Polydopamine-Assisted Surface Modification of Ti-6Al-4V Alloy with Anti-Biofilm Activity for Dental Implantology Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the PDA Samples Used in Structural Investigations
2.2. Deposition of PDA to Ti-6Al-4V
2.2.1. Characterization Techniques
Scanning Electron Microscope
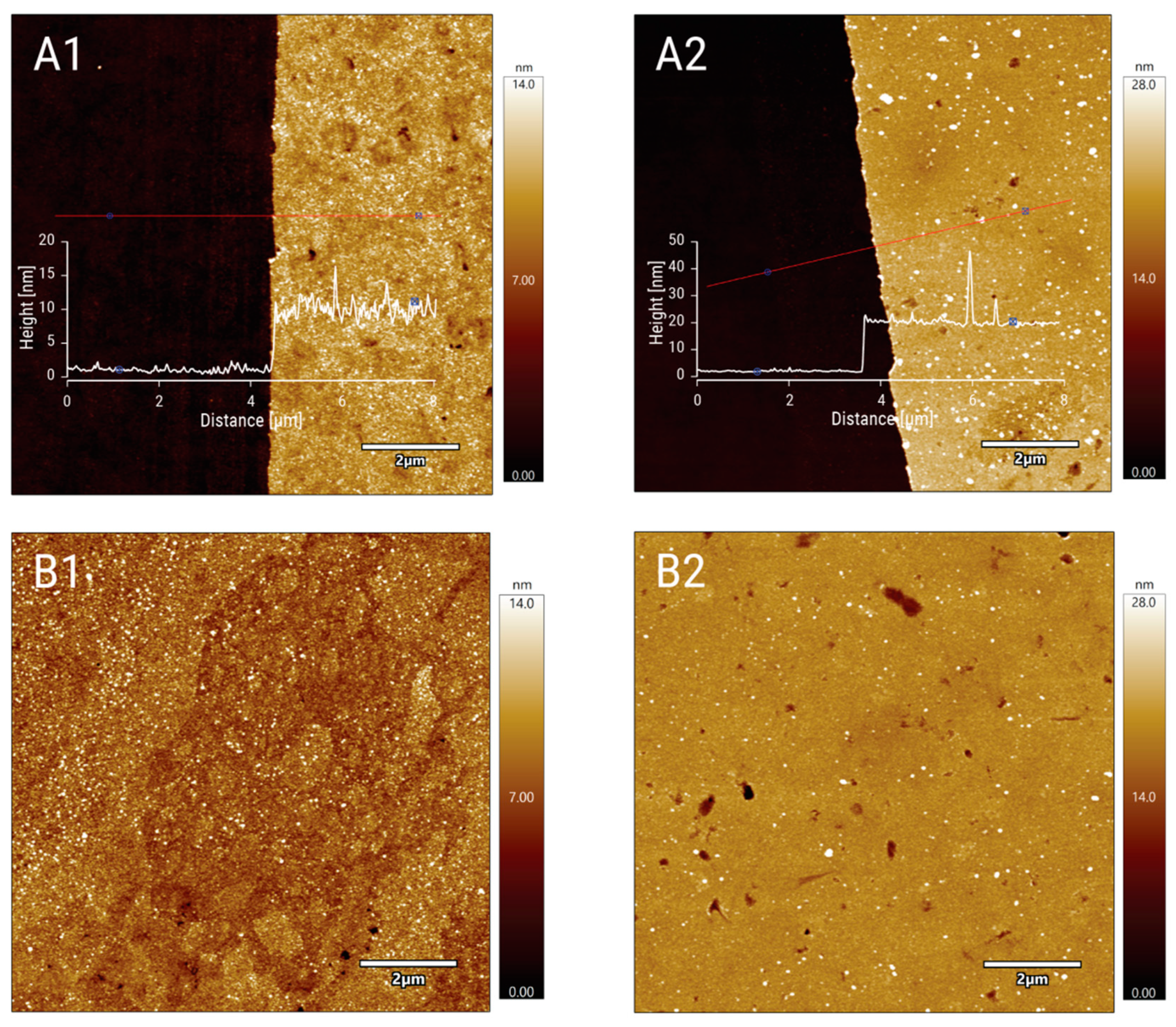
Atomic Force Microscopy (AFM)
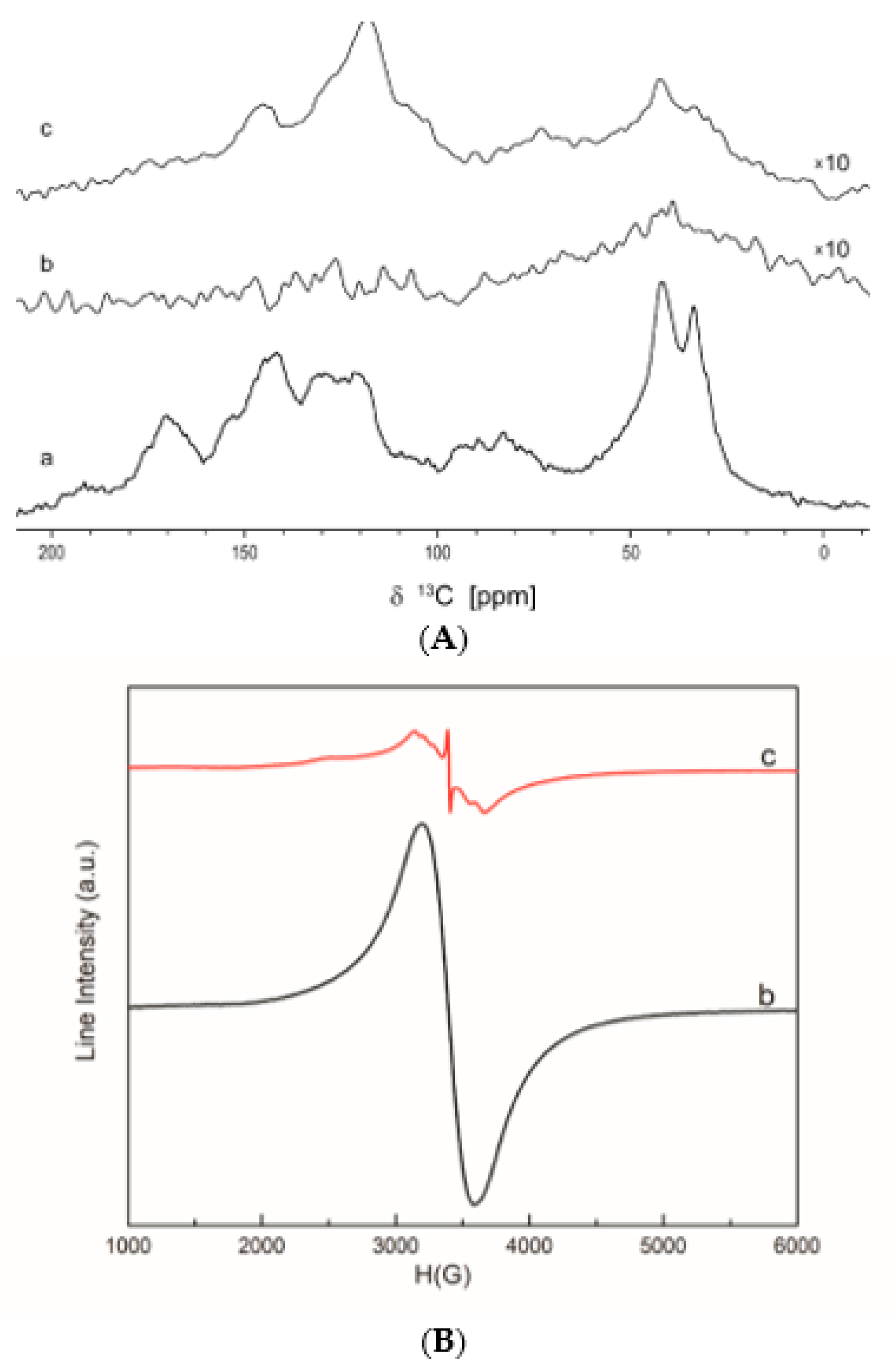
Solid-State 13C NMR Spectrometry
Electron Paramagnetic Resonance Spectrometry (EPR)
2.2.2. Assessment of Antimicrobial Activity
Logarithmic Reduction
In Vitro Quantification of Reactive Nitrogen Intermediates (RNI)
Study of Antibiofilm Activity of Ti-6Al-4V-PDA Disks
2.2.3. Determination of Cytotoxicity
2.3. Statistical Analysis
3. Results
3.1. Structural and Morphological Characterization
3.2. Experimental Design for Optimal Logarithmic Microbial Reduction with Low Cytotoxicity
3.3. Antibiofilm Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghensi, P.; Bettio, E.; Maniglio, D.; Bonomi, E.; Piccoli, F.; Gross, S.; Caciagli, P.; Segata, N.; Nollo, G.; Tessarolo, F. Dental Implants with Anti-Biofilm Properties: A Pilot Study for Developing a New Sericin-Based Coating. Materials 2019, 12, 2429. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.G.; Richards, R.G. Staphylococci and implant surfaces: A review. Injury 2006, 37, 3–14. [Google Scholar] [CrossRef]
- Kato, K.; Uchida, E.; Kang, E.T.; Uyama, Y.; Ikada, Y. Polymer surface with graft chain. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Chen, W.; Thomas, J.M. Layer-by-Layer Deposition: A Tool for Polymer Surface Modification. Macromolecules 1997, 30, 78–86. [Google Scholar] [CrossRef]
- Luo, M.L.; Zhao, J.Q.; Tang, W.; Pu, C.S. Hydrophilic modification of poly (ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Hegemann, D.; Brunner, H.; Oehr, C. Plasma treatment of polymers for surface and adhesion improvement. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 208, 281–286. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface Characteristics of a Self-Polymerized Dopamine Coating Deposited on Hydrophobic Polymer Films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of History. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Salomäki, M.; Martilla, L.; Kivellä, H.; Ouvinen, T.; Lukkari, H. Effects of pH and Oxidants on the First Steps of Polydopamine Formation: A Thermodynamic Approach. J. Phys. Chem. B 2018, 122, 6314–6327. