Functionality of Films from Nigella sativa Defatted Seed Cake Proteins Plasticized with Grape Juice: Use in Wrapping Sweet Cherries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Protein Extraction
2.2.2. Proximate Analysis
2.2.3. Film Preparation
2.2.4. Film Characteristics
2.2.5. Sweet Cherry Wrapping
2.2.6. Physicochemical Analyses of Wrapped and Unwrapped Cherries
3. Results and Discussion
3.1. Proximate Analysis of Nigella Sativa Seeds and Their Derivatives
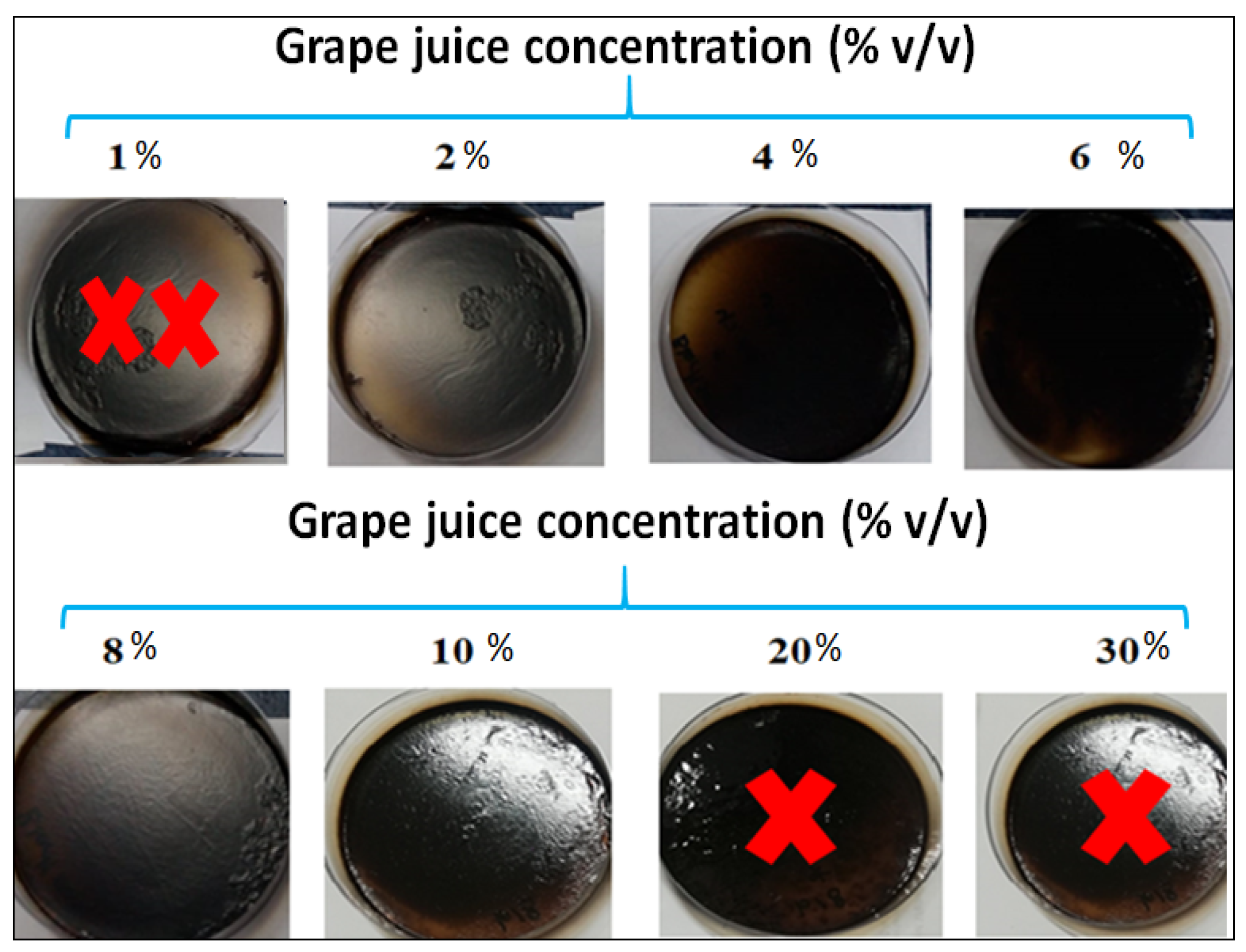
3.2. Nigella Sativa Edible Films Obtained in the Presence of Different Concentrations of Grape Juice
3.3. Film Characterizations
3.3.1. Film Thickness and Mechanical Properties
