence of the Preparation Temperature on Phase, Microstructure and Anti-Oxidation Property of SiC Coating for C/C Composites. Carbon 2004, 42, 1517–1521. [Google Scholar] [CrossRef]
- Smeacetto, F.; Salvo, M.; Ferraris, M. Oxidation Protective Multilayer Coatings for Carbon–Carbon Composites. Carbon 2002, 40, 583–587. [Google Scholar] [CrossRef]
- Grilli, M.L.; Valerini, D.; Slobozeanu, A.E.; Postolnyi, B.O.; Balos, S.; Rizzo, A.; Piticescu, R.R. Critical Raw Materials Saving by Protective Coatings under Extreme Conditions: A Review of Last Trends in Alloys and Coatings for Aerospace Engine Applications. Materials 2021, 14, 1656. [Google Scholar] [CrossRef]
- Yao, X.Y.; Li, H.J.; Zhang, Y.L.; Ren, J.-J.; Yao, D.-J.; Tao, J. A SiC/ZrB2–SiC/SiC Oxidation Resistance Multilayer Coating for Carbon/Carbon Composites. Corros. Sci. 2012, 57, 148–153. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Yi, Z.; Lemuel, G.; Xiang, X.; Bai-Yun, H. Preparation and Oxidation Property of ZrB2-MoSi2/SiC Coating on Carbon/Carbon Composites. Trans. Nonferr. Met. Soc. China 2011, 21, 1538–1544. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, H.J.; Yao, X.Y.; Li, K.-Z.; Qiang, X.-F. Oxidation Protection of C/SiC Coated Carbon/Carbon Composites with Si–Mo Coating at High Temperature. Corros. Sci. 2011, 53, 2075–2079. [Google Scholar] [CrossRef]
- Liu, G.H.; Cheng, L.F.; Li, K.Z.; Chen, Z.; Xiong, X.; Luana, X. Damage Behavior of Atomic Oxygen on Zirconium Carbide Coating Modified Carbon/Carbon Composite. Ceram. Int. 2020, 46, 3324–3331. [Google Scholar] [CrossRef]
- Hu, D.; Fu, Q.G.; Liu, T.Y.; Tong, M. Structural Design and Ablation Performance of ZrB2/MoSi2 Laminated Coating for SiC Coated Carbon/Carbon Composites. J. Eur. Ceram. Soc. 2020, 40, 212–219. [Google Scholar] [CrossRef]
- Ren, X.R.; Chu, H.A.; Wu, K.Y.; Zhang, A.; Huang, M.; Ma, C.; Liu, H.; Feng, P. Effect of the ZrB2 Content on the Oxygen Blocking Ability of ZrB2-SiC Coating at 1973 K. J. Eur. Ceram. Soc. 2020, 41, 1059–1070. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, G.B.; Ouyang, H.B.; Lu, J. ZrB2 Particles Reinforced Glass Coating for Oxidation Protection of Carbon/Carbon Composites. J. Adv. Ceram. 2019, 8, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Min-Kang, S. The Effect of MoSi2 on the Oxidation Behavior of Carbon/Carbon Composites. Carbon 2001, 39, 1229–1235. [Google Scholar] [CrossRef]