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrowczynski, R.; Scheidt, H.A.; Filip, C.; Hadade, D.N.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story. Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Cîrcu, M.; Filip, C. Closer to the polydopamine structure: New insights from a combined 13C/1H/2H solid-state NMR study on deuterated sample. Polym. Chem. 2018, 9, 3379–3387. [Google Scholar] [CrossRef]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine Mediated Antimicrobial Adhesion System. Front. Microbiol. 2021, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Physicochemical perspective on polydopamine and poly(catechoamine) films for their applications in biomaterial coatings. Biointerphases 2014, 9, 030801. [Google Scholar] [CrossRef]
- Shu, H.; Zhou, P.; Wang, L.; Xiong, X.; Zhang, Y.; Deng, Y.; Wei, S. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc. Interface 2014, 11, 20140169. [Google Scholar]
- Lim, K.; Chua, R.R.Y.; Ho, B.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Neethu, S.; Midhun, S.J.; Radhakrishnan, E.; Jyothis, M. Surface functionalization of central venous catheterwith mycofabricated silver nanoparticles and its antibiofilm activity on multidrug resistant Acinetobacter baumannii. Microb. Pathog. 2020, 138, 103832. [Google Scholar] [CrossRef]
- Petran, A.; Mrowczynski, R.; Filip, C.; Turcu, R.; Liebscher, J. Melanin-like polydopa amides-synthesis and applications in functionalization of magnetic nanoparticles. Polym. Chem. 2015, 6, 2139–2149. [Google Scholar] [CrossRef]
- Zhang, C.; Ou, Y.; Lei, W.-X.; Wan, L.-S.; Ji, J.; Xu, Z.-K. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angew. Chem. Int. Ed. 2016, 55, 3054–3057. [Google Scholar] [CrossRef]
- Tahroudia, Z.M.; Razmjoua, A.; Bagheriana, M.; Asadniac, M. Polydopamine surface modification with UV-shielding effect using KMnO4 as an efficient oxidizing agent. Colloids Surf. A 2018, 559, 68–73. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Rehman, S.; Leu, S.Y.; An, A.K. Evaluation of anti-bacterial adhesion performance of polydopamine cross-linked graphene oxide RO membrane via in situ optical coherence tomography. Desalination 2020, 479, 114339. [Google Scholar] [CrossRef]