3.3.2. Moisture Content and Uptake
3.3.3. Antioxidant Activity of the Film
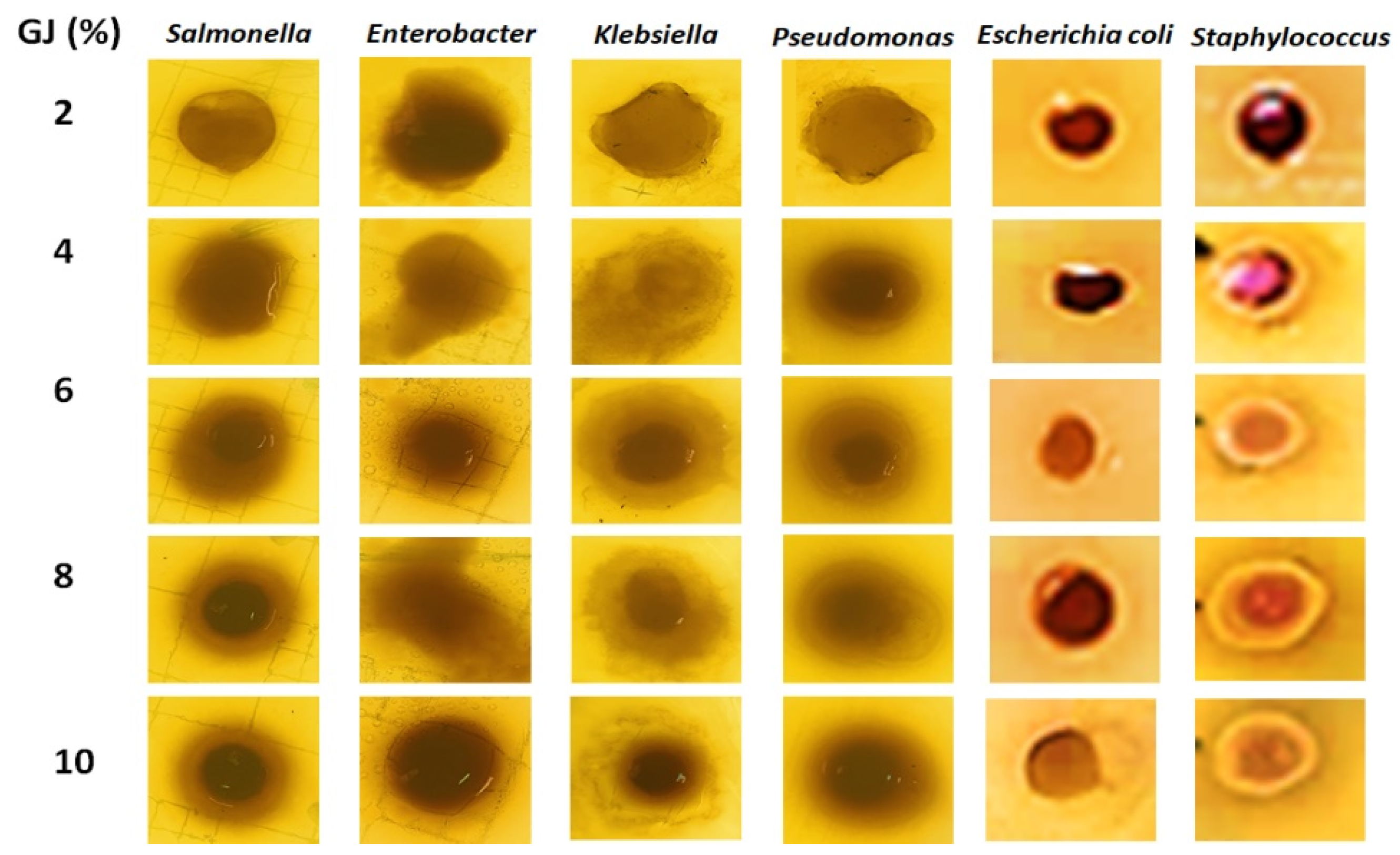
3.3.4. NSDSC Protein Film Antimicrobial Activity
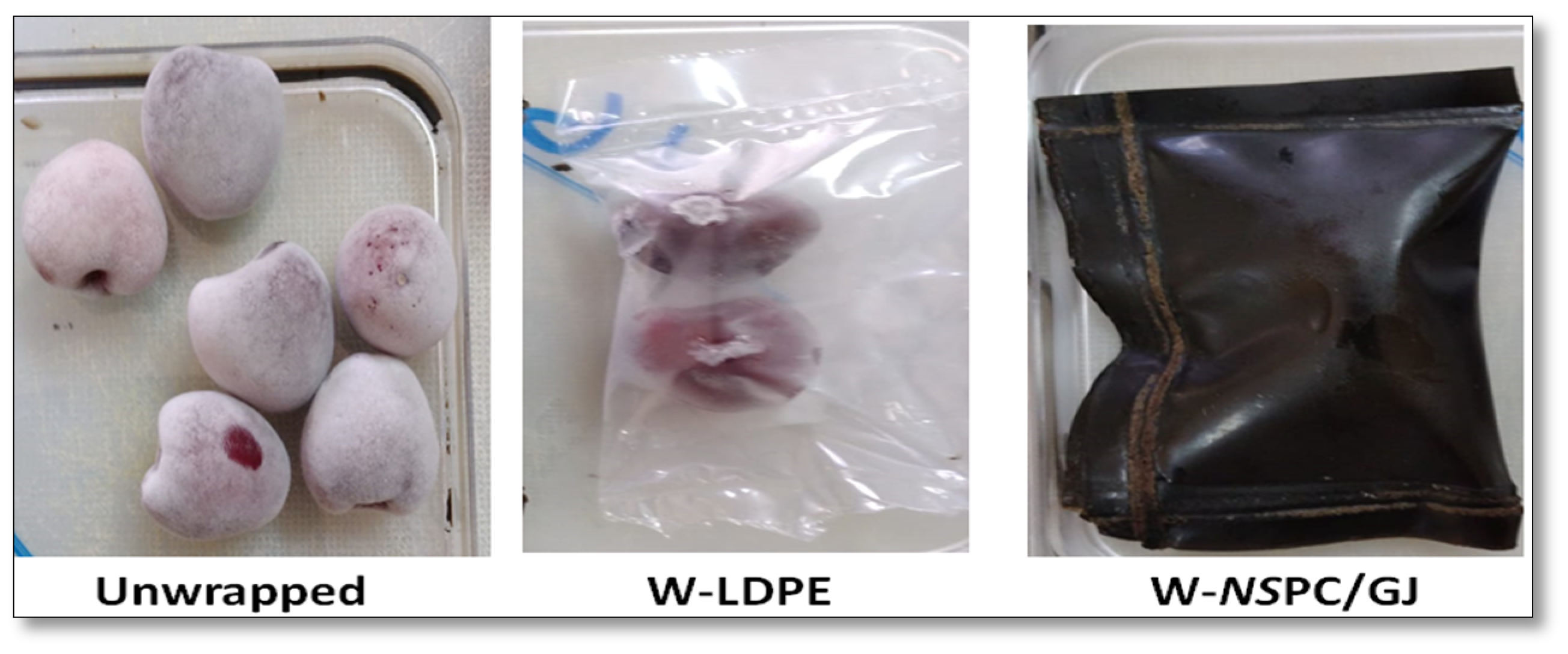
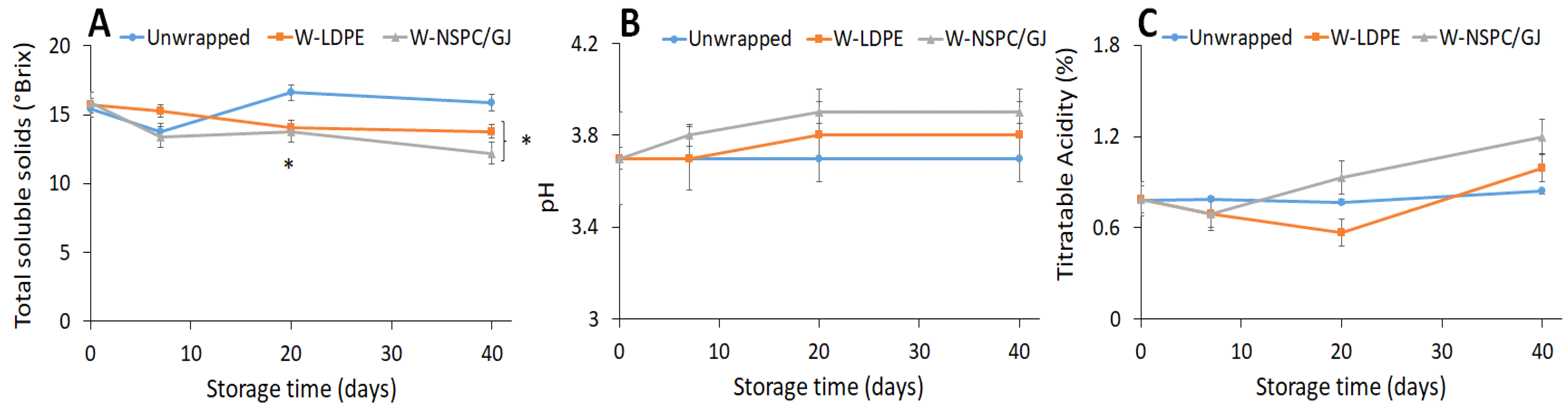
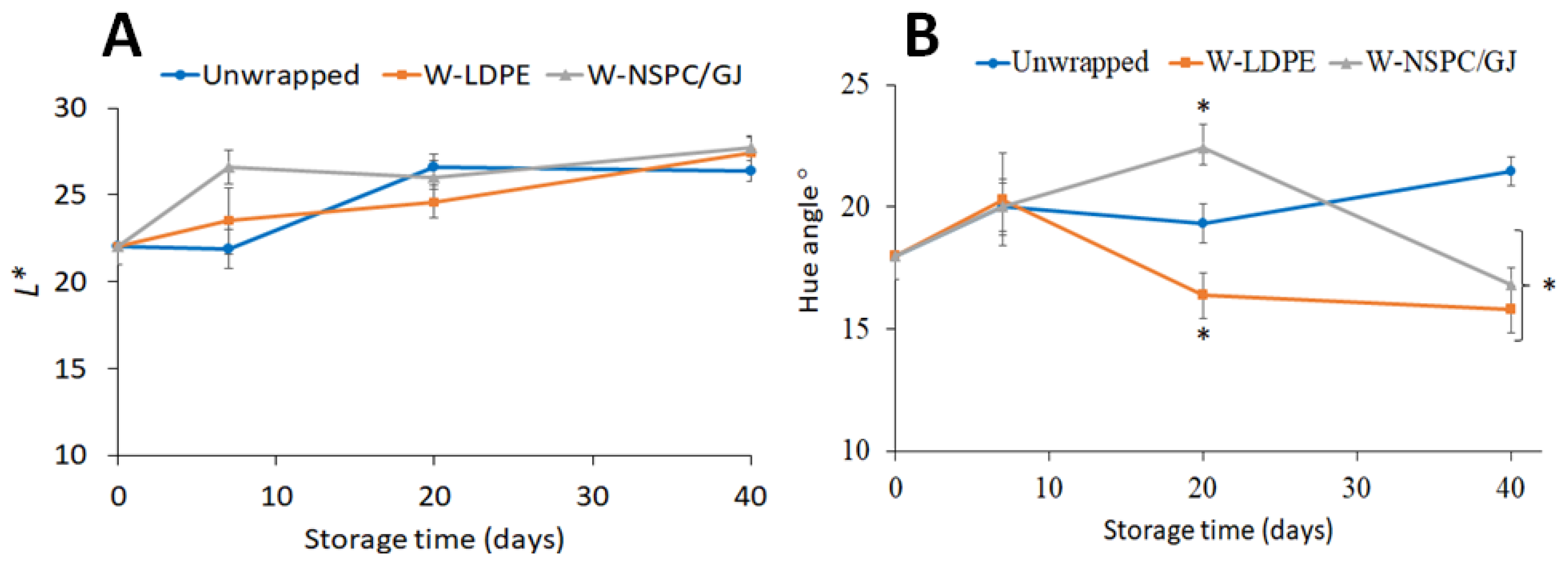
3.4. Effect of Wrapping with or without NSPC/GJ Film on Sweet Cherries Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Phil. Trans. R. Soc. B 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [Green Version]
- Bandoim, L. Can Edible Food Wrappers Solve the Plastic Crisis? Available online: https://sciencing.com/can-edible-food-wrappers-solve-the-plastic-crisis-13717334.html (accessed on 29 February 2020).