- Mao, J.Y.; Liu, M.; Mao, J. Oxidation-resistance of ZrB2-MoSi2 Composite Coatings Prepared by Atmospheric Plasma Spraying. J. Inorg. Mater. 2015, 39, 282–286. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.F.; Cao, L.Y.; Li, C.; Hao, W.; Zhu, K. Influence of Solvothermal Treatment Time on Oxidation of Carbon/Carbon Composites Containing ZrB2 Microparticles. Ceram. Int. 2014, 40, 13529–13535. [Google Scholar] [CrossRef]
- Yao, X.Y.; Li, H.J.; Zhang, Y.L.; Wang, Y. Oxidation and Mechanical Properties of SiC/SiCMoSi2-ZrB2 Coating for Carbon/Carbon Composites. J. Mater. Sci. Technol. 2014, 30, 123–127. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, D.; Ru, H.Q.; Wang, W.; Zhang, C. Oxidation Protective ZrB2-MoSi2-SiC-Si Coating for Graphite Materials Prepared by Slurry Dipping and Vapor Silicon Infiltration. Surf. Coat. Technol. 2018, 339, 91–100. [Google Scholar] [CrossRef]
- Yang, X.; Wei, L.; Song, W.; Bi-Feng, Z.; Zhao-Hui, C. ZrB2/SiC as a Protective Coating for C/SiC Composites: Effect of High Temperature Oxidation on Mechanical Properties and Anti-Ablation Property. Compos. Part B Eng. 2013, 45, 1391–1396. [Google Scholar] [CrossRef]
- Yang, X.; Feng, C.; Qing, W. ZrB2-SiC as a Protective Coating for C/SiC Composites: Effect of High Temperature Oxidation on Thermal Shock Property and Protection Mechanism. J. Asian Ceram. 2016, 4, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Wang, Z.; Zhao, J.; Zheng, X.; Zeng, Y.; Ding, C. Comparison of ZrB2-MoSi2 Composite Coatings Fabricated by Atmospheric and Vacuum Plasma Spray Processes. J. Therm. Spray Technol. 2017, 26, 100–107. [Google Scholar] [CrossRef]
- Liu, X.; Han, W.; Wen, K.; Deng, C.; Deng, C.; Liu, M.; Zhou, K. Bimodal Microstructure ZrB2-MoSi2 Coating Prepared by Atmospheric Plasma Spraying for Carbon/Carbon Composites Against Long-Term Ablation. Ceram. Int. 2017, 43, 16659–16667. [Google Scholar] [CrossRef]
- Podchernyaeva, I.A.; Grigoriev, O.N.; Panasyuk, A.D.; Evdokimenko, Y.I.; Kisel’, V.M.; Yurechko, D.V.; Panashenko, V.M. High-Temperature ZrB2-Based Coatings on Metallic Alloys Produced by High-Velocity Air-Fuel Thermal Spraying. Powder Metall. Met. Ceram. 2017, 55, 689–697. [Google Scholar] [CrossRef]
