Preparation and Characterization of Bioactive Chitosan-Based Films Incorporated with Olive Leaves Extract for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
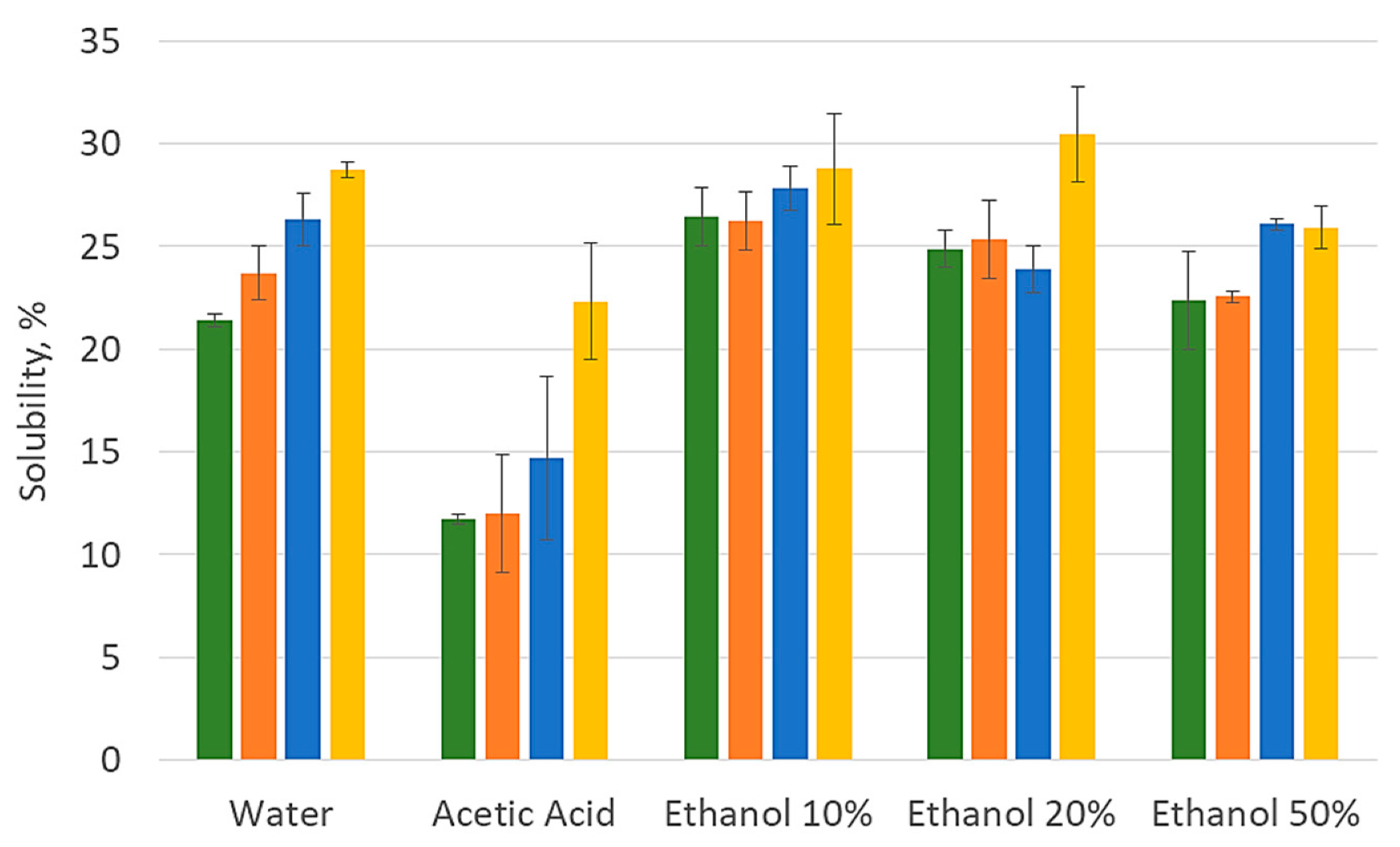
2.2. Preparation of Chitosan and Chitosan-Olive Leaf Extract Films
2.3. Analyses
2.3.1. OLE and Chitosan Characterization
2.3.2. Evaluation of Active Properties
2.3.3. Physical Characteristics of the Films
3. Results and Discussion
3.1. Deacetylation Degree (DD)
3.2. Oleuropein Content in the OLE
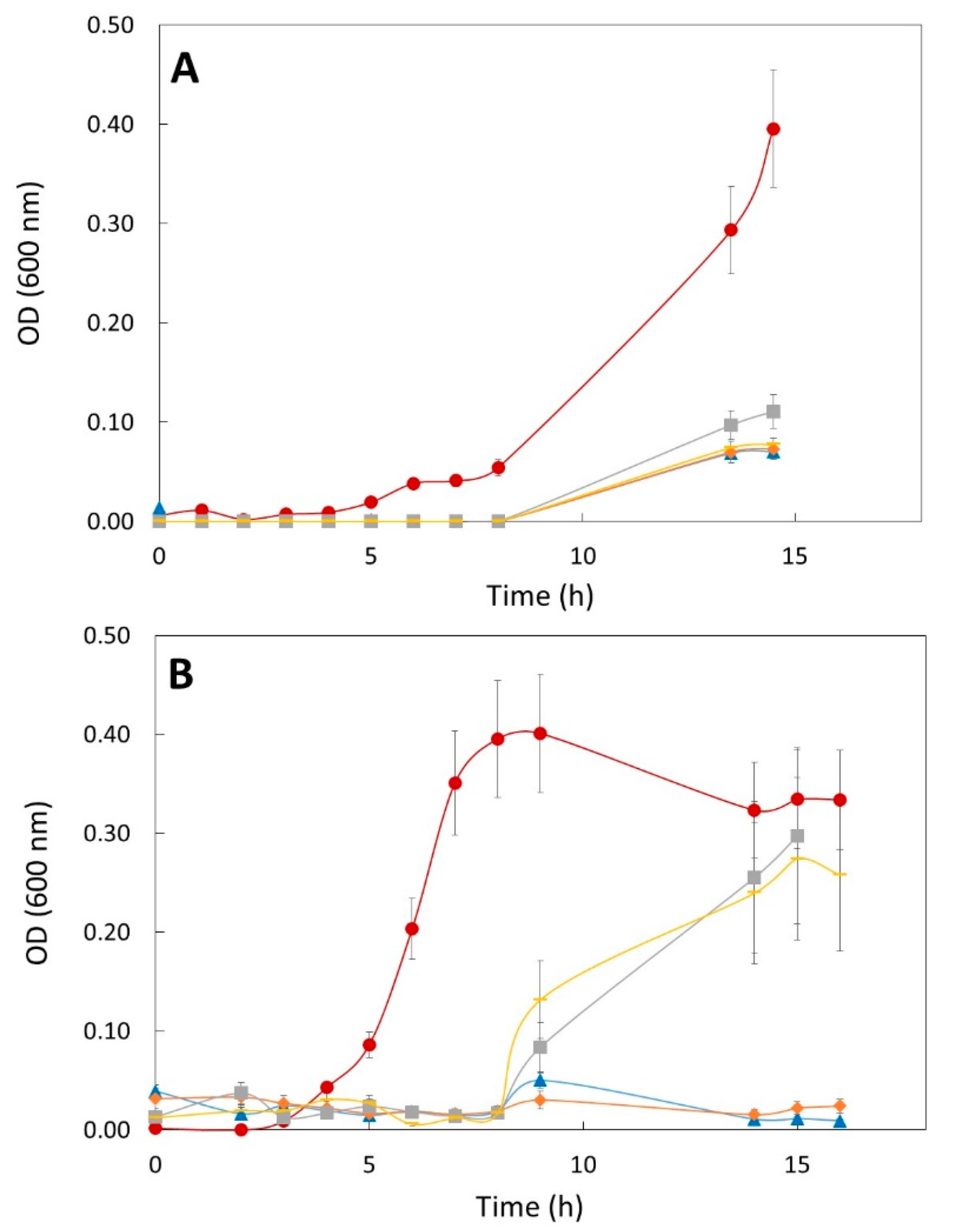
3.3. Anti-Microbial Effect of OLE in L. monocytogenes, E. coli and C. jejuni
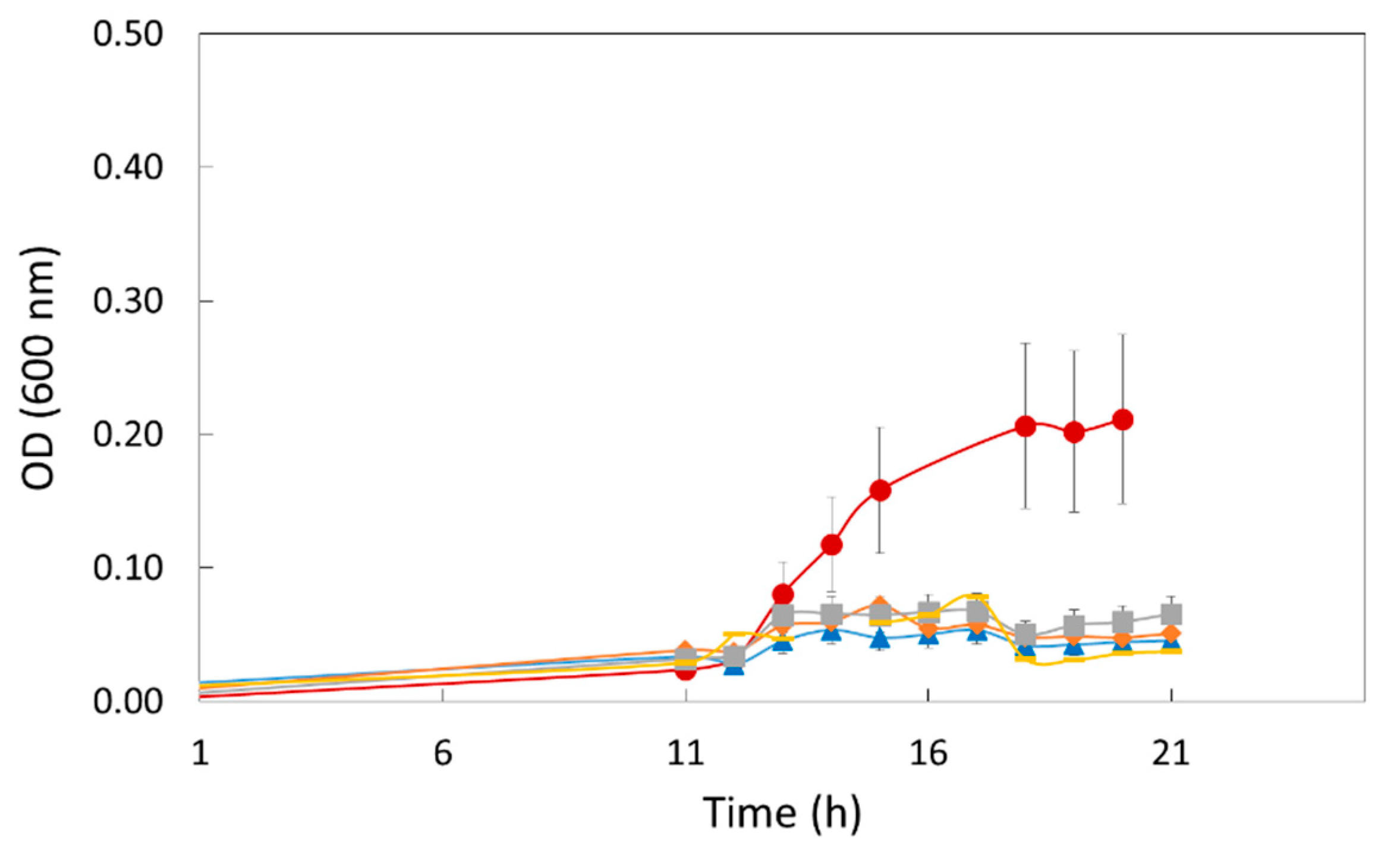
3.4. In Situ Test Using Chicken Pieces
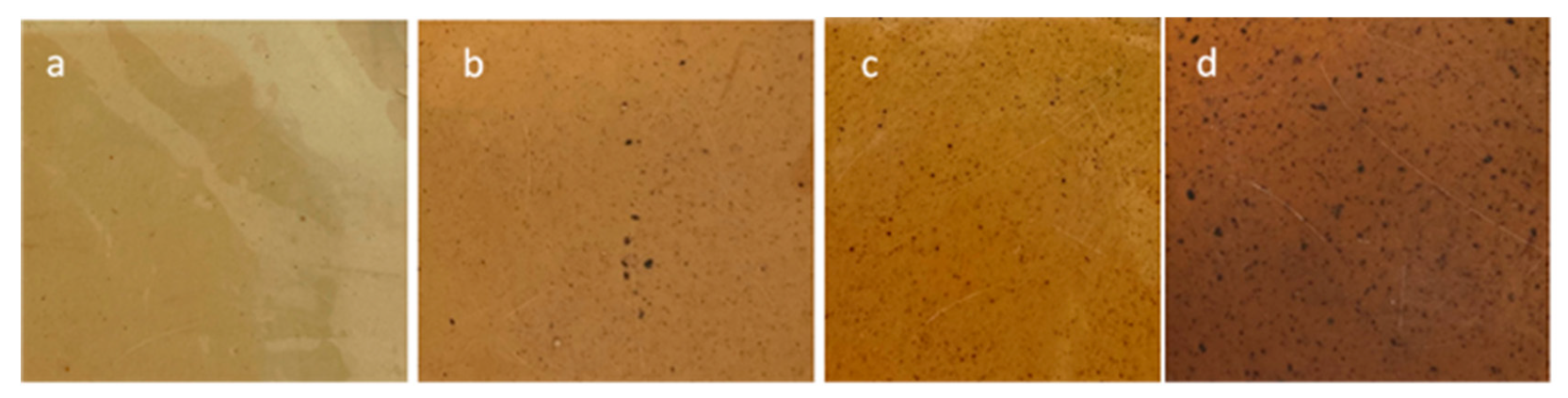
3.5. Antioxidant Activity of the Films
3.6. Physical Characteristics of the Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherhaufer, S.; Moates, G.; Hartikainen, H.; Waldron, K.; Obersteiner, G. Environmental impacts of food waste in europe. Waste Manag. 2018, 77, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Miteluț, A.C.; Tănase, E.E.; Popa, V.I.; Popa, M.E. Sustainable alternative for food packaging: Chitosan biopolymer—A review. AgroLife Sci. J. 2015, 4, 52–61. [Google Scholar]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, S.; Scallan, E. Epidemiology, Cost, and Risk Analysis of Foodborne Disease (Chapter 2) in Foodborne Diseases, 3rd ed.; Christine, E.R.D., Tim, A., Richard, A.S., Dean, O.C., Hans, P.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 31–63. [Google Scholar]