- Verbeek, C.J.R.; Van den Berg, L.E. Extrusion processing and properties of protein-based thermoplastics. Macromol. Mater. Eng. 2010, 295, 10–21. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Gniewosz, M. Substances with antimicrobial activity in edible films. A review. Pol. J. Food Nutr. Sci. 2012, 62, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Muzaffar, H.; Ajesh Kumar, V.; Chirag Maheshwari, S.; Mangraj. Biodegradable and edible film: A counter to plastic pollution. Int. J. Chem. Stud. 2020, 8, 2242–2245. [Google Scholar]
- Shokri, S.; Ehsani, A.; Jasour, M.S. Efficacy of lactoperoxidase system-whey protein coating on shelf-life extension of rainbow trout fillets during cold storage (4 °C). Food Bioprocess Technol. 2015, 8, 54–62. [Google Scholar] [CrossRef]
- Mohareb, E.; Mittal, G.S. Formulation and process conditions for biodegradable/edible soy-based packaging trays. Packag. Technol. Sci. 2007, 20, 1–15. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P.M. Edible polymers: Challenges and opportunities. J. Polym. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fangfang, Z.; Xinpeng, B.; Wei, G.; Wang, G.; Shi, Z.; Jun, C. Effects of virgin coconut oil on the physicochemical, morphological and antibacterial properties of potato starch-based biodegradable films. Int. J. Food Sci. Technol. 2020, 55, 192–200. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Emiroğlu, Z.K.; Yemiş, G.P.; Coşkun, B.K.; Candoğan, K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010, 86, 283–288. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López de Lacey, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hyd. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Dubick, M.A. Historical perspectives on the use of herbal preparations to promote health. J. Nutr. 1986, 116, 1348–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Paarakh, P.M. Nigella sativa Linn. A comprehensive review. Indian J. Nat. Prod. Resour. 2010, 1, 409–429. [Google Scholar]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Aljabre, S.H.; Alakloby, O.M.; Randhawa, M.A. Dermatological effects of Nigella sativa. J. Dermatol. Dermatol. Surg. 2015, 19, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Dubey, P.N.; Singh, B.; Mishra, B.K.; Kant, K.; Solanki, R.K. Nigella (Nigella sativa): A high value seed spice with immense medicinal potential. Indian J. Agric. Sci. 2016, 86, 967–979. [Google Scholar]
- Meddah, B.; Ducroc, R.; Faouzi, M.E.A.; Eto, B.; Mahraoui, L.; Benhaddou-Andaloussi, A.; Martineau, L.; Cherrah, Y.; Haddad, P.S. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J. Ethnopharmacol. 2009, 121, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, A.; Mahdian, V.; Razavi, B.M.; Hosseinzadeh, H. Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone. J. Pharmacopuncture. 2017, 20, 179–193. [Google Scholar] [PubMed]
- Leong, X.F.; Rais Mustafa, M.; Jaarin, K. Nigella sativa and its protective role in oxidative stress and hypertension. Evid. Based Complementary Altern. Med. 2013, 2013, 1–9. [Google Scholar]
- Hosseinzadeh, H.; Parvardeh, S.; Nassiri-Asl, M.; Mansouri, M.T. Intracerebroventricular administration of thymoquinone, the major constituent of Nigella sativa seeds, suppresses epileptic seizures in rats. Med. Sci. Monit. 2005, 11, 106–110. [Google Scholar]
- El-Dakhakhny, M.; Barakat, M.; Abd El-Halim, M.; Aly, S.M. Effects of Nigella sativa oil on gastric secretion and ethanol induced ulcer in rats. J. Ethnopharmacol. 2000, 72, 299–304. [Google Scholar] [CrossRef]
- Koshak, A.; Wei, L.; Koshak, E.; Wali, S.; Alamoudi, O.; Demerdash, A.; Majdy, Q.; Peter, N.; Heinrich, M. Nigella sativa supplementation improves asthma control and biomarkers: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2017, 31, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majdalawieh, A.F.; Fayyad, M.W.; Nasrallah, G.K. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Food Sci. Nutr. 2017, 57, 3911–3928. [Google Scholar]
- Mollazadeh, H.; Afshari, A.R.; Hosseinzadeh, H. Review on the potential therapeutic roles of Nigella sativa in the treatment of patients with cancer: Involvement of apoptosis:-black cumin and cancer. J. Pharmacopunct. 2017, 20, 158–172. [Google Scholar]
- Woo, C.C.; Kumar, A.P.; Sethi, G.; Tan, K.H.B. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012, 83, 443–451. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Zoheir, A.D.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Haseena, S.; Aithal, M.; Das, K.K.; Saheb, S.H. Phytochemical analysis of Nigella sativa and its effect on reproductive system. J. Pharm. Sci. Res. 2015, 7, 514–517. [Google Scholar]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (black cumin): A promising natural remedy for wide range of illnesses. Evid. Based Complementary Altern. Med. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [Green Version]
