Novel Approaches to Combat Medical Device-Associated BioFilms
Abstract
1. Introduction
2. Medical Device-Related Biofilm Infection and the Challenges of Treatment
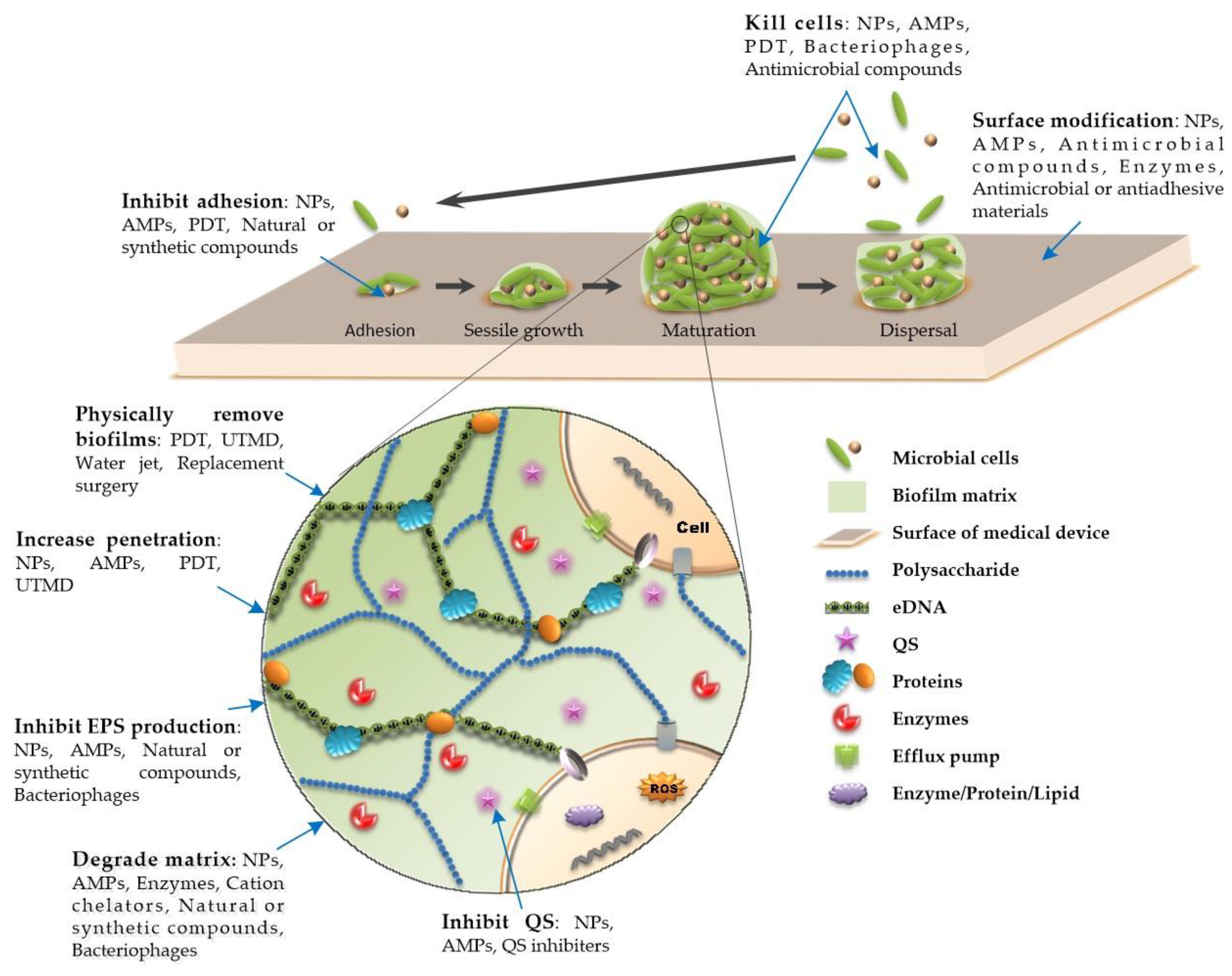
3. Novel Approaches to Combat Medical Device-Associated Biofilms
3.1. Physical Therapy
3.2. Surface Modification
| Surface-Modifying Compounds | Pathogens | Mechanism of Action | Reference |
|---|---|---|---|
| Isoeugenol | S. aureus, L. monocytogenes, and P. fluorescens | Antibacterial | [141] |
| N-acetyl cysteine and chitosan film | S. aureus | Antibacterial and antiadhesion | [144] |
| AgNP | E. coli and S. aureus | Antibacterial and antiadhesion | [146] |
| Silver-containing phosphonate monolayers | E. coli and S. epidermidis | Antiadhesion | [147] |
| Hydrophobin (Vmh2 and Pac3) | S. epidermidis | Antiadhesion | [152] |
| ECA | C. albicans | Antiadhesion | [153] |
| Poly(glycidol) | S. aureus | Antiadhesion | [154] |
| tPA | S. aureus | Antiadhesion, increases the sensitivity of biofilm infections to antibiotics | [157] |
| TMS/O2 | S. aureus | Antiadhesion | [158] |
| Direct thrombin inhibitors (argatroban, hirudin, and dabigatran) | staphylococcal | Antiadhesion | [159] |
| DNase I | S. mutans and S. aureus | Affect the structural integrity of the biofilm | [160] |
| Polypyrrole | S. mutans and S. sanguinis | Affect the integrity of biofilms | [161] |
3.3. Antimicrobial Peptides
3.4. Nanotechnology
3.5. Agents for Degradation of the Extracellular Matrix of Biofilms
3.6. Bacteriophage Therapy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies Against Microbial Biofilm Challenges. Front. Cell. Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Genet. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Tseng, B.S.; Majerczyk, C.D.; Da Silva, D.P.; Chandler, J.R.; Greenberg, E.P.; Parsek, M.R. Quorum Sensing Influences Burkholderia thailandensis Biofilm Development and Matrix Production. J. Bacteriol. 2016, 198, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Van Gennip, M.; Christensen, L.D.; Alhede, M.; Phipps, R.; Jensen, P.Ø.; Christophersen, L.; Pamp, S.J.; Moser, C.; Mikkelsen, P.J.; Koh, A.Y.; et al. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS 2009, 117, 537–546. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Otoole, G.; Kaplan, H.B.; Kolter, R. Biofilm Formation as Microbial Development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med. Microbiol. 2017, 307, 481–489. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.C.; Leonard, A.; Schäuble, M.; Rieckmann, L.M.; Hoyer, J.; Maass, S.; Lalk, M.; Becher, D.; Pané-Farré, J.; Riedel, K. Virulence Factors Produced by Staphylococcus aureus Biofilms Have a Moonlighting Function Contributing to Biofilm Integrity. Mol. Cell. Proteom. 2019, 18, 1036–1053. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Lam, A.K.; Wouters, C.L.; Moen, E.L.; Pusavat, J.; Rice, C.V. Antibiofilm Synergy of β-Lactams and Branched Polyethylenimine against Methicillin-ResistantStaphylococcus epidermidis. Biomacromolecules 2019, 20, 3778–3785. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Vanepps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhão, F.J.M.; Gomes, L.C. The Use of Probiotics to Fight Biofilms in Medical Devices: A Systematic Review and Meta-Analysis. Microorganisms 2020, 9, 27. [Google Scholar] [CrossRef]
- Sánchez-Gómez, S.; Martínez-De-Tejada, G. Antimicrobial Peptides as Anti-biofilm Agents in Medical Implants. Curr. Top. Med. Chem. 2016, 17, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.M.; Tran, H.; Booth, M.A.; Fox, K.E.; Nguyen, T.H.; Tran, N.; Tran, P.A. Nanomaterials for Treating Bacterial Biofilms on Implantable Medical Devices. Nanomaterials 2020, 10, 2253. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barat, L.; Torres, A. Biofilms in ventilator-associated pneumonia. Futur. Microbiol. 2016, 11, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Bjarnsholt, T.; McBain, A.; James, G.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, J.; Machado, D.; Gomes, A.M.; Machado, I.; Santos, C.; Lima, N.; Carvalho, M.J.; Cabrita, A.; Rodrigues, A.; Martins, M. Deciphering the Contribution of Biofilm to the Pathogenesis of Peritoneal Dialysis Infections: Characterization and Microbial Behaviour on Dialysis Fluids. PLoS ONE 2016, 11, e0157870. [Google Scholar] [CrossRef]
- Gominet, M.; Compain, F.; Beloin, C.; Lebeaux, D. Central venous catheters and biofilms: Where do we stand in 2017? APMIS 2017, 125, 365–375. [Google Scholar] [CrossRef]
- Septimus, E.J.; Schweizer, M.L. Decolonization in Prevention of Health Care-Associated Infections. Clin. Microbiol. Rev. 2016, 29, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.; Silva, P.; Silva, R.; Silva, G.; Machado, G.; Coelho, L.; Correia, M. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kelkar, A.; Agarwal, A.G.; Jayaswal, D.; Schultz, C.; Jayaswal, A.; Goel, V.K.; Agarwal, A.K.; Gidvani, S. Implant Retention or Removal for Management of Surgical Site Infection After Spinal Surgery. Glob. Spine J. 2019, 10, 640–646. [Google Scholar] [CrossRef]
- Sabir, N.; Ikram, A.; Zaman, G.; Satti, L.; Gardezi, A.; Ahmed, A.; Ahmed, P. Bacterial biofilm-based catheter-associated urinary tract infections: Causative pathogens and antibiotic resistance. Am. J. Infect. Control. 2017, 45, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, S.S. Pseudomonas aeruginosa Biofilm, a Programmed Bacterial Life for Fitness. J. Microbiol. Biotechnol. 2017, 27, 1053–1064. [Google Scholar] [CrossRef]
- Piperaki, E.-T.; Syrogiannopoulos, G.A.; Tzouvelekis, L.S.; Daikos, G.L. Klebsiella pneumoniae. Pediatr. Infect. Dis. J. 2017, 36, 1002–1005. [Google Scholar] [CrossRef]
- Eze, E.C.; Chenia, H.Y.; E El Zowalaty, M. Acinetobacter baumannii biofilms: Effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018, 11, 2277–2299. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Genet. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Rao, Y.; Shang, W.; Yang, Y.; Zhou, R.; Rao, X. Fighting Mixed-Species Microbial Biofilms with Cold Atmospheric Plasma. Front. Microbiol. 2020, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Wu, H.; Høiby, N.; Molin, S.; Song, Z. Current understanding of multi-species biofilms. Int. J. Oral Sci. 2011, 3, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Merritt, J.; Qi, F. Bacterial and Host Interactions of Oral Streptococci. DNA Cell Biol. 2009, 28, 397–403. [Google Scholar] [CrossRef]
- Santos, A.P.A.; Watanabe, E.; De Andrade, D. Biofilm on artificial pacemaker: Fiction or reality? Arquivos Brasileiros de Cardiologia 2011, 97, e113–e120. [Google Scholar] [CrossRef] [PubMed]
- Salmeri, M.; Sorbello, M.G.; Mastrojeni, S.; Santanocita, A.; Milazzo, M.; Di Stefano, G.; Scalia, M.; Addamo, A.; Toscano, M.A.; Stefani, S.; et al. Infections of cardiovascular implantable electronic devices: 14 years of experience in an Italian hospital. Infez. Med. 2016, 24, 131–136. [Google Scholar] [PubMed]
- Del Río, A.; Anguera, I.; Miró, J.M.; Mont, L.; Fowler, V.G.; Azqueta, M.; Mestres, C.A.; Hospital Clínic Endocarditis Study Group. Surgical Treatment of Pacemaker and Defibrillator Lead Endocarditis. Chest 2003, 124, 1451–1459. [Google Scholar] [CrossRef]
- Blomström-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.-C.; et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eurospace 2020, 22, 515–549. [Google Scholar] [CrossRef]
- Cahill, T.J.; Baddour, L.M.; Habib, G.; Hoen, B.; Salaun, E.; Pettersson, G.B.; Schäfers, H.J.; Prendergast, B.D. Challenges in Infective Endocarditis. J. Am. Coll. Cardiol. 2017, 69, 325–344. [Google Scholar] [CrossRef]
- Conklin, E.F.; Giannelli, S., Jr.; Nealon, T.F., Jr. Four hundred consecutive patients with permanent transvenous pacemakers. J Thorac. Cardiovasc. Surg. 1975, 69, 1–7. [Google Scholar] [CrossRef]
- Bluhm, G. Pacemaker infections. A clinical study with special reference to prophylactic use of some isoxazolyl penicillins. Acta Med. Scand. Suppl. 1985, 699, 1–62. [Google Scholar] [PubMed]