- Volpe, S.; Cavella, S.; Torrieri, E. Biopolymer coatings as alternative to modified atmosphere packaging for shelf life extension of minimally processed apples. Coatings 2019, 9, 569. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macr. 2015, 74, 289–296. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU), No. 10/2011 of 14 January 2011. Off. J. Eur. Union 2011, 54, 1–89. [Google Scholar]
- Ashrafi, A.; Jokar, M.; Mohammadi Nafchi, A. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macr. 2018, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-starch films with natural extracts: Physical, chemical, morphological and thermal properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treviño-Garza, M.Z.; Correa-Cerón, R.C.; Ortiz-Lechuga, E.G.; Solís-Arévalo, K.K.; Castillo-Hernández, S.L.; Gallardo-Rivera, C.T.; Arévalo Niño, K. Effect of linseed (Linum usitatissimum) mucilage and chitosan edible coatings on quality and shelf-life of fresh-cut cantaloupe (Cucumis melo). Coatings 2019, 9, 368. [Google Scholar] [CrossRef] [Green Version]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macr. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macr. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Coma, V.; Deschamps, A.; Martial-Gros, A. Bioactive packaging materials from edible chitosan polymer—Antimicrobial activity assessment on dairy-related contaminants. J. Food Sci. 2003, 68, 2788–2792. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Lekjing, S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Freitas Rodrigues, P.; Lopes, A.; Silva, R.J.; Caldeira, J.; Duarte, M.P.; Braz Fernandes, F.; Coelhoso, I.M.; et al. Physical and morphological characterization of chitosan/montmorillonite films incorporated with ginger essential oil. Coatings 2019, 9, 700. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xu, T.; Zhang, Y.; Zhang, C.; Lu, Z.; Lu, F.; Zhao, H. Effect of tea polyphenols on curdlan/chitosan blending film properties and its application to chilled meat preservation. Coatings 2019, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Liu, W.; Shei, Y.; Mei, J.; Xie, J. Coating effects of ε-polylysine and rosmarinic acid combined with chitosan on the storage quality of fresh half-smooth tongue sole (Cynoglossus semilaevis Günther) fillets. Coatings 2019, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Li, C.; Pei, J.; Zhu, S.; Song, Y.; Xiong, X.; Xue, F. Development of chitosan/peptide films: Physical, antibacterial and antioxidant properties. Coatings 2020, 10, 1193. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and antioxidant activity of olive leaf extracts from Greek Olive cultivars. J. Am. Oil Chem. Soc. 2010, 87, 369–376. [Google Scholar] [CrossRef]
- Dua, S.; Bhat, Z.F.; Kumar, S. Effect of oleuropein on the oxidative stability and storage quality of Tabaq-Maz, fried mutton ribs. Food Biosci. 2015, 12, 84–92. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Yingyuad, S.; Ruamsin, S.; Reekprkhon, D.; Suwassa, P.; Siripatrawan, U. Effect of chitosan coating and vacuum packaging on the quality of refrigerated grilled pork. Packag. Technol. Sci. 2006, 19, 149–157. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Szymański, M.; Witkowska-Banaszczak, E.; Matławska, I.; Szymański, A. Determination of oleuropein in herbal preparation and Olea europaea L. extracts by HPLC. Ars Sep. Acta 2012, 9–10, 55–63. [Google Scholar]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of degree of deacetylation of chitosan—Comparision of methods. Prog. Chem. Appl. Chitin Deriv. 