- El-Hack, M.E.A.; Alagawany, M.; Saeed, M.; Arif, M.; Arain, M.A.; Bhutto, Z.A.; Fazlani, S.A. Effect of gradual substitution of soyabean meal by Nigella sativa meal on growth performance, carcass traits and blood lipid profile of growing Japanese quail. J. Anim. Feed Sci. 2016, 25, 244–249. [Google Scholar] [CrossRef]
- Haq, A.; Lobo, P.I.; Al-Tufail, M.; Rama, N.R.; Al-Sedairy, S.T. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Int. J. Immunopharmacol. 1999, 21, 283–295. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Taiari, S.; Nassiri-Asl, M. Effect of thymoquinone, a constituent of Nigella sativa L., on ischemia-reperfusion in rat skeletal muscle. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 503–508. [Google Scholar] [CrossRef]
- Farah, I.O.; Begum, R.A. Effect of Nigella sativa (N. sativa L.) and oxidative stress on the survival pattern of MCF-7 breast cancer cells. Biomed. Sci. Instrum. 2003, 39, 359–364. [Google Scholar]
- Sabbah, M.; Altamimi, M.; Di Pierro, P.; Schiraldi, C.; Cammarota, M.; Porta, R. Black edible films from protein-containing defatted cake of nigella sativa seeds. Int. J. Mol. Sci. 2020, 21, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Esti, M.; Cinquanta, L.; Sinesio, F.; Moneta, E.; Di Matteo, M. Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. Food Chem. 2002, 76, 399–405. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of “Brooks” and “Bing” cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Alonso, J.; Alique, R. Influence of edible coating on shelf life and quality of “Picota” sweet cherries. Eur. Food Res. Technol. 2004, 218, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.P.; Jiang, A.L.; Xu, Y.; Wang, Y.S. Responses of physiology and quality of sweet cherry fruit to different atmospheres in storage. Food Chem. 2004, 87, 43–49. [Google Scholar] [CrossRef]
- Meheriuk, M.; Girard, B.; Moyls, L.; Beveridge, H.J.T.; McKenzie, D.L.; Harrison, J.; Weintraub, S.E.; Hocking, R. Modified atmosphere packaging of “Lapins” sweet cherry. Food Res. Int. 1995, 28, 239–244. [Google Scholar] [CrossRef]
- Petriccione, M.; De Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess Technol. 2014, 8, 394–408. [Google Scholar] [CrossRef]
- Pham, Q.T.; Mawson, R.F. Moisture migration and ice recrystallization in frozen foods. Qual. Frozen Food. 1997, 67–91. [Google Scholar]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshi, R.; Riahi, Z.; Rhim, J.-W. Antioxidant pectin/pullulan edible coating incorporated with Vitis vinifera grape seed extract for extending the shelf life of peanuts. Postharvest Biol. Technol. 2022, 183, 111740. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lee, C.Y. Isolation and HPLC determination of phenolic compounds in red grapes. Am. J. Enol. Vitic. 1990, 39, 259–262. [Google Scholar]
- Somers, T.C.; Ziemelis, G. Spectral evaluation of total phenolic components in Vitis vinifera: Grapes and wines. J. Sci. Food Agric. 1985, 36, 1275–1284. [Google Scholar] [CrossRef]
- Alonso, A.M.; Guillén, D.A.; Barroso, C.G.; Puertas, B.; García, A. Determination of the antioxidant activity of wine byproducts and its correlation with polyphenolic content. J. Agric. Food Chem. 2002, 50, 5832–5836. [Google Scholar] [CrossRef] [PubMed]
- De Campos, L.M.; Leimann, F.V.; Pedrosa, R.C.; Ferreira, S.R. Free radical scavenging of grape pomace extracts from cabernet sauvingnon (Vitis vinifera). Bioresour. Technol. 2008, 99, 8413–8420. [Google Scholar] [CrossRef]
- Pedreschi, R.; Cisneros-Zevallos, L. Antimutagenic and antioxidant properties of phenolic fractions from Andean purple corn (Zea mays L.). J. Agric. Food Chem. 2006, 54, 4557–4567. [Google Scholar] [CrossRef] [PubMed]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Porta, R. Biorefining of seed oil cakes as industrial co-streams for production of innovative bioplastics. A review. Trends Food Sci. Technol. 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Sabbah, M.; Di Pierro, P.; Giosafatto, C.V.L.; Esposito, M.; Mariniello, L.; Regalado-Gonzales, C.; Porta, R. Plasticizing effects of polyamines in protein-based films. Int. J. Mol. Sci. 2017, 18, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Association of Cereal Chemists (AACC). Approved Methods of AACC; The Association: St. Paul, MN, USA, 2003. [Google Scholar]
- ASTM Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM: West Conshohocken, PA, USA, 1995.