- Blanco-Guzman, M.; Wang, X.; Vader, J.M.; Olsen, M.A.; Dubberke, E.R. Epidemiology of Left Ventricular Assist Device Infections: Findings From a Large Nonregistry Cohort. Clin. Infect. Dis. 2021, 72, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Novosad, S.A.; Lake, J.; Nguyen, D.; Soda, E.; Moulton-Meissner, H.; Pho, M.T.; Gualandi, N.; Bepo, L.; Stanton, R.A.; Daniels, J.B.; et al. Multicenter Outbreak of Gram-Negative Bloodstream Infections in Hemodialysis Patients. Am. J. Kidney Dis. 2019, 74, 610–619. [Google Scholar] [CrossRef]
- Martin, K.; Lorenzo, Y.S.P.; Leung, P.Y.M.; Chung, S.; O’Flaherty, E.; Barker, N.; Ierino, F. Clinical Outcomes and Risk Factors for Tunneled Hemodialysis Catheter-Related Bloodstream Infections. Open Forum Infect. Dis. 2020, 7, ofaa117. [Google Scholar] [CrossRef]
- Taylor, G.; Gravel, D.; Johnston, L.; Embil, J.; Holton, D.; Paton, S.; Canadian Nosocomial Infection Surveillance Program; Canadian Hospital Epidemiology Committee. Incidence of bloodstream infection in multicenter inception cohorts of hemodialysis patients. Am. J. Infect. Control. 2004, 32, 155–160. [Google Scholar] [CrossRef]
- Parameswaran, R.; Sherchan, J.B.; Varma, D.M.; Mukhopadhyay, C.; Vidyasagar, S. Intravascular catheter-related infections in an Indian tertiary care hospital. J. Infect. Dev. Ctries. 2010, 5, 452–458. [Google Scholar] [CrossRef]
- Ikram, S.; Heikal, A.; Finke, S.; Hofgaard, A.; Rehman, Y.; Sabri, A.N.; Økstad, O.A. Bacillus cereus biofilm formation on central venous catheters of hospitalised cardiac patients. Biofouling 2019, 35, 204–216. [Google Scholar] [CrossRef]
- Cobrado, L.; Silva-Dias, A.; Azevedo, M.M.; Rodrigues, A. Anti-Candida activity of antimicrobial impregnated central venous catheters. Antimicrob. Resist. Infect. Control 2017, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hansra, S.; Crockford, G.; Köster, W.; Allan, B.J.; Blondeau, J.M.; Lainesse, C.; White, A.P. Tetrasodium EDTA Is Effective at Eradicating Biofilms Formed by Clinically Relevant Microorganisms from Patients’ Central Venous Catheters. mSphere 2018, 3, e00525-18. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.; Brown, M.; Faria, R.; Fraser, C.; Donohue, C.; Rainford, N.; Grosso, A.; Sinha, A.K.; Dorling, J.; Gray, J.; et al. Antimicrobial-impregnated central venous catheters for preventing neonatal bloodstream infection: The PREVAIL RCT. Health Technol. Assess. 2020, 24, 1–190. [Google Scholar] [CrossRef]
- Khaled, J.M.; Alyahya, S.A.; Chenthis Kanisha, C.; Alharbi, N.S.; Kadaikunnan, S.; Ramachandran, G.; Alanzi, K.F.; Rajivgandhi, G.; Vimala, R.; Manoharan, N. Anti-biofilm activity of LC-MS based Solanum nigrum essential oils against multi drug resistant biofilm forming P. mirabilis. Saudi J. Biol. Sci. 2021, 28, 302–309. [Google Scholar] [CrossRef]
- Townsend, E.M.; Moat, J.; Jameson, E. CAUTI’s next top model—Model dependent Klebsiella biofilm inhibition by bacteriophages and antimicrobials. Biofilm 2020, 2, 100038. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.G.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Genet. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Obručová, H.; Kotásková, I.; Tihelková, R.; Holá, V.; Růžička, F.; Freiberger, T. Fluorescent Capillary Electrophoresis Is Superior to Culture in Detecting Candida Species from Samples of Urinary Catheters and Ureteral Stents with Mono- or Polyfungal Biofilm Growth. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Singhai, M.; Malik, A.; Shahid, M.; Malik, M.A.; Goyal, R. A study on device-related infections with special reference to biofilm production and antibiotic resistance. J. Glob. Infect. Dis. 2012, 4, 193–198. [Google Scholar] [CrossRef]
- Stensballe, J.; Tvede, M.; Looms, D.; Lippert, F.K.; Dahl, B.; Tønnesen, E.; Rasmussen, L.S. Infection Risk with Nitrofurazone-Impregnated Urinary Catheters in Trauma Patients. Ann. Intern. Med. 2007, 147, 285–293. [Google Scholar] [CrossRef]
- Alonso, B.; Fernández-Barat, L.; Di Domenico, E.G.; Marín, M.; Cercenado, E.; Merino, I.; De Pablos, M.; Muñoz, P.; Guembe, M. Characterization of the virulence of Pseudomonas aeruginosa strains causing ventilator-associated pneumonia. BMC Infect. Dis. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Thorarinsdottir, H.R.; Kander, T.; Holmberg, A.; Petronis, S.; Klarin, B. Biofilm formation on three different endotracheal tubes: A prospective clinical trial. Crit. Care 2020, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-associated pneumonia in adults: A narrative review. Intensiv. Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.; Al Talhi, Y.M.; Aldabbagh, M.; Baksh, M.; Osman, M.; Azzam, M. The incidence of ventilator-associated pneumonia (VAP) in a tertiary-care center: Comparison between pre- and post-VAP prevention bundle. J. Infect. Public Health 2020, 13, 552–557. [Google Scholar] [CrossRef]
- Hart, R.; McNeill, S.; MacLean, S.; Hornsby, J.; Ramsay, S. The prevalence of suspected ventilator-associated pneumonia in Scottish intensive care units. J. Intensiv. Care Soc. 2019, 21, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Metersky, M.L.; Wang, Y.; Klompas, M.; Eckenrode, S.; Bakullari, A.; Eldridge, N. Trend in Ventilator-Associated Pneumonia Rates Between 2005 and 2013. JAMA 2016, 316, 2427–2429. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Pesce, M.; Franchelli, S.; Baldelli, I.; De Maria, A.; Marchese, A. Phenotypic and genotypic characterization of Staphylococci causing breast peri-implant infections in oncologic patients. BMC Microbiol. 2015, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.D. Breast Augmentation, Antibiotic Prophylaxis, and Infection: Comparative Analysis of 1,628 Primary Augmentation Mammoplasties Assessing the Role and Efficacy of Antibiotics Prophylaxis Duration. Aesthetic Plast. Surg. 2009, 34, 42–47. [Google Scholar] [CrossRef]
- Basile, A.R.; Basile, F. Late infection following breast augmentation with textured silicone gel–filled implants. Aesthetic Surg. J. 2005, 25, 249–254. [Google Scholar] [CrossRef]
- Chen, C.-F.; Lin, S.-F.; Hung, C.-F.; Chou, P. Risk of infection is associated more with drain duration than daily drainage volume in prosthesis-based breast reconstruction. Medicine 2016, 95, e5605. [Google Scholar] [CrossRef] [PubMed]
- Kıvanç, S.A.; Arık, G.; Akova-Budak, B.; Kıvanç, M. Biofilm forming capacity and antibiotic susceptibility of Staphylococcus spp. with the icaA/icaD/bap genotype isolated from ocular surface of patients with diabetes. Malawi Med. J. 2018, 30, 243–249. [Google Scholar] [CrossRef]
- Oliver, J.C.; Bredarioli, P.A.P.; Leandro, F.D.; Ferreira, C.B.R.J.; Veiga, S.M.O.M.; Dias, A.L.T. Ozone against Pseudomonas aeruginosa biofilms in contact lenses storage cases. Revista do Instituto de Medicina Tropical de São Paulo 2019, 61, e23. [Google Scholar] [CrossRef] [PubMed]
- Onurdağ, F.K.; Özkan, S.; Ozgen, S.; Olmuş, H.; Abbasoglu, U. Candida albicans and Pseudomonas aeruginosa adhesion on soft contact lenses. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 249, 559–564. [Google Scholar] [CrossRef]
- Steele, K.R.; Szczotka-Flynn, L. Epidemiology of contact lens-induced infiltrates: An updated review. Clin. Exp. Optom. 2017, 100, 473–481. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Dhir, S. Biofilm and dental implant: The microbial link. J. Indian Soc. Periodontol. 2013, 17, 5–11. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alshagroud, R.S.; Alsahhaf, A.; Almojaly, S.A.; Abduljabbar, T.; Javed, F. Presence of Candida species in the subgingival oral biofilm of patients with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2019, 21, 781–785. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J.; Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Zatorska, B.; Groger, M.; Moser, D.; Diab-Elschahawi, M.; Lusignani, L.S.; Presterl, E. Does Extracellular DNA Production Vary in Staphylococcal Biofilms Isolated from Infected Implants versus Controls? Clin. Orthop. Relat. Res. 2017, 475, 2105–2113. [Google Scholar] [CrossRef]
- Fily, F.; Jolivet-Gougeon, A.; Polard, E.; Gicquel, T.; Dupont, M.; Verdier, M.; Arvieux, C. Moxifloxacin-rifampicin combination for the treatment of non-staphylococcal Gram-positive orthopedic implant-related infections. Médecine et Maladies Infectieuses 2019, 49, 540–544. [Google Scholar] [CrossRef]
- Blom, A.W.; Taylor, A.H.; Pattison, G.; Whitehouse, S.L.; Bannister, G.C. Infection after total hip arthroplasty. J. Bone Jt. Surg. Br. Vol. 2003, 85, 956–959. [Google Scholar] [CrossRef]