- Kovaleva, M.G.; Goncharov, I.Y.; Novikov, V.Y.; Yapryntsev, M.N.; Vagina, O.N.; Pavlenko, I.N.; Sirota, V.V.; Tyurin, Y.N.; Kolisnichenko, O.V. Characteristics of ZrB2-ZrO2-MoSi2-Al Coating on Carbon/Carbon Composite Obtained by a New Multi-chamber Detonation Accelerator. IOP Conf. Ser. Mater. Sci. Eng. 2020, 872, 012053. [Google Scholar] [CrossRef]
- Kovaleva, M.; Goncharov, I.; Novikov, V.; Yapryntsev, M.; Vagina, O.; Pavlenko, I.; Sirota, V.; Tyurin, Y.; Kolisnichenko, O. Effect of Heat Treatment on the Microstructure and Phase Composition of ZrB2–MoSi2 Coating. Coatings 2019, 9, 779. [Google Scholar] [CrossRef] [Green Version]
- Vasilik, N.; Tyurin, Y.; Kolisnichenko, O. Method for Gas-Dynamic Detonating Speedup of Powders and Device for Its Implementation. RU Patent 2,506,341, 11 July 2012. [Google Scholar]
- Kovaleva, M.; Tyurin, Y.; Vasilik, N.; Kolisnichenko, O.; Prozorova, M.; Arseenko, M.; Danshina, E. Deposition and Characterization of Al2O3 Coatings by Multi-Chamber Gas-Dynamic Accelerator. Surf. Coat. Technol. 2013, 232, 719–725. [Google Scholar] [CrossRef]
- Zhang, X.C.; Xu, B.S.; Wu, Y.X.; Xuan, F.Z.; Tu, S.-T. Porosity, mechanical properties, residual stresses of supersonic plasma-sprayed Ni-Based alloy coatings prepared at different powder feed rates. Appl. Surf. Sci. 2008, 254, 3879–3889. [Google Scholar] [CrossRef]
- Kovaleva, M.; Sirota, V.; Goncharov, I.; Novikov, V.; Yapryntsev, M.; Vagina, O.; Pavlenko, I.; Tyurin, Y.; Mogucheva, A. Kinetics Investigation of the Formation of a Gas-Resistant Glass-Forming Layer During the Oxidation of ZrB2-MoSi2-Y2O3-Al Coatings in the Air Atmosphere. Coatings 2021, 11, 1018. [Google Scholar] [CrossRef]
- Kovaleva, M.; Tyurin, Y.; Vasilik, N.; Kolisnichenko, O.; Prozorova, M.; Arseenko, M.; Yapryntsev, M.; Sirota, V.; Pavlenko, I. Effect of Processing Parameters on the Microstructure and Properties of WC–10Co–4Cr Coatings Formed by a New Multi-chamber Gas-Dynamic Accelerator. Ceram. Int. 2015, 41, 15067–15074. [Google Scholar] [CrossRef]
- Kovaleva, M.; Tyurin, Y.; Vasilik, N.; Kolisnichenko, O.; Prozorova, M.; Arseenko, M.; Sirota, V.; Pavlenko, I. Effect of Heat Treatment on the Microstructure and Microhardness of Nanostructural Al2O3 Coatings. J. Therm. Spray Technol. 2014, 23, 1199–1209. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, H.J.; Qiang, X.F.; Li, K.-Z.; Zhang, S.-Y. C/SiC/MoSi2-Si Multilayer Coatings for Carbon/Carbon Composites for Protection Against Oxidation. Corros. Sci. 2011, 53, 3840–3844. [Google Scholar] [CrossRef]