- Krochta, J.M. Proteins as raw materials for films and coatings: Definitions, current status, and opportunities. Protein-Based Film. Coat. 2002, 1, 1–40. [Google Scholar]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacterial properties of alginate-based edible film incorporated with garlic oil. Int. Food Res. J. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. Hort. Sci. 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Kabir, Y.; Shirakawa, H.; Komai, M. Nutritional composition of the indigenous cultivar of black cumin seeds from Bangladesh. Prog. Nutr. 2019, 21, 428–434. [Google Scholar]
- Dangaran, K.L.; Krochta, J.M. Preventing the loss of tensile, barrier and appearances properties caused by plasticizer crystallization in whey protein films. Int. J. Food Sci. Technol. 2007, 42, 1094–1100. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Krochta, J.M. Thermoplastic processing of proteins for film formation—A review. J. Food Sci. 2008, 73, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, T.; Hervet, H.; Léger, L.; Champion, D.; Debeaufort, F.; Voilley, A. Effect of plasticizers (water and glycerol) on the diffusion of a small molecule in κcarragennan biopolymer films for edible coating application. Biomacromolecules 2006, 7, 2011–2019. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizer effect on oxygen permeability of betalactoglobulin films. J. Agric. Food Chem. 2000, 48, 6298–6302. [Google Scholar] [CrossRef] [PubMed]
- Dangaran, K.L.; Krochta, J.M. Aqueous whey protein coatings for panned products. Manuf. Confect. 2003, 83, 61–65. [Google Scholar]
- Veiga-Santos, P.; Oliveira, L.M.; Cereda, M.P.; Scamparini, A.R.P. Sucrose and inverted sugar as plasticizer. Effect on cassava starch–gelatin film mechanical properties, hydrophilicity and water activity. Food Chem. 2007, 103, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 2007, 104, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.K.; Dhakal, M.R. Phytomelanin in compositae. Curr. Sci. 2001, 80, 933–940. [Google Scholar]
- Eid, A.M.; Elmarzugi, N.A.; Abu Ayyash, L.M.; Sawafta, M.N.; Daana, H.I. A review on the cosmeceutical and external applications of Nigella sativa. J. Trop. Med. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özdemir, Ö.; Keles, Y. Extraction, purification, antioxidant properties and stability conditions of phytomelanin pigment on the sunflower seeds. Int. J. Second Metab. 2017, 5, 140–148. [Google Scholar]
- Ozdemir, M.; Floros, J.D. Optimization of edible whey protein films containing preservatives for mechanical and optical properties. J. Food Eng. 2008, 84, 116–123. [Google Scholar] [CrossRef]
- Hopkins, E.J.; Stone, A.K.; Wang, J.; Korber, D.R.; Nickerson, M.T. Effect of glycerol on the physicochemical properties of films based on legume protein concentrates: A comparative study. J. Texture Stud. 2019, 50, 539–546. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Roviello, V.; Sabbah, M. Tuning the functional properties of bitter vetch (Vicia ervilia) protein films grafted with spermidine. Int. J. Mol. Sci. 2017, 18, 2658. [Google Scholar] [CrossRef] [Green Version]
- Vejdan, A.; Mahdi Ojagh, S.; Adeli, A.; Abdollahi, M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Effect of various plasticizers and concentration on the physical, thermal, mechanical, and structural properties of cassava-starch-based films. Starch-Stärke 2017, 69, 1500366. [Google Scholar] [CrossRef]
- Bamdad, F.; Goli, A.H.; Kadivar, M. Preparation and characterization of proteinous film from lentils (Lens culinaris). Food Res. Int. 2006, 39, 106–111. [Google Scholar] [CrossRef]
- Mahmoud, R.; Savello, P.A. Mechanical properties of and water vapor transferability through whey protein films. J. Dairy Sci. 1992, 75, 942–946. [Google Scholar] [CrossRef]