- Marmor, S.; Kerroumi, Y.; Meyssonnier, V.; Lhotellier, L.; Mouton, A.; Graff, W.; Zeller, V. One-Stage Exchange Arthroplasty for Fistulizing Periprosthetic Joint Infection of the Hip: An Effective Strategy. Front. Med. 2020, 7, 540929. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D.; Team, I. Re-Infection Outcomes following One- and Two-Stage Surgical Revision of Infected Hip Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0139166. [Google Scholar] [CrossRef]
- Fowler, T.J.; Sayers, A.; Whitehouse, M.R. Two-stage revision surgery for periprosthetic joint infection following total hip arthroplasty. Ann. Transl. Med. 2019, 7, S261. [Google Scholar] [CrossRef]
- Kisil, O.V.; Efimenko, T.A.; Gabrielyan, N.I.; Efremenkova, O.V. Development of antimicrobial therapy methods to overcome the antibiotic resistance of Acinetobacter baumannii. Acta Nat. 2020, 12, 34–45. [Google Scholar] [CrossRef]
- Tseng, B.S.; Zhang, W.; Harrison, J.J.; Quach, T.P.; Song, J.L.; Penterman, J.; Singh, P.K.; Chopp, D.L.; Packman, A.I.; Parsek, M.R. The extracellular matrix protects Pseudomonas aeruginosabiofilms by limiting the penetration of tobramycin. Environ. Microbiol. 2013, 15, 2865–2878. [Google Scholar] [CrossRef]
- Wilton, M.; Charron-Mazenod, L.; Moore, R.; Lewenza, S. Extracellular DNA Acidifies Biofilms and Induces Aminoglycoside Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 60, 544–553. [Google Scholar] [CrossRef]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA Chelates Cations and Induces Antibiotic Resistance in Pseudomonas aeruginosa Biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef]
- Driffield, K.; Miller, K.; Bostock, J.M.; O’Neill, A.J.; Chopra, I. Increased mutability of Pseudomonas aeruginosa in biofilms. J. Antimicrob. Chemother. 2008, 61, 1053–1056. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, P.; Wang, Y.; Hao, Y. Mechanisms and Control Measures of Mature Biofilm Resistance to Antimicrobial Agents in the Clinical Context. ACS Omega 2020, 5, 22684–22690. [Google Scholar] [CrossRef]
- Avila-Novoa, M.-G.; Solís-Velázquez, O.-A.; Rangel-López, D.-E.; González-Gómez, J.-P.; Guerrero-Medina, P.-J.; Gutiérrez-Lomelí, M. Biofilm Formation and Detection of Fluoroquinolone- and Carbapenem-Resistant Genes in Multidrug-ResistantAcinetobacter baumannii. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Coquet, L.; Junter, G.A.; Jouenne, T. Resistance of artificial biofilms of Pseudomonas aeruginosa to imipenem and tobramycin. J. Antimicrob. Chemother. 1998, 42, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [PubMed]
- Sirijant, N.; Sermswan, R.W.; Wongratanacheewin, S. Burkholderia pseudomallei resistance to antibiotics in biofilm-induced conditions is related to efflux pumps. J. Med. Microbiol. 2016, 65, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ling, N.; Gao, J.; Zhang, M.; Zhang, X.; Tong, L.; Ou, D.; Wang, Y.; Zhang, J.; Wu, Q. Short communication: Roles of outer membrane protein W (OmpW) on survival and biofilm formation of Cronobacter sakazakii under neomycin sulfate stress. J. Dairy Sci. 2018, 101, 2927–2931. [Google Scholar] [CrossRef]
- Mah, T.-F. Biofilm-specific antibiotic resistance. Futur. Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The Complex Relationship between Virulence and Antibiotic Resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus Biofilms Promote Horizontal Transfer of Antibiotic Resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef]
- Kouzel, N.; Oldewurtel, E.R.; Maier, B. Gene Transfer Efficiency in Gonococcal Biofilms: Role of Biofilm Age, Architecture, and Pilin Antigenic Variation. J. Bacteriol. 2015, 197, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Águila-Arcos, S.; Álvarez-Rodríguez, I.; Garaiyurrebaso, O.; Garbisu, C.; Grohmann, E.; Alkorta, I. Biofilm-Forming Clinical Staphylococcus Isolates Harbor Horizontal Transfer and Antibiotic Resistance Genes. Front. Microbiol. 2017, 8, 2018. [Google Scholar] [CrossRef] [PubMed]
- Saginur, R.; Stdenis, M.; Ferris, W.; Aaron, S.D.; Chan, F.; Lee, C.; Ramotar, K. Multiple Combination Bactericidal Testing of Staphylococcal Biofilms from Implant-Associated Infections. Antimicrob. Agents Chemother. 2006, 50, 55–61. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, X.; Tian, Y.; Tian, D.; Zhang, B. Incidence of surgical-site infection following open reduction and internal fixation of a distal femur fracture. Medicine 2019, 98, e14547. [Google Scholar] [CrossRef]
- Hedequist, D.; Haugen, A.; Hresko, T.; Emans, J. Failure of Attempted Implant Retention in Spinal Deformity Delayed Surgical Site Infections. Spine 2009, 34, 60–64. [Google Scholar] [CrossRef]
- Darouiche, R.O. Device-Associated Infections: A Macroproblem that Starts with Microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef]
- Pitiriga, V.; Kanellopoulos, P.; Bakalis, I.; Kampos, E.; Sagris, I.; Saroglou, G.; Tsakris, A. Central venous catheter-related bloodstream infection and colonization: The impact of insertion site and distribution of multidrug-resistant pathogens. Antimicrob. Resist. Infect. Control. 2020, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blot, S.I.; Depuydt, P.; Annemans, L.; Benoit, D.; Hoste, E.; De Waele, J.J.; Decruyenaere, J.; Vogelaers, D.; Colardyn, F.; Vandewoude, K.H. Clinical and Economic Outcomes in Critically Ill Patients with Nosocomial Catheter-Related Bloodstream Infections. Clin. Infect. Dis. 2005, 41, 1591–1598. [Google Scholar] [CrossRef]
- Subbarao, E.K.; Tarpay, M.M.; Marks, M.I. Soft-Tissue Infections Caused by Mycobacterium fortuitum Complex Following Penetrating Injury. Arch. Pediatr. Adolesc. Med. 1987, 141, 1018–1020. [Google Scholar] [CrossRef]
- Prażmo, E.J.; Kwaśny, M.; Łapiński, M.; Mielczarek, A. Photodynamic Therapy as a Promising Method Used in the Treatment of Oral Diseases. Adv. Clin. Exp. Med. 2016, 25, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, R.; Assadian, H.; Chiniforush, N.; Parker, S.; Pourakbari, B.; Ehsani, B.; Alikhani, M.Y.; Bahador, A. Modulation of virulence in Enterococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: An ex vivo biofilm model. Photodiagnosis Photodyn. Ther. 2020, 29, 101643. [Google Scholar] [CrossRef]
- Yuan, Z.; Tao, B.; He, Y.; Mu, C.; Liu, G.; Zhang, J.; Liao, Q.; Liu, P.; Cai, K. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials 2019, 223, 119479. [Google Scholar] [CrossRef] [PubMed]
- Teirlinck, E.; Barras, A.; Liu, J.; Fraire, J.C.; Lajunen, T.; Xiong, R.; Forier, K.; Li, C.; Urtti, A.; Boukherroub, R.; et al. Exploring Light-Sensitive Nanocarriers for Simultaneous Triggered Antibiotic Release and Disruption of Biofilms Upon Generation of Laser-Induced Vapor Nanobubbles. Pharmaceutics 2019, 11, 201. [Google Scholar] [CrossRef]
- Sayar, F.; Chiniforush, N.; Bahador, A.; Etemadi, A.; Akhondi, N.; Azimi, C. Efficacy of antimicrobial photodynamic therapy for elimination of Aggregatibacter actinomycetemcomitans biofilm on Laser-Lok titanium discs. Photodiagnosis Photodyn. Ther. 2019, 27, 462–466. [Google Scholar] [CrossRef]
- Briggs, T.; Blunn, G.; Hislop, S.; Ramalhete, R.; Bagley, C.; McKenna, D.; Coathup, M. Antimicrobial photodynamic therapy-a promising treatment for prosthetic joint infections. Lasers Med. Sci. 2017, 33, 523–532. [Google Scholar] [CrossRef]
- Geralde, M.C.; Leite, I.S.; Inada, N.M.; Salina, A.C.G.; Medeiros, A.I.; Kuebler, W.M.; Kurachi, C.; Bagnato, V.S. Pneumonia treatment by photodynamic therapy with extracorporeal illumination—An experimental model. Physiol. Rep. 2017, 5, e13190. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.X. A clinical review of phototherapy for psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef]
- Granick, M.S.; Paribathan, C.; Shanmugam, M.; Ramasubbu, N. Direct-Contact Low-Frequency Ultrasound Clearance of Biofilm from Metallic Implant Materials. Eplasty 2017, 17, e13. [Google Scholar]
- Dong, Y.; Chen, S.; Wang, Z.; Peng, N.; Yu, J. Synergy of ultrasound microbubbles and vancomycin against Staphylococcus epidermidis biofilm. J. Antimicrob. Chemother. 2012, 68, 816–826. [Google Scholar] [CrossRef]