- Fu, Q.G.; Li, H.J.; Wang, Y.J.; Li, K.-Z.; Shi, X.-H. B2O3 Modified SiC-MoSi2 Oxidation Resistant Coating for Carbon/Carbon Composites by a Two-Step Pack Cementation. Corros. Sci. 2009, 51, 2450–2454. [Google Scholar] [CrossRef]
- Enzo, S.; Frattini, R.; Canton, P.; Monagheddu, M.; Delogu, F. Neutron Diffraction Study of Mechanically Alloyed and In Situ Annealed Al75Mo25 Powders. J. Appl. Phys. 2000, 87, 2753–2759. [Google Scholar] [CrossRef] [Green Version]
- Grushko, B.; Velikanova, T.Y. Formation of Quasicrystals and Related Structures in Systems of Aluminum with Transition Metals. I. Binary Systems Formed by Aluminum with 3d Metals. Powder Metall. Met. Ceram. 2004, 43, 72–86. [Google Scholar] [CrossRef]
- Bian, Y.; Gao, T.; Li, Z.; Sun, Q.; Ma, X.; Liu, X. In–Situ Synthesis of an Al Composite Reinforced with Multi–Scale Al12Mo, (Al, Zr, Si) and Al2O3 Particles through a Multi–Stage Reaction. Mater. Sci. Eng. A 2019, 762, 138069. [Google Scholar] [CrossRef]
- Colgan, E.G.; Nastasi, M.; Mayer, J.W. Initial Phase Formation and Dissociation in the Thin Film Ni/Al System. J. Appl. Phys. 1985, 58, 4125–4129. [Google Scholar] [CrossRef]
- Kaiser, A.; Lobert, M.; Telle, R. Thermal Stability of Zircon (ZrSiO4). J. Eur. Ceram. Soc. 2008, 28, 2199–2211. [Google Scholar] [CrossRef]
- Elsandika, G.; Putri, A.D.C.; Musyarofah, M.; Pratapa, S. Synthesis of ZrSiO4 Powders by a Sol-Gel Method with Varied Calcination Temperatures. IOP Conf. Ser. Mater. Sci. Eng. 2019, 496, 012047. [Google Scholar] [CrossRef]
- Ferrari, S.; Grinblat, F.; Bilovol, V.; Pampillo, L.G.; Saccone, F.D.; Errandonea, D.; Chanquía, C.M. Synthesis and Characterization of Ti-Doped ZrSiO4 at Ambient and High-Pressure Conditions. J. Mater. Sci. 2018, 53, 8817–8825. [Google Scholar] [CrossRef]
- Yan, S.; Yang, Q.; Wang, H.; Zhang, Q. Depression of Synthesis Temperature and Structure Characterization of ZrSiO4 Used in Ceramic Pigments. Mater. Chem. Phys. 2018, 205, 97–101. [Google Scholar] [CrossRef]
- Wang, C.; Li, K.; He, Q.; He, D.; Huo, C.; Su, Y.; Shi, X.; Li, H. Oxidation and Ablation Protection of Plasma Sprayed LaB6-MoSi2-ZrB2 Coating for Carbon/Carbon Composites. Corros. Sci. 2019, 151, 57–68. [Google Scholar] [CrossRef]