- Luo, C.; Liu, W.; Luo, B.; Tian, J.; Wen, W.; Liu, M.; Zhou, C. Antibacterial activity and cytocompatibility of chitooligosaccharide-modified polyurethane membrane via polydopamine adhesive layer. Carbohydr. Polym. 2017, 156, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.B.; Hasane, A.; Wang, Z.; Mansurov, A.; Castrilloìn, S.R.V. Bacterial adhesion to ultrafiltration membranes: Role of hydrophilicity, natural organic matter, and cell-surface macromolecules. Environ. Sci. Technol. 2018, 52, 162–172. [Google Scholar]
- Quinteros, M.A.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. In Vitro 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Coy, L.E.; Scheibe, B.; Czechowski, T.; Augustyniak-Jabłokow, M.; Jurga, S.; Tadyszak, K. Electron Paramagnetic Resonance Imaging and Spectroscopy of Polydopamine Radicals. J. Phys. Chem. B 2015, 119, 10341–10347. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.; Raita, O.; Stan, M.; Pana, O.; Borodi, G.; Giurgiu, L.M. Electron Paramagnetic Resonance of Mn-Doped Sn1−xMnxO2 Powders. Appl. Magn. Reson. 2012, 42, 453–462. [Google Scholar] [CrossRef]
- Miranda, J.E.A.; Sotomayor, C.E.; Albesa, I.; Paraje, M.G. Oxidative and nitrosative stress in Staphylococcus aureus biofilm. FEMS Microbiol. Lett. 2011, 315, 23–29. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Zhang, M.; Abdallah, Y.; Ahmed, T.; Qiu, W.; Ali, M.A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. The Bio-Synthesis of Three Metal Oxide Nanoparticles (ZnO, MnO2, and MgO) and Their Antibacterial Activity against the Bacterial Leaf Blight Pathogen. Front. Microbiol. 2020, 11, 588326. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.; Garcia-Godoy, F.; Brambilla, E. Surface properties of resin-based composite materials and biofilm formation: A review of the current literature. Am. J. Dent. 2015, 28, 311–320. [Google Scholar]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria–material interactions. Eur. Cell. Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Lei, W.; Ren, K.; Chen, T.; Chen, X.; Li, B.; Chang, H.; Ji, J. Polydopamine Nanocoating for Effective Photothermal Killing of Bacteria, and Fungus upon Near-Infrared Irradiation. Adv. Mater. Interfaces 2016, 3, 1600767. [Google Scholar] [CrossRef]
- Koopaie, M.; Bordbar-Khiabani, A.; Kolahdooz, S.; Darbandsari, A.K.; Mozafari, M. Advanced surface treatment techniques counteract biofilm-associated infections on dental implants. Mater. Res. Express 2020, 7, 015417. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, X.; Chang, L.; Zhu, S.; Guan, S. Characterization and cytocompatibility of polydopamine on MAO-HA coating supported on Mg-Zn-Ca alloy. Surf. Interface Anal. 2017, 49, 1115–1123. [Google Scholar] [CrossRef]