- Carmen, J.C.; Runyan, C.M.; Robison, R.A.; Nelson, J.L.; Beckstead, B.L.; Pitt, W.G.; Schaalje, G.B. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J. Infect. Chemother. 2004, 10, 193–199. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, N.; Li, L.; Ma, Y.; Zhao, C.; Wu, Q.; Li, Y.; He, N.; Wang, X. The synergistic bactericidal effect of vancomycin on UTMD treated biofilm involves damage to bacterial cells and enhancement of metabolic activities. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Li, P.; Yu, J. Ultrasound Microbubbles Enhance the Activity of Vancomycin Against Staphylococcus epidermidis Biofilms In Vivo. J. Ultrasound Med. 2018, 37, 1379–1387. [Google Scholar] [CrossRef]
- LuTheryn, G.; Glynne-Jones, P.; Webb, J.S.; Carugo, D. Ultrasound-mediated therapies for the treatment of biofilms in chronic wounds: A review of present knowledge. Microb. Biotechnol. 2019, 13, 613–628. [Google Scholar] [CrossRef]
- He, N.; Hu, J.; Liu, H.; Zhu, T.; Huang, B.; Wang, X.; Wu, Y.; Wang, W.; Qu, D. Enhancement of Vancomycin Activity against Biofilms by Using Ultrasound-Targeted Microbubble Destruction. Antimicrob. Agents Chemother. 2011, 55, 5331–5337. [Google Scholar] [CrossRef]
- Rediske, A.M.; Roeder, B.L.; Nelson, J.L.; Robison, R.L.; Schaalje, G.B.; Robison, R.A.; Pitt, W.G. Pulsed Ultrasound Enhances the Killing ofEscherichia coli Biofilms by Aminoglycoside Antibiotics In Vivo. Antimicrob. Agents Chemother. 2000, 44, 771–772. [Google Scholar] [CrossRef]
- Bigelow, T.A.; Thomas, C.L.; Wu, H.; Itani, K.M.F. Impact of High-Intensity Ultrasound on Strength of Surgical Mesh When Treating Biofilm Infections. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 66, 38–44. [Google Scholar] [CrossRef]
- Ng, E.; Lim, L.P. An Overview of Different Interdental Cleaning Aids and Their Effectiveness. Dent. J. 2019, 7, 56. [Google Scholar] [CrossRef]
- Frascella, J.A.; Fernández, P.; Gilbert, R.D.; Cugini, M. A randomized, clinical evaluation of the safety and efficacy of a novel oral irrigator. Am. J. Dent. 2000, 13, 55–58. [Google Scholar]
- Kato, K.; Tamura, K.; Nakagaki, H. Quantitative evaluation of the oral biofilm-removing capacity of a dental water jet using an electron-probe microanalyzer. Arch. Oral Biol. 2012, 57, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sharab, L.; Baier, R.; Ciancio, S.; Mang, T. Influence of Photodynamic Therapy on Bacterial Attachment to Titanium Surface. J. Oral Implant. 2020. [Google Scholar] [CrossRef]
- Yamada, J.; Takiguchi, T.; Saito, A.; Odanaka, H.; Soyama, H.; Yamamoto, M. Removal of Oral Biofilm on an Implant Fixture by a Cavitating Jet. Implant. Dent. 2017, 26, 904–910. [Google Scholar] [CrossRef]
- Toma, S.; Behets, C.; Brecx, M.C.; Lasserre, J.F. In Vitro Comparison of the Efficacy of Peri-Implantitis Treatments on the Removal and Recolonization of Streptococcus gordonii Biofilm on Titanium Disks. Materials 2018, 11, 2484. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Rudolph, M.; Bause, V.; Terfort, A. Electrochemical removal of biofilms from titanium dental implant surfaces. Bioelectrochemistry 2018, 121, 84–94. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Black, R.; Kum, J.; Berbel, L.; Sadr, A.; Karoussis, I.; Simopoulou, M.; Daubert, D. Effect of implant cleaning on titanium particle dissolution and cytocompatibility. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Li, H.; Fairfax, M.R.; Dubocq, F.; Darouiche, R.O.; Rajpurkar, A.; Thompson, M.; Tefilli, M.V.; Dhabuwala, C.B. Antibacterial Activity of Antibiotic Coated Silicone Grafts. J. Urol. 1998, 160, 1910–1913. [Google Scholar] [CrossRef]
- Dwyer, A. Reducing Tunneled Hemodialysis Catheter Morbidity: Surface-Treated Catheters—A Review. Semin. Dial. 2008, 21, 542–546. [Google Scholar] [CrossRef]
- Jennings, J.A.; Carpenter, D.P.; Troxel, K.S.; Beenken, K.E.; Smeltzer, M.S.; Courtney, H.S.; Haggard, W.O. Novel Antibiotic-loaded Point-of-care Implant Coating Inhibits Biofilm. Clin. Orthop. Relat. Res. 2015, 473, 2270–2282. [Google Scholar] [CrossRef]
- Nielsen, C.; Subbiahdoss, G.; Zeng, G.; Salmi, Z.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R. Antibacterial isoeugenol coating on stainless steel and polyethylene surfaces prevents biofilm growth. J. Appl. Microbiol. 2017, 124, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef]
- Costa, F.; Sousa, D.M.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Clement, J.L.; Jarrett, P.S. Antibacterial Silver. Met. Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M.; Deponti, D.; Di Giancamillo, A.; Peretti, G.; Sannino, A. Effect of silver nanocoatings on catheters for haemodialysis in terms of cell viability, proliferation, morphology and antibacterial activity. J. Mater. Sci. Mater. Med. 2013, 24, 1105–1112. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Mathieu, M.; Lavigne, J.-P.; Toupet, K.; Guerrero, G.; Ponche, A.; Amalric, J.; Noël, D.; Mutin, P.H. In vitro and in vivo characterization of antibacterial activity and biocompatibility: A study on silver-containing phosphonate monolayers on titanium. Acta Biomater. 2015, 15, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zuber, F.; Maniura-Weber, K.; Brugger, J.; Ren, Q. Nanostructured surface topographies have an effect on bactericidal activity. J. Nanobiotechnology 2018, 16, 1–9. [Google Scholar] [CrossRef]
- Liu, R.; Tang, Y.; Zeng, L.; Zhao, Y.; Ma, Z.; Sun, Z.; Xiang, L.; Ren, L.; Yang, K. In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application. Dent. Mater. 2018, 34, 1112–1126. [Google Scholar] [CrossRef]
- Sopata, M.; Karpiński, T.M.; Jakubowicz, J.; Sopata, M. Development of tantalum with highly hydrophilic surface and antimicrobial properties obtained by micro-arc oxidation process. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020. [Google Scholar] [CrossRef]
- Bottino, M.A.; Pereira, S.; Amaral, M.; Milhan, N.; Pereira, C.A.; Camargo, S.; Carvalho, A.; Melo, R.M. Streptococcus mutans Biofilm Formation and Cell Viability on Polymer-infiltrated Ceramic and Yttria-stabilized Polycrystalline Zirconium Dioxide Ceramic. Oper. Dent. 2019, 44, E271–E278. [Google Scholar] [CrossRef]
- Artini, M.; Cicatiello, P.; Ricciardelli, A.; Papa, R.; Selan, L.; Dardano, P.; Tilotta, M.; Vrenna, G.; Tutino, M.L.; Giardina, P.; et al. Hydrophobin coating prevents Staphylococcus epidermidis biofilm formation on different surfaces. Biofouling 2017, 33, 601–611. [Google Scholar] [CrossRef]
- Távora, F.F.F.; Chocano, A.P.C.; De Oliveira, D.G.; Pereira, J.R.; Almeida, R.S.; Neppelenbroek, K.H.; Porto, V.C. Beneficial Effects of Ethyl-Cyanoacrylate Coating Against Candida Albicans Biofilm Formation. Braz. Dent. J. 2019, 30, 266–271. [Google Scholar] [CrossRef]
- Lockhart, J.N.; Spoonmore, T.J.; McCurdy, M.W.; Rogers, B.R.; Guelcher, S.A.; Harth, E. Poly(glycidol) Coating on Ultrahigh Molecular Weight Polyethylene for Reduced Biofilm Growth. ACS Appl. Mater. Interfaces 2018, 10, 4050–4056. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment ofStaphylococcus aureusbiofilms. Expert Rev. Anti-Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef] [PubMed]
- Gotz, F. Staphylococcus and biofilms. Mol. Microbiol. 2002, 43, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Na, M.; Jarneborn, A.; Jacobsson, G.; Peetermans, M.; Verhamme, P.; Jin, T. Tissue Plasminogen Activator Coating on Implant Surfaces Reduces Staphylococcus Aureus Biofilm Formation. Appl. Environ. Microbiol. 2015, 82, 394–401. [Google Scholar] [CrossRef]
- Xu, Y.; Jones, J.E.; Yu, H.; Yu, Q.; Christensen, G.D.; Chen, M.; Sun, H. Nanoscale Plasma Coating Inhibits Formation of Staphylococcus aureus Biofilm. Antimicrob. Agents Chemother. 2015, 59, 7308–7315. [Google Scholar] [CrossRef]
- Hogan, S.; Kasotakis, E.; Maher, S.; Cavanagh, B.; O’Gara, J.P.; Pandit, A.; Keyes, T.E.; Devocelle, M.; O’Neill, E. A novel medical device coating prevents Staphylococcus aureus biofilm formation on medical device surfaces. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Ye, J.; Shao, C.; Zhang, X.; Guo, X.; Gao, P.; Cen, Y.; Ma, S.; Liu, Y. Effects of DNase I coating of titanium on bacteria adhesion and biofilm formation. Mater. Sci. Eng. C 2017, 78, 738–747. [Google Scholar] [CrossRef]