- Zhuo, G.; Su, L.; Jiang, K.; Yang, J. Effect of Spraying Power on Oxidation Resistance Of MoSi2-ZrB2 Coating for Nb-Si Based Alloy Prepared by Atmospheric Plasma. Materials 2020, 13, 5060. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.G.; Li, H.J.; Shi, X.H.; Li, K.-Z.; Sun, G.-D. Silicon Carbide Coating to Protect Carbon/Carbon Composites Against Oxidation. Script. Mater. 2005, 52, 923–927. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, H.; Liu, Z.; Hu, C.; Wang, X.; Zheng, X.; Ding, C. Microstructure Evolution of ZrB2–MoSi2 Composite Coatings at Middle and High Temperatures. Surf. Coat. Technol. 2015, 273, 30–38. [Google Scholar] [CrossRef]
- Fei, X.; Niu, Y.; Ji, H.; Huang, L.; Zheng, X. A Comparative Study of MoSi2 Coatings Manufactured by Atmospheric and Vacuum Plasma Spray Processes. Ceram. Int. 2011, 37, 813–817. [Google Scholar] [CrossRef]

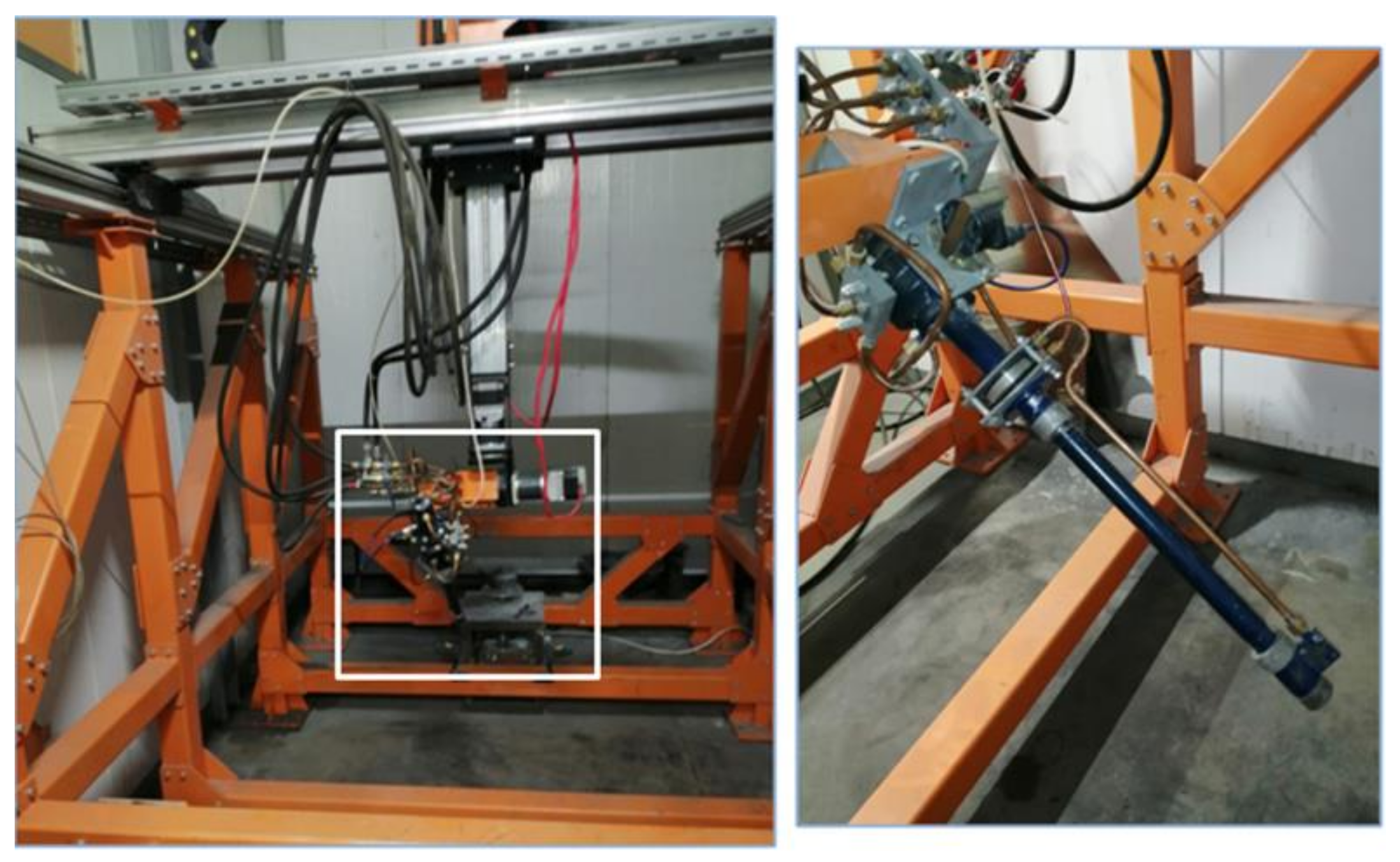



| Barrel Length (mm) | Barrel Diameter (mm) | Powder Feed Rate (g/h) | Flow Rate of Fuel Mixture Components, m3/h | Oxygen/Fuel Ratio | ||
|---|---|---|---|---|---|---|
| Oxygen | C3H8 + C4H10 | Air | ||||
| 500 | 16 | 600 | 4.00 */3.60 ** | 0.75 */0.68 ** | 0.12 */0.12 ** | 5.28 */5.38 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovaleva, M.; Sirota, V.; Goncharov, I.; Novikov, V.; Yapryntsev, M.; Vagina, O.; Pavlenko, I.; Tyurin, Y. Oxidation Behavior and Microstructural Evolution of ZrB2–35MoSi2–10Al Composite Coating. Coatings 2021, 11, 1531. https://doi.org/10.3390/coatings11121531
Kovaleva M, Sirota V, Goncharov I, Novikov V, Yapryntsev M, Vagina O, Pavlenko I, Tyurin Y. Oxidation Behavior and Microstructural Evolution of ZrB2–35MoSi2–10Al Composite Coating. Coatings. 2021; 11(12):1531. https://doi.org/10.3390/coatings11121531
Chicago/Turabian StyleKovaleva, Marina, Viacheslav Sirota, Igor Goncharov, Vseslav Novikov, Maxim Yapryntsev, Olga Vagina, Ivan Pavlenko, and Yuri Tyurin. 2021. "Oxidation Behavior and Microstructural Evolution of ZrB2–35MoSi2–10Al Composite Coating" Coatings 11, no. 12: 1531. https://doi.org/10.3390/coatings11121531
APA StyleKovaleva, M., Sirota, V., Goncharov, I., Novikov, V., Yapryntsev, M., Vagina, O., Pavlenko, I., & Tyurin, Y. (2021). Oxidation Behavior and Microstructural Evolution of ZrB2–35MoSi2–10Al Composite Coating. Coatings, 11(12), 1531. https://doi.org/10.3390/coatings11121531