- Jangchud, A.; Chinnan, M.S. Peanut protein film as affected by drying temperature and ph of film forming solution. J. Food Sci. 1990, 64, 153–157. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Park, P.; Je, J.; Kim, S. Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr. Polym. 2004, 55, 17–22. [Google Scholar] [CrossRef]
- Ramchandani, A.G.; Chettiyar, R.S.; Pakhale, S.S. Evaluation of antioxidant and anti-initiating activities of crude polyphenolic extracts from seedless and seeded Indian grapes. Food Chem. 2010, 119, 298–305. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Aires, A.; Marques, E.; Carvalho, R.; Rosa, E.A.; Saavedra, M.J. Evaluation of biological value and appraisal of polyphenols and glucosinolates from organic baby-leaf salads as antioxidants and antimicrobials against important human pathogenic bacteria. Molecules 2013, 18, 4651–4668. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef] [Green Version]
- Kappel, F.; Toivonen, P.; McKenzie, D.L.; Stan, S. Storage characteristics of new sweet cherry cultivars. HortScience 2002, 37, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, R.L.; Pandey, S. Juice blends—A way of utilization of under-utilized fruits, vegetables, and spices: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Gutiérrez-Jara, C.; Bilbao-Sainz, C.; McHugh, T.; Chiou, B.S.; Williams, T.; Villalobos-Carvajal, R. Effect of cross-linked alginate/oil nanoemulsion coating on cracking and quality parameters of sweet cherries. Foods 2021, 10, 449. [Google Scholar] [CrossRef]


| Compositions (%) | NS Seed * | NSDSC | NSPC |
|---|---|---|---|
| Protein | 20.3 ± 0.6 | 34.0 ± 2.7 | 45.1 ± 2.5 |
| Moisture | 7.1 ± 0.2 | 7.5 ± 0. 1 | 5.0 ± 0.3 |
| Ash | 7.4 ± 0.3 | 5.5 ± 0.1 | 3.7 ± 0.7 |
| Fats | 45.4 ± 0.5 | 18.2 ± 0.5 | 3.1 ± 0.3 |
| Carbohydrate | 19.7 ± 0.4 | 34.8 ± 2.3 | 43.1 ± 1.4 |
| Film | Thickness (µm) | TS (MPa) | EB (%) | YM (MPa) |
|---|---|---|---|---|
| NSPC + GJ 2% | 67.5 ± 1.5 | 24.6 ± 1.1 | 5.6 ± 2.3 | 409.5 ± 50.0 |
| NSPC + GJ 6% | 73.3 ± 0.81 | 6.90 ± 1.5 | 39.3 ± 7.6 | 74.5 ± 23.10 |
| Viscofan (NDX) * | 30.0 ± 0.4 | 36.6 ± 8.1 | 13.1 ± 2.9 | 356.0 ± 29.0 |
| HDPE * | 36.2 ± 1.7 | 13.1 ± 1.4 | 501.9 ± 43.3 | 75.2 ± 2.70 |
| Film | Water Content (%) | Water Uptake (%) |
|---|---|---|
| NSPC + GJ (2%) | 12.1 ± 1.4 a | 7.6 ± 1.1 a,b |
| NSPC + GJ (4%) | 17.4 ± 1.4 a | 11.1 ± 0.3 a |
| NSPC + GJ (6%) | 18.0 ± 0.9 a | 8.9 ± 0.8 a,b |
| NSPC + GJ (8%) | 15.5 ± 3.7 a | 9.8 ± 0.7 a,b |
| NSPC + GJ (10%) | 16.7 ± 3.4 a | 6.4 ± 1.8 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaseen, D.; Sabbah, M.; Al-Asmar, A.; Altamimi, M.; Famiglietti, M.; Giosafatto, C.V.L.; Mariniello, L. Functionality of Films from Nigella sativa Defatted Seed Cake Proteins Plasticized with Grape Juice: Use in Wrapping Sweet Cherries. Coatings 2021, 11, 1383. https://doi.org/10.3390/coatings11111383
Yaseen D, Sabbah M, Al-Asmar A, Altamimi M, Famiglietti M, Giosafatto CVL, Mariniello L. Functionality of Films from Nigella sativa Defatted Seed Cake Proteins Plasticized with Grape Juice: Use in Wrapping Sweet Cherries. Coatings. 2021; 11(11):1383. https://doi.org/10.3390/coatings11111383
Chicago/Turabian StyleYaseen, Dana, Mohammed Sabbah, Asmaa Al-Asmar, Mohammad Altamimi, Michela Famiglietti, C. Valeria L. Giosafatto, and Loredana Mariniello. 2021. "Functionality of Films from Nigella sativa Defatted Seed Cake Proteins Plasticized with Grape Juice: Use in Wrapping Sweet Cherries" Coatings 11, no. 11: 1383. https://doi.org/10.3390/coatings11111383
APA StyleYaseen, D., Sabbah, M., Al-Asmar, A., Altamimi, M., Famiglietti, M., Giosafatto, C. V. L., & Mariniello, L. (2021). Functionality of Films from Nigella sativa Defatted Seed Cake Proteins Plasticized with Grape Juice: Use in Wrapping Sweet Cherries. Coatings, 11(11), 1383. https://doi.org/10.3390/coatings11111383