- Senpuku, H.; Tuna, E.B.; Nagasawa, R.; Nakao, R.; Ohnishi, M. The inhibitory effects of polypyrrole on the biofilm formation of Streptococcus mutans. PLoS ONE 2019, 14, e0225584. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.M.; Costa, R.C.; Nagay, B.E.; Dongari-Bagtzoglou, A.; Barão, V.A.R. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. iScience 2021, 24, 102008. [Google Scholar] [CrossRef]
- Zoccali, C.; Scoccianti, G.; Biagini, R.; Daolio, P.A.; Giardina, F.L.; Campanacci, D.A. Antibacterial hydrogel coating in joint mega-prosthesis: Results of a comparative series. Eur. J. Orthop. Surg. Traumatol. 2021, 1–9. [Google Scholar] [CrossRef]
- Fiore, M.; Sambri, A.; Zucchini, R.; Giannini, C.; Donati, D.M.; De Paolis, M. Silver-coated megaprosthesis in prevention and treatment of peri-prosthetic infections: A systematic review and meta-analysis about efficacy and toxicity in primary and revision surgery. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 201–220. [Google Scholar] [CrossRef]
- Sochacki, K.A.; Barns, K.J.; Bucki, R.; Weisshaar, J.C. Real-time attack on single Escherichia coli cells by the human antimicrobial peptide LL-37. Proc. Natl. Acad. Sci. USA 2011, 108, E77–E81. [Google Scholar] [CrossRef]
- Wang, G.; Hanke, M.L.; Mishra, B.; Lushnikova, T.; Heim, C.E.; Chittezham Thomas, V.; Bayles, K.W.; Kielian, T. Transformation of Human Cathelicidin LL-37 into Selective, Stable, and Potent Antimicrobial Compounds. ACS Chem. Biol. 2014, 9, 1997–2002. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, G.; Lu, S.; Chen, D.; Fan, S.; Xu, J.; Wu, B.; He, J. Design and antimicrobial activities of LL-37 derivatives inhibiting the formation of Streptococcus mutans biofilm. Chem. Biol. Drug Des. 2019, 93, 1175–1185. [Google Scholar] [CrossRef]
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Kaplan, C.W.; Sim, J.H.; Shah, K.R.; Kolesnikova-Kaplan, A.; Shi, W.; Eckert, R. Selective Membrane Disruption: Mode of Action of C16G2, a Specifically Targeted Antimicrobial Peptide. Antimicrob. Agents Chemother. 2011, 55, 3446–3452. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Wang, C.; Qiao, X.; Wu, S.; Wang, H.; Feng, L.; Wang, Y. Assessing the potential of four cathelicidins for the management of mouse candidiasis and Candida albicans biofilms. Biochimie 2016, 121, 268–277. [Google Scholar] [CrossRef]
- Rowe-Magnus, D.A.; Kao, A.Y.; Prieto, A.C.; Pu, M.; Kao, C. Cathelicidin Peptides Restrict Bacterial Growth via Membrane Perturbation and Induction of Reactive Oxygen Species. mBio 2019, 10. [Google Scholar] [CrossRef]
- Dean, S.N.; Bishop, B.M.; Van Hoek, M.L. Susceptibility of Pseudomonas aeruginosa Biofilm to Alpha-Helical Peptides: D-enantiomer of LL-37. Front. Microbiol. 2011, 2, 128. [Google Scholar] [CrossRef]
- Zhu, C.; Tan, H.; Cheng, T.; Shen, H.; Shao, J.; Guo, Y.; Shi, S.; Zhang, X. Human β-defensin 3 inhibits antibiotic-resistant Staphylococcus biofilm formation. J. Surg. Res. 2013, 183, 204–213. [Google Scholar] [CrossRef]
- Geng, H.; Yuan, Y.; Adayi, A.; Zhang, X.; Song, X.; Gong, L.; Zhang, X.; Gao, P. Engineered chimeric peptides with antimicrobial and titanium-binding functions to inhibit biofilm formation on Ti implants. Mater. Sci. Eng. C 2018, 82, 141–154. [Google Scholar] [CrossRef]
- Li, S.; Zhu, C.; Fang, S.; Zhang, W.; He, N.; Xu, W.; Kong, R.; Shang, X. Ultrasound microbubbles enhance human β-defensin 3 against biofilms. J. Surg. Res. 2015, 199, 458–469. [Google Scholar] [CrossRef]
- Parducho, K.R.; Beadell, B.; Ybarra, T.K.; Bush, M.; Escalera, E.; Trejos, A.T.; Chieng, A.; Mendez, M.; Anderson, C.; Park, H.; et al. The Antimicrobial Peptide Human Beta-Defensin 2 Inhibits Biofilm Production of Pseudomonas aeruginosa Without Compromising Metabolic Activity. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Moazzezy, N.; Karam, M.R.A.; Rafati, S.; Bouzari, S.; Oloomi, M. Inhibition and eradication activity of truncated α-defensin analogs against multidrug resistant uropathogenic Escherichia coli biofilm. PLoS ONE 2020, 15, e0235892. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Lin, L.; Tan, L.-S.; Yu, H.-Y.; Cheng, J.-W.; Pan, Y.-P. Molecular pathways underlying inhibitory effect of antimicrobial peptide Nal-P-113 on bacteria biofilms formation of Porphyromonas gingivalis W83 by DNA microarray. BMC Microbiol. 2017, 17, 1–7. [Google Scholar] [CrossRef]
- Hinsa, S.M.; Espinosa-Urgel, M.; Ramos, J.L.; O’Toole, G.A. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol. Microbiol. 2003, 49, 905–918. [Google Scholar] [CrossRef]
- Di Somma, A.; Recupido, F.; Cirillo, A.; Romano, A.; Romanelli, A.; Caserta, S.; Guido, S.; Duilio, A. Antibiofilm Properties of Temporin-L on Pseudomonas fluorescens in Static and In-Flow Conditions. Int. J. Mol. Sci. 2020, 21, 8526. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, A.; Avitabile, C.; Cirillo, A.; Moretta, A.; Merlino, A.; Paduano, L.; Duilio, A.; Romanelli, A. The antimicrobial peptide Temporin L impairs E. coli cell division by interacting with FtsZ and the divisome complex. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129606. [Google Scholar] [CrossRef]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Hobbs, M.; Livingston, S.P.; Krishnapillai, V.; Mattick, J.S. Characterisation of a Pseudomonas aeruginosa twitching motility gene and evidence for a specialised protein export system widespread in eubacteria. Gene 1991, 101, 33–44. [Google Scholar] [CrossRef]
- Haisma, E.M.; De Breij, A.; Chan, H.; Van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; El Ghalbzouri, A.; Nibbering, P.H. LL-37-Derived Peptides Eradicate Multidrug-Resistant Staphylococcus aureus from Thermally Wounded Human Skin Equivalents. Antimicrob. Agents Chemother. 2014, 58, 4411–4419. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.M.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of Bacterial Biofilm Formation and Swarming Motility by a Small Synthetic Cationic Peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Giacomucci, S.; Cros, C.D.-N.; Perron, X.; Mathieu-Denoncourt, A.; Duperthuy, M. Flagella-dependent inhibition of biofilm formation by sub-inhibitory concentration of polymyxin B in Vibrio cholerae. PLoS ONE 2019, 14, e0221431. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente-Núñez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nat. Cell Biol. 2013, 503, 365–370. [Google Scholar] [CrossRef]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; De Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; Van Der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef]
- Maisetta, G.; Grassi, L.; Di Luca, M.; Bombardelli, S.; Medici, C.; Brancatisano, F.L.; Esin, S.; Batoni, G. Anti-biofilm properties of the antimicrobial peptide temporin 1Tb and its ability, in combination with EDTA, to eradicate Staphylococcus epidermidis biofilms on silicone catheters. Biofouling 2016, 32, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.M.; Abraham, N.M.; Massaro, J.; Murphy, K.; Smith-Carpenter, J.; Fikrig, E. Anti-Biofilm Activity of a Self-Aggregating Peptide against Streptococcus mutans. Front. Microbiol. 2017, 8, 488. [Google Scholar] [CrossRef]
- Brancatisano, F.L.; Maisetta, G.; Di Luca, M.; Esin, S.; Bottai, D.; Bizzarri, R.; Campa, M.; Batoni, G. Inhibitory effect of the human liver-derived antimicrobial peptide hepcidin 20 on biofilms of polysaccharide intercellular adhesin (PIA)-positive and PIA-negative strains of Staphylococcus epidermidis. Biofouling 2014, 30, 435–446. [Google Scholar] [CrossRef]
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M.; et al. Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms. FEBS J. 2017, 284, 3662–3683. [Google Scholar] [CrossRef]
- Grassi, L.; Batoni, G.; Ostyn, L.; Rigole, P.; Van den Bossche, S.; Rinaldi, A.C.; Maisetta, G.; Esin, S.; Coenye, T.; Crabbé, A. The Antimicrobial Peptide lin-SB056-1 and Its Dendrimeric Derivative Prevent Pseudomonas aeruginosa Biofilm Formation in Physiologically Relevant Models of Chronic Infections. Front. Microbiol. 2019, 10, 198. [Google Scholar] [CrossRef]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Shai, Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1610–1619. [Google Scholar] [CrossRef]