- Francolini, I.; Donelli, G.; Crisante, F.; Taresco, V.; Piozzi, A. Antimicrobial Polymers for Anti-Biofilm Medical Devices: State-of-Art and Perspectives. Adv. Exp. Med. Biol. 2015, 831, 93–117. [Google Scholar]
- Sadrearhami, Z.; Nguyen, T.-K.; Namivandi-Zangeneh, R.; Jung, K.; Wong, E.H.H.; Boyer, C. Recent Advances in Nitric Oxide Delivery for Antimicrobial Applications Using Polymer-Based Systems. J. Mater. Chem. B 2018, 6, 2945–2959. [Google Scholar] [CrossRef]
- Sadrearhami, Z.; Shafiee, F.N.; Ho, K.K.K.; Kumar, N.; Krasowska, M.; Blencowe, A.; Wong, E.H.H.; Boyer, C. Antibiofilm Nitric Oxide-Releasing Polydopamine Coatings. ACS Appl. Mater. Interfaces 2019, 11, 7320–7329. [Google Scholar] [CrossRef]
- Pant, J.; Gao, J.; Goudie, M.J.; Hopkins, S.P.; Locklin, J.; Handa, H. A Multi-Defense Strategy: Enhancing Bactericidal Activity of a Medical Grade Polymer with a Nitric Oxide Donor and Surface-Immobilized Quaternary Ammonium Compound. Acta Biomater. 2017, 58, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.; Aveyard, J.; Fothergill, J.; McBride, F.; Raval, R.; D’Sa, R. Nitric Oxide Releasing Polymeric Coatings for the Prevention of Biofilm Formation. Polymers 2017, 9, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta, M.A.; da Silva, M.A.; Ortega, M.G.; Cabrera, J.L.; Paraje, M.G. Usnic Acid Activity on Oxidative and Nitrosative Stress of Azole-Resistant Candida albicans Biofilm. Planta Med. 2017, 83, 326–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mendoza, I.L.I.; Cayero-Garay, A.; Quindós-Andrés, G.; Aguirre-Urizar, J.M. A systematic review on the implication of Candida in peri-implantitis. Int. J. Implant Dent. 2021, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Bürgers, R.; Hahnel, S.; Reichert, T.E.; Rosentritt, M.; Behr, M.; Gerlach, T.; Handel, G.; Gosau, M. Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater. 2010, 6, 2307–2313. [Google Scholar] [CrossRef] [PubMed]




| Nr. | Cod | Factor 1 (Dopamine Molar Echiv) | Factor 2 (KMnO4 Molar Echiv) | Reduction of S. aureus (lgCFU/mL) | Reduction of E. coli (lgCFU/mL) | Reduction of C. albicans (lgCFU/mL) | MTT (%Viability) | LDH (%LDH Release) |
|---|---|---|---|---|---|---|---|---|
| 1 | P1 | 0.5 | 0.1 | 0.38 ± 0.07 | 0.55 ± 0.10 | 0.38 ± 0.06 | 69.50 ± 4.35 | 24.99 ± 3.54 |
| 2 | P2 | 0.5 | 0.5 | 0.58 ± 0.10 | 0.24 ± 0.05 | 0.84 ± 0.17 | 90.51 ± 0.31 | 23.04 ± 2.35 |