- Shurko, J.F.; Galega, R.S.; Li, C.; Lee, G.C. Evaluation of LL-37 antimicrobial peptide derivatives alone and in combination with vancomycin against S. aureus. J. Antibiot. 2018, 71, 971–974. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Murillo, O.; Ariza, J. Clinical Use of Colistin in Biofilm-Associated Infections. Adv. Exp. Med. Biol. 2019, 1145, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 10–39. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Shen, T.; Chen, L.; Zhou, J.; Shi, S.; Wang, Y.; Zhao, Z.; Liao, C.; Wang, C. N-terminal Myristoylation Enhanced the Antimicrobial Activity of Antimicrobial Peptide PMAP-36PW. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The antimicrobial peptide ZY4 combats multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2019, 116, 26516–26522. [Google Scholar] [CrossRef]
- Miao, X.; Zhou, T.; Zhang, J.; Xu, J.; Guo, X.; Hu, H.; Zhang, X.; Hu, M.; Li, J.; Yang, W.; et al. Enhanced cell selectivity of hybrid peptides with potential antimicrobial activity and immunomodulatory effect. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129532. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Su, P.-Y.; Kuo, S.-C.; Lauderdale, T.-L.Y.; Shih, C. Adding a C-terminal Cysteine (CTC) Can Enhance the Bactericidal Activity of Three Different Antimicrobial Peptides. Front. Microbiol. 2018, 9, 1440. [Google Scholar] [CrossRef]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. D- and Unnatural Amino Acid Substituted Antimicrobial Peptides with Improved Proteolytic Resistance and Their Proteolytic Degradation Characteristics. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Kao, C.; Lin, X.; Yi, G.; Zhang, Y.; Rowe-Magnus, D.A.; Bush, K. Cathelicidin Antimicrobial Peptides with Reduced Activation of Toll-Like Receptor Signaling Have Potent Bactericidal Activity against Colistin-Resistant Bacteria. mBio 2016, 7. [Google Scholar] [CrossRef]
- Almaaytah, A.; Mohammed, G.K.; Abualhaijaa, A.; Al-Balas, Q. Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Des. Dev. Ther. 2017, 11, 3159–3170. [Google Scholar] [CrossRef]
- Kim, H.; Beack, S.; Han, S.; Shin, M.; Lee, T.; Park, Y.; Kim, K.S.; Yetisen, A.K.; Yun, S.H.; Kwon, W.; et al. Multifunctional Photonic Nanomaterials for Diagnostic, Therapeutic, and Theranostic Applications. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: Current status and potential future applications. J. Nanobiotechnol. 2020, 18, 1–18. [Google Scholar] [CrossRef]
- Manosalva, N.; Tortella, G.; Cristina Diez, M.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green synthesis of silver nanoparticles: Effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef]
- Estevez, M.B.; Raffaelli, S.; Mitchell, S.G.; Faccio, R.; Alborés, S. Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules 2020, 25, 2023. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, C.; Guzmán-Moreno, J.; Ángeles-Chávez, C.; Rodríguez-González, V.; Ortega-Sigala, J.J.; Ramírez-Santoyo, R.M.; Vidales-Rodríguez, L.E. Biosynthesis of silver nanoparticles by Fusarium scirpi and its potential as antimicrobial agent against uropathogenic Escherichia coli biofilms. PLoS ONE 2020, 15, e0230275. [Google Scholar] [CrossRef]
- Teirlinck, E.; Xiong, R.; Brans, T.; Forier, K.; Fraire, J.; Van Acker, H.; Matthijs, N.; De Rycke, R.; De Smedt, S.C.; Coenye, T.; et al. Laser-induced vapour nanobubbles improve drug diffusion and efficiency in bacterial biofilms. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Habibipour, R.; Moradi-Haghgou, L.; Farmany, A. Green synthesis of AgNPs@PPE and its Pseudomonas aeruginosa biofilm formation activity compared to pomegranate peel extract. Int. J. Nanomed. 2019, 14, 6891–6899. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; James, B.; Devadathan, A.; Johny, M.K.; Mathew, J.; Jacob, J. Comparative Evaluation of Antibiofilm Efficacy of Chitosan Nanoparticle- and Zinc Oxide Nanoparticle-Incorporated Calcium Hydroxide-Based Sealer: An In vitro Study. Contemp. Clin. Dent. 2018, 9, 434–439. [Google Scholar]
- Niemirowicz, K.; Piktel, E.; Wilczewska, A.Z.; Markiewicz, K.H.; Durnaś, B.; Wątek, M.; Puszkarz, I.; Wróblewska, M.; Niklińska, W.; Savage, P.B.; et al. Core–shell magnetic nanoparticles display synergistic antibacterial effects against Pseudomonas aeruginosa and Staphylococcus aureus when combined with cathelicidin LL-37 or selected ceragenins. Int. J. Nanomed. 2016, 11, 5443–5455. [Google Scholar] [CrossRef] [PubMed]
- Esteban Florez, F.L.; Hiers, R.D.; Larson, P.; Johnson, M.; O’Rear, E.; Rondinone, A.J.; Khajotia, S.S. Antibacterial dental adhesive resins containing nitrogen-doped titanium dioxide nanoparticles. Mater. Sci. Eng. C 2018, 93, 931–943. [Google Scholar] [CrossRef]
- Duncan, B.; Li, X.; Landis, R.F.; Kim, S.T.; Gupta, A.; Wang, L.-S.; Ramanathan, R.; Tang, R.; Boerth, J.A.; Rotello, V.M. Nanoparticle-Stabilized Capsules for the Treatment of Bacterial Biofilms. ACS Nano 2015, 9, 7775–7782. [Google Scholar] [CrossRef]
- Siddhardha, B.; Pandey, U.; Kaviyarasu, K.; Pala, R.; Syed, A.; Bahkali, A.H.; Elgorban, A.M. Chrysin-Loaded Chitosan Nanoparticles Potentiates Antibiofilm Activity against Staphylococcus aureus. Pathogens 2020, 9, 115. [Google Scholar] [CrossRef]
- Scott, C.J.; Abdelghany, S.M.; Quinn, D.J.; Ingram, R.J.; Gilmore, B.F.; Donnelly, R.F.; Taggart, C.C. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063. [Google Scholar] [CrossRef]
- Liakos, I.L.; Grumezescu, A.M.; Holban, A.M.; Florin, I.; D’Autilia, F.; Carzino, R.; Bianchini, P.; Athanassiou, A. Polylactic Acid—Lemongrass Essential Oil Nanocapsules with Antimicrobial Properties. Pharmaceuticals 2016, 9, 42. [Google Scholar] [CrossRef]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15. [Google Scholar] [CrossRef]
- Guo, P.; Buttaro, B.A.; Xue, H.Y.; Tran, N.T.; Wong, H.L. Lipid-polymer hybrid nanoparticles carrying linezolid improve treatment of methicillin-resistant Staphylococcus aureus (MRSA) harbored inside bone cells and biofilms. Eur. J. Pharm. Biopharm. 2020, 151, 189–198. [Google Scholar] [CrossRef]
- Vera-González, N.; Bailey-Hytholt, C.M.; Langlois, L.; de Camargo Ribeiro, F.; de Souza Santos, E.L.; Junqueira, J.C.; Shukla, A. Anidulafungin liposome nanoparticles exhibit antifungal activity against planktonic and biofilm Candida albicans. J. Biomed. Mater. Res. Part A 2020, 108, 2263–2276. [Google Scholar] [CrossRef]
- Hu, D.; Deng, Y.; Jia, F.; Jin, Q.; Ji, J. Surface Charge Switchable Supramolecular Nanocarriers for Nitric Oxide Synergistic Photodynamic Eradication of Biofilms. ACS Nano 2019, 14, 347–359. [Google Scholar] [CrossRef]
- Gondil, V.S.; Kalaiyarasan, T.; Bharti, V.K.; Chhibber, S. Antibiofilm potential of Seabuckthorn silver nanoparticles (SBT@AgNPs) against Pseudomonas aeruginosa. 3 Biotech 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Manivasagan, P.; Lee, J.-W.; Pham, D.T.N.; Oh, J.; Kim, Y.-M. Fucoidan-Stabilized Gold Nanoparticle-Mediated Biofilm Inhibition, Attenuation of Virulence and Motility Properties in Pseudomonas aeruginosa PAO1. Mar. Drugs 2019, 17, 208. [Google Scholar] [CrossRef]
- Mu, H.; Guo, F.; Niu, H.; Liu, Q.; Wang, S.; Duan, J. Chitosan Improves Anti-Biofilm Efficacy of Gentamicin through Facilitating Antibiotic Penetration. Int. J. Mol. Sci. 2014, 15, 22296–22308. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmad, T.; Khan, A.; Sarwar, R.; Shafiq, J.; Raza, Y.; Ahmed, A.; Ullah, S.; Ur Rehman, N.; Al-Harrasi, A. Rifampicin conjugated silver nanoparticles: A new arena for development of antibiofilm potential against methicillin resistant Staphylococcus aureus and Klebsiella pneumoniae. Int. J. Nanomed. 2019, 14, 3983–3993. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.E.; Gouyau, J.; Lamouroux, E. Limitations of Recent Studies Dealing with the Antibacterial Properties of Silver Nanoparticles: Fact and Opinion. Nanomaterials 2019, 9, 1775. [Google Scholar] [CrossRef]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative Strategies Toward the Disassembly of the EPS Matrix in Bacterial Biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Eddenden, A.; Kitova, E.N.; Klassen, J.S.; Nitz, M. An Inactive Dispersin B Probe for Monitoring PNAG Production in Biofilm Formation. ACS Chem. Biol. 2020, 15, 1204–1211. [Google Scholar] [CrossRef]
- Donelli, G.; Francolini, I.; Romoli, D.; Guaglianone, E.; Piozzi, A.; Ragunath, C.; Kaplan, J.B. Synergistic Activity of Dispersin B and Cefamandole Nafate in Inhibition of Staphylococcal Biofilm Growth on Polyurethanes. Antimicrob. Agents Chemother. 2007, 51, 2733–2740. [Google Scholar] [CrossRef]
- Chen, K.-J.; Lee, C.-K. Twofold enhanced dispersin B activity by N-terminal fusion to silver-binding peptide for biofilm eradication. Int. J. Biol. Macromol. 2018, 118, 419–426. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Fleming, D.; Rumbaugh, K.P. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, A.P. Antibiofilm Effect of DNase against Single and Mixed Species Biofilm. Foods 2018, 7, 42. [Google Scholar] [CrossRef]
- ElAdawy, M.; El-Mowafy, M.; El-Sokkary, M.M.A.; Barwa, R. Effects of Lysozyme, Proteinase K, and Cephalosporins on Biofilm Formation by Clinical Isolates of Pseudomonas aeruginosa. Interdiscip. Perspect. Infect. Dis. 2020, 2020, 6156720. [Google Scholar] [CrossRef] [PubMed]
- Borghi, G.N.; Rodrigues, L.P.; Lopes, L.M.; Parisotto, T.M.; Steiner-Oliveira, C.; Nobre-Dos-Santos, M. Relationship amongαamylase and carbonic anhydrase VI in saliva, visible biofilm, and early childhood caries: A longitudinal study. Int. J. Paediatr. Dent. 2017, 27, 174–182. [Google Scholar] [CrossRef]
- Baker, P.; Hill, P.J.; Snarr, B.D.; Alnabelseya, N.; Pestrak, M.J.; Lee, M.J.; Jennings, L.K.; Tam, J.; Melnyk, R.A.; Parsek, M.R.; et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci. Adv. 2016, 2, e1501632. [Google Scholar] [CrossRef]
- Raad, I.; Chatzinikolaou, I.; Chaiban, G.; Hanna, H.; Hachem, R.; Dvorak, T.; Cook, G.; Costerton, W. In Vitro and Ex Vivo Activities of Minocycline and EDTA against Microorganisms Embedded in Biofilm on Catheter Surfaces. Antimicrob. Agents Chemother. 2003, 47, 3580–3585. [Google Scholar] [CrossRef]
- Casalinuovo, I.A.; Sorge, R.; Bonelli, G.; Di Francesco, P. Evaluation of the antifungal effect of EDTA, a metal chelator agent, on Candida albicans biofilm. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1413–1420. [Google Scholar]
- Shao, C.; Zhang, X.; Ye, J.; Li, Y.-C.; Bao, Y.-J.; Li, Z.-H.; Huang, Y.; Liu, Y. Surface functionalization of titanium substrates with Deoxyribonuclease I inhibit peri-implant bacterial infection. Dent. Mater. J. 2020, 2020–2055. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Ludwig, R.; Schneider-Stickler, B. Co-immobilization of cellobiose dehydrogenase and deoxyribonuclease I on chitosan nanoparticles against fungal/bacterial polymicrobial biofilms targeting both biofilm matrix and microorganisms. Mater. Sci. Eng. C 2020, 108, 110499. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Černáková, L. Farnesol and Tyrosol: Secondary Metabolites with a Crucial quorum-sensing Role in Candida Biofilm Development. Genes 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, B.; Souza, G.K.; Chiavelli, L.U.R.; Pomini, A.M.; Svidzinski, T.I.E.; Negri, M. The ability of farnesol to prevent adhesion and disrupt Fusarium keratoplasticum biofilm. Appl. Microbiol. Biotechnol. 2020, 104, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Shirtliff, M.; James, C.; Meiller, T. Effect of farnesol onCandida dubliniensisbiofilm formation and fluconazole resistance. FEMS Yeast Res. 2006, 6, 1063–1073. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dastidar, D.G.; Paul, P.; Dutta, S.; Basu, D.; Sharma, S.R.; Basu, S.; Sarker, R.K.; Sen, A.; Sarkar, A.; et al. Inhibition of biofilm formation of Pseudomonas aeruginosa by caffeine: A potential approach for sustainable management of biofilm. Arch. Microbiol. 2019, 202, 623–635. [Google Scholar] [CrossRef]
- Fernandes, A.W.C.; Santos, V.L.D.A.; Araújo, C.R.M.; De Oliveira, H.P.; Da Costa, M.M. Anti-biofilm Effect of β-Lapachone and Lapachol Oxime Against Isolates of Staphylococcus aureus. Curr. Microbiol. 2019, 77, 204–209. [Google Scholar] [CrossRef]
- Kipanga, P.N.; Liu, M.; Panda, S.K.; Mai, A.H.; Veryser, C.; Van Puyvelde, L.; De Borggraeve, W.M.; Van Dijck, P.; Matasyoh, J.; Luyten, W. Biofilm inhibiting properties of compounds from the leaves of Warburgia ugandensis Sprague subsp ugandensis against Candida and staphylococcal biofilms. J. Ethnopharmacol. 2020, 248, 112352. [Google Scholar] [CrossRef]
- Shi, L.; Wu, Y.; Yang, C.; Ma, Y.; Zhang, Q.-Z.; Huang, W.; Zhu, X.-Y.; Yan, Y.-J.; Wang, J.-X.; Zhu, T.; et al. Effect of nicotine on Staphylococcus aureus biofilm formation and virulence factors. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Lombardi, L.; Klatt, A.-B.; Trampuz, A.; Di Luca, M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 842–853. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, M.; Zhang, H.; Dai, J.; Guo, Z.; Li, X.; Ji, Y.; Cai, R.; Xi, H.; Wang, X.; et al. Antibacterial Effects of Phage Lysin LysGH15 on Planktonic Cells and Biofilms of Diverse Staphylococci. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Sosa, B.R.; Niu, Y.; Turajane, K.; Staats, K.; Suhardi, V.; Carli, A.; Fischetti, V.; Bostrom, M.; Yang, X. 2020 John Charnley Award: The antimicrobial potential of bacteriophage-derived lysin in a murine debridement, antibiotics, and implant retention model of prosthetic joint infection. Bone Jt. J. 2020, 102 (Suppl. B), 3–10. [Google Scholar] [CrossRef]
- Corbellino, M.; Kieffer, N.; Kutateladze, M.; Balarjishvili, N.; Leshkasheli, L.; Askilashvili, L.; Tsertsvadze, G.; Rimoldi, S.G.; Nizharadze, D.; Hoyle, N.; et al. Eradication of a Multidrug-Resistant, Carbapenemase-Producing Klebsiella pneumoniae Isolate Following Oral and Intra-rectal Therapy with a Custom Made, Lytic Bacteriophage Preparation. Clin. Infect. Dis. 2019, 70, 1998–2001. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Kelly, N.; Elliott, L.; Grant, A.; Wilkinson, M.; Hazratwala, K.; McEwen, P. Evaluation of Bacteriophage Anti-Biofilm Activity for Potential Control of Orthopedic Implant-Related Infections Caused by Staphylococcus aureus. Surg. Infect. 2019, 20, 16–24. [Google Scholar] [CrossRef]
- Qin, J.; Wu, N.; Bao, J.; Shi, X.; Ou, H.; Ye, S.; Zhao, W.; Wei, Z.; Cai, J.; Li, L.; et al. Heterogeneous Klebsiella pneumoniae Co-infections Complicate Personalized Bacteriophage Therapy. Front. Cell. Infect. Microbiol. 2021, 10. [Google Scholar] [CrossRef]
- Cesta, N.; Di Luca, M.; Corbellino, M.; Tavio, M.; Galli, M.; Andreoni, M. Bacteriophage therapy: An overview and the position of Italian Society of Infectious and Tropical Diseases. Le Infezioni Medicina 2020, 28, 322–331. [Google Scholar]
| Medical Devices | Main Microorganisms | Infection Rates | Reference |
|---|---|---|---|
| Cardiac implantable devices | S. epidermidis, S. aureus, S. hominis | 0.13%–38.6% | [42,43,44,45,46,47,48,49] |
| Hemodialyzers | Staphylococci, P. aeruginosa, S. marcescens | ~18% | [28,50,51,52] |
| Center venous catheters | S. aureus, S. epidermidis, P. aeruginosa, candida, B. cereus | 3%–14% | [17,53,54,55,56,57] |
| Urinary catheters | E. coli, E. cloacae, P. mirabilis, Klebsiella, Enterococcus spp., P. aeruginosa, Candida | 9.1%–26.6% | [33,58,59,60,61,62,63] |
| Ventilator | P. aeruginosa, A. baumannii, Klebsiella species, S. maltophilia, Enterococcus spp., Candida spp. | 5%–40% | [37,62,64,65,66,67,68,69] |
| Artificial breasts | S. aureus and S. epidermidis | 0.5%–5.1% | [70,71,72,73] |
| Contact lenses | Staphylococcus spp., P. aeruginosa, C. albicans | 2.5%–6% | [74,75,76,77] |
| Dental prosthetics | Streptococcus, Fusobacterium, Capnocytophaga, Actinomyces and Candida species | 5.9%–56% | [78,79,80,81] |
| Orthopedic prosthetics | MRSA, MRSE, S. epidermis, S. aureus, P. acnes, E. faecalis | 1%–15% | [32,82,83,84,85,86,87] |
| AMP | Pathogen | Infection Model | MIC | MBIC/EC | Effects and Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| IG-13-1 IG-13-2 (M) | S. mutans | in vitro | 5 μM 5 μM | 6.92 μM 7.58 μM | Disrupt the bacterial membrane, causing leakage of the contents; regulate the inflammatory response | [167] |
| B22 (M) | P. aeruginosa V. cholerae | in vitro | 4 μM 2 μM | 100 nM | Penetrate the membrane and kill bacteria by regulating physiological metabolic processes, including protein synthesis, peptide glycan biosynthesis, respiration, and detoxification of ROS | [172] |