| 3 | P3 | 1.5 | 0.1 | 0.33 ± 0.02 | 0.70 ± 0.06 | 0.78 ± 0.14 | 88.52 ± 10.46 | 35.31 ± 4.13 |
| 4 | P4 | 1.5 | 0.5 | 0.38 ± 0.08 | 0.24 ± 0.05 | 0.84 ± 0.18 | 94.31 ± 3.65 | 13.614 |
| Model (p-value) | 0.0153 | <0.0001 | 0.0018 | 0.0039 | 0.0042 | |||
| F-value | 6.52 | 31.18 | 13.29 | 10.38 | 10.16 | |||
| R2 | 0.7099 | 0.9212 | 0.8329 | 0.7955 | 0.7921 | |||
| Adjusted R2 | 0.6011 | 0.8917 | 0.7702 | 0.7189 | 0.7142 | |||
| Parameter | Lower Limit | Upper Limit | Goal | Importance | Optimal Value | Predicted Value |
|---|---|---|---|---|---|---|
| Dopamine | 0.5 | 1.5 | 3 | 0.88 | - | |
| KMnO4 | 0.1 | 0.5 | 3 | 0.45 | - | |
| Reduction log CFU/mL S. aureus | 0.30 | 0.64 | Maximize | 4 | 0.47 | |
| Reduction log CFU/mL E. coli | 0.19 | 0.76 | Maximize | 4 | 0.27 | |
| reduction log CFU/mL C. albicans | 0.34 | 1.05 | Maximize | 4 | 0.78 | |
| % Viability | 65.13 | 99.00 | Maximize | 5 | 90.17 | |
| % LDH release | 10.12 | 39.88 | Minimize | 5 | 20.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinas, I.C.; Tihauan, B.M.; Diaconu, A.G.; Filip, X.; Petran, A.; Grosu, I.-G.; Bogdan, D.; Barbu, L.; Ivanof, A.M.; Angheloiu, M.; et al. Polydopamine-Assisted Surface Modification of Ti-6Al-4V Alloy with Anti-Biofilm Activity for Dental Implantology Applications. Coatings 2021, 11, 1385. https://doi.org/10.3390/coatings11111385
Marinas IC, Tihauan BM, Diaconu AG, Filip X, Petran A, Grosu I-G, Bogdan D, Barbu L, Ivanof AM, Angheloiu M, et al. Polydopamine-Assisted Surface Modification of Ti-6Al-4V Alloy with Anti-Biofilm Activity for Dental Implantology Applications. Coatings. 2021; 11(11):1385. https://doi.org/10.3390/coatings11111385
Chicago/Turabian StyleMarinas, Ioana Cristina, Bianca Maria Tihauan, Andreea Gabriela Diaconu, Xenia Filip, Anca Petran, Ioana-Georgeta Grosu, Diana Bogdan, Lucian Barbu, Ana Maria Ivanof, Marin Angheloiu, and et al. 2021. "Polydopamine-Assisted Surface Modification of Ti-6Al-4V Alloy with Anti-Biofilm Activity for Dental Implantology Applications" Coatings 11, no. 11: 1385. https://doi.org/10.3390/coatings11111385
APA StyleMarinas, I. C., Tihauan, B. M., Diaconu, A. G., Filip, X., Petran, A., Grosu, I.-G., Bogdan, D., Barbu, L., Ivanof, A. M., Angheloiu, M., Pircalabioru, G. G., & Filip, C. (2021). Polydopamine-Assisted Surface Modification of Ti-6Al-4V Alloy with Anti-Biofilm Activity for Dental Implantology Applications. Coatings, 11(11), 1385. https://doi.org/10.3390/coatings11111385