| Nal-P-113 (M) | P. gingivalis | in vitro | NP | 6.25 μg/mL | Inhibit biofilm formation by inhibiting the synthesis of proteins that promote bacterial adhesion | [179] |
| Temporin-L (N) | P. fluorescens | ex vivo | >512 μM | 25 μM | Inhibit biofilm formation and disrupt biofilm structure | [181] |
| Temporin 1Tb (N) | S. epidermidis | in vitro | 25–50 μg/mL | 25–50 μg/mL | Eradicate established biofilms | [191] |
| 1037 (S) | P. aeruginosa B. cenocepacia L. monocytogenes | in vitro | 304 μg/mL >608 μg/mL 25 μg/mL | 10 μg/mL 5 μg/mL 0.63 μg/mL | Reduce swimming and swarming motilities, stimulate twitching motility, and suppress the expression of genes involved in biofilm formation | [186] |
| D-LL-37 (M) | P. aeruginosa | in vitro | > 1 μg/mL | 1 μg/mL | Promote bacterial twitching motility and inhibit biofilm formation | [173] |
| LL-37 (N) | P. aeruginosa | in vitro | 64 μg/mL | 0.5 μg/mL | Reduce swimming and swarming motilities, stimulate twitching motility, and interfere with QS | [183] |
| 1018 (S) | P. aeruginosa, E. coli A. baumannii B. cenocepacia S. enterica serovar Typhimurium K. pneumoniae, MRSA | in vitro | 64 µg/mL 32 µg/mL 128 µg/mL >256 µg/mL 64 µg/mL 8 µg/mL 64 µg/mL | 10 µg/mL 10 µg/mL 10 µg/mL 10 µg/mL 20 µg/mL 2 µg/mL 2.5 µg/mL | Inhibit biofilm formation by blocking the signal molecule (p)ppGpp, kill bacteria | [188] |
| ADEP4 (S) | MRSA | in vivo | 0.5 µg/mL | 5 µg/mL | Kill persister cells and eradicate biofilm infections by activating the ClpP protease | [189] |
| SAAP-148 (S) | S. aureus A. baumannii | in vivo | NP | 3.2 μM 1.6 μM | Prevent the formation of the extracellular matrix and promote its breakdown and eradication, eradicate persister cells | [190] |
| P1 (S) | S. mutans | in vitro | NP | 25 μg/mL | Disturb the biofilm architecture, resulting in a drastic reduction in attached biofilm biomass | [192] |
| Hepcidin 20 (S) | S. epidermidis | in vitro | >50 μM | 25 μM | Reduce the mass of the extracellular matrix and change the structure of biofilms by targeting PIA | [193] |
| HBD-3 (N) | S. epidermidis S. aureus | in vivo | 4 μg/mL 8 μg/mL | 40 μg/mL 80 μg/mL | Inhibit biofilm formation | [176] |
| TBP-1-GGG-hBD3-3 (S) | S. oralis S. gordonii S. sanguinis | in vitro | 320 μg/mL 500 μg/mL 500 μg/mL | 640 μg/mL 800 μg/mL 850 μg/ml | Reduce the expression of adhesion protein and inhibit biofilm formation | [175] |
| HDP3-Cu (M) | P. aeruginosa | in vitro | 32 μM | 2 μM | Destroy extracellular DNA | [194] |
| C16G2 (S) | S. mutans | in vitro | ~5 μM | 20 μM | Kill bacteria through disruption of the cell membrane | [170] |
| lin-SB056-1 (lin-SB056-1)2-K (S) | P. aeruginosa PAO1 | ex vivo | 4.8 μM 19.25 μM | NP 19.25 μM | Inhibit biofilm formation, kill bacteria, disrupt biofilm structure | [195] |
| Polymyxin B (N) | V. cholerae | in vitro | 100 μg/mL | 25 μg/mL | Affect flagella, reduce motility, inhibit biofilm formation | [187] |
| BF Pc-CATH1 Cc-CATH2 Cc-CATH3 (N) | C. albicans | in vivo | 1–16 μg/mL | 2.5 μg/mL NP NP NP | Inhibit biofilm formation, destroy preformed biofilms | [171] |
| Category | Material | Shape and Size | Pathogens | MIC | MBIC/EC | Anti-Biofilm Mode | Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| Inorganic | Biosynthesized AgNPs from Phanerochaete chrysosporium | spherical, ~45 nm | E. coli C. albicans | 0.25 nM NP | NP NP | Intrinsic antibiofilm activity | Reduce biomass of mature biofilms: 29% and 80% in E. coli and C. albicans biofilms, respectively | [212] |
| Biosynthesized AgNPs from Fusarium scirpi | spherical, 2–20 nm | E. coli | 25 mg/mL | 7.5 mg/L | Intrinsic antibiofilm activity | Inhibit 97% biofilm formation, kill 80% bacteria | [213] | |
| Biosynthesized AgNPs from pomegranate peel | spherical, 32–85 nm | P. aeruginosa | NP | 0.1 mg/mL | Intrinsic antibiofilm activity | Inhibit biofilm formation | [215] | |
| Biosynthesized AgNPs from seabuckthorn | spherical, 10–40 nm | P. aeruginosa | 2 μg/mL | 2 μg/mL | Intrinsic antibiofilm activity | Kill bacteria, disrupt biofilm structure and cell wall, interfere with cell membrane, and inhibit QS | [227] | |
| Biosynthesized AuNPs from brown seaweed | spherical, 15–119 nm | P. aeruginosa | 512 µg/mL | 128 µg/mL | Intrinsic antibiofilm activity | Inhibit>80% biofilm formation; eradicate mature biofilm; attenuate the production of virulence factors; impair bacterial swarming, swimming, and twitching motilities | [228] | |
| AgNPs + rifampicin | ellipsoidal and clumpy, 15–18 ± 4 nm | MRSA K. pneumoniae | 4 µg/mL 0.025 µg/mL | 1 µg/mL 0.003 µg/mL | Intrinsic antibiofilm activity and carrier | Inhibit >90% biofilm formation, eradicate 40-70% mature biofilms, kill ~50% bacteria, enhance the biofilm penetrating power of the drug | [230] | |
| ZnO NPs | NR | E. faecalis | NP | NP | Intrinsic antibiofilm activity | Disrupt biofilm structure, kill bacteria | [216] | |
| Core-shell MNPs (MNP@NH2, MNP@Au, MNP@PQAS) + LL-37 or LL-37 derivatives | spherical, 12, 11, and 13 nm | S. aureus P. aeruginosa | 128, 128, 128 μg/mL 128, 16, 64 μg/mL | 64, 64, 128 μg/mL 256, 64, 128 μg/mL | Carrier and magnetic field catalyst | Inhibit biofilm formation by 40%–85% | [217] | |
| AuNPs | 70 mm | B. multivorans, P. aeruginosa, and S. aureus | NP | 1.4E + 10 AuNP/mL | Light/thermal catalyst | Produce VNB that interfere with biofilm structure and disrupt biofilms | [214] | |
| TiO2 NPs+ nitrogen | spherical, 12 nm | S. mutans | NA | NA | Light/thermal catalyst | Kill bacteria | [218] | |
| SiO2 NPs+ peppermint oil + cinnamaldehyde | ~150 nm | E. coli, P. aeruginosa, and E. cloacae complex and S. aureus | NA | NA | Carrier | Deliver the essential oil payloads, effectively eradicate biofilms | [219] | |
| Polymer-based | Chitosan NPs | NR | E. faecalis | NP | NP | Intrinsic antibiofilm activity | Disrupt biofilm structure, kill bacteria | [216] |
| Chitosan NPs + chrysin | spherical, ~355 nm | S. aureus | 1024 µg/mL | 768 µg/mL | Intrinsic antibiofilm activity and carrier | Inhibit ~66% biofilm formation, disturb ~43% mature biofilm, reduce EPS production and bacterial cell surface hydrophobicity | [220] | |
| Mesoporous polydopamine NPs | 15.6 mm | S. aureus | NA | NA | Light/thermal catalyst | Inhibit biofilm formation by 95%, synergistic PDT/PTT effect (ROS generation/local hyperthermia) | [115] | |
| PLGA NPs + gentamicin | 251 nm | P. aeruginosa | 3 µg/mL | 6 μg/mL | Carrier | Control drug release for up to 16 days and enhance antimicrobial activity | [221] | |
| PLA NCs | spherical, 300 ± 110 nm | S. aureus, P. aeruginosa, E. coli, and C. albicans | NA | NA | Intrinsic antibiofilm activity and carrier | Inhibit biofilm formation | [222] | |
| Carbon-based | Graphene nanoplatelets | Thickness: ~1–~25 nm | S. mutans | NA | NA | Intrinsic antibiofilm activity | Inhibit bacterial adhesion, mechanical damage of cell wall, inhibit biofilm formation | [223] |
| Lipid | Lipid-polymer hybrid NPs + linezolid | - | MRSA | 0.9–1.9 µg/mL | 32 µg/mL | Carrier | Control drug release, suppress MRSA biofilm growth to 35%–60% | [224] |
| Liposomal NPs + anidulafungin | ~100 nm | C. albicans | 1.56–12.50 µg/mL | 1.25 mg/mL | Carrier | Disrupt preformed C. albicans biofilms, reduce fungal burden by as much as 99% | [225] | |
| Molecular polymer | Supramolecular polymer α-CD-Ce6-NO-DA | spherical ~136 nm | MRSA | NA | NA | Antibiofilm activity, carrier, respond to acid and light | Promote effective penetration of biofilms at pH 5.5, trigger the rapid release of NO, kill bacteria and improve the efficiency of PDT by reducing GSH and generating RNS, eradicate biofilms | [226] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Sun, L.; Zhang, P.; Wang, Y. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings 2021, 11, 294. https://doi.org/10.3390/coatings11030294
Li X, Sun L, Zhang P, Wang Y. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings. 2021; 11(3):294. https://doi.org/10.3390/coatings11030294
Chicago/Turabian StyleLi, Xin, Luyao Sun, Peng Zhang, and Yang Wang. 2021. "Novel Approaches to Combat Medical Device-Associated BioFilms" Coatings 11, no. 3: 294. https://doi.org/10.3390/coatings11030294
APA StyleLi, X., Sun, L., Zhang, P., & Wang, Y. (2021). Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings, 11(3), 294. https://doi.org/10.3390/coatings11030294