2012, 17, 5–20. [Google Scholar]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3 ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1- picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Younes, Y.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef]
- Croisier, F.; Jerome, C. Chitosan based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.; Mulas, S.; Heimler, D. Polyphenols and secoiridoids in raw material (Olea europaea L. leaves) and commercial food supplements. Eur. Food Res. Technol. 2017, 243, 429–435. [Google Scholar] [CrossRef]
- Liu, Y.; McKeever, L.C.; Malik, N.S.A. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markin, D.; Duek, L.; Berdicevsky, I. In vitro antibacterial activity of olive leaves. Mycoses 2003, 46, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentao, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antibacterial activity of olive (Olea europaea L. Cv. Cobrancosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Nassrullah Malik, S. Antibacterial activity of olive (Olea europaea) leaves and arugula (Eruca sativa) seeds extract. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 307–310. [Google Scholar]
- Azizollahi Aliabadi, M.; Kazemi Darsanaki, R.; Laleh Rokhi, M.; Nourbakhsh, M.; Raeisi, G. Antimicrobial activity of olive leaf aqueous extract. Ann. Biol. Res. 2012, 3, 4189–4191. [Google Scholar]
- Erdohan, Z.O.; Çam, B.; Turhan, K.N. Characterization of antimicrobial polylactic acid based films. J. Food Eng. 2013, 119, 308–315. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.E.; Piette, G.; Bégin, A.; Holley, R.A. Diffusion of acetic and propionic acids from chitosan-based antimicrobial packaging films. J. Food Sci. 2000, 65, 768–773. [Google Scholar] [CrossRef]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Hammer, K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Ag. 2009, 33, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Crizel, M.T.; Oliveira Rios, A.; Alves, V.; Bandarra, N.; Moldão-Martins, M.; Flôres, S.H. Active food packaging prepared with chitosan and olive pomace. Food Hydrocoll. 2018, 74, 139–150. [Google Scholar] [CrossRef]
- Moudache, M.; Colon, M.; Nerín, C.; Zaidi, F. Phenolic content and antioxidant activity of olive by-products and antioxidant film containing olive leaf extract. Food Chem. 2016, 212, 521–527. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Antioxidant effects of chitin, chitosan, and their derivatives. Mar. Carbohydr. Fundam. Appl. Part B 2014, 15–31. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohyd. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 56–67. [Google Scholar] [CrossRef]
- Razavi, M.S.; Golmohammadi, A.; Nematollahzadeh, A.; Rovera, C.; Farris, S. Fish gelatin-bacterial cellulose nanocrystals active films containing cinnamon essential oil nanocapsules. Food Biophys. 2021. [Google Scholar] [CrossRef]

| Sample | AAEAC, g/L |
|---|---|
| OLE powder | 0.25 ± 0.01 |
| CH 2% film | 0.01 ± 0.01 |
| OLE film (10%) | 0.04 ± 0.00 |
| OLE film (20%) | 0.08 ± 0.01 |
| OLE film (30%) | 0.15 ± 0.01 |
| Sample | WVP (g·Pa−1·s−1·m−1) | TS (MPa) | E (%) |
|---|---|---|---|
| CH 2% film | (4.92 ± 0.12) × 10−11 | 17.02 ± 2.27 | 4.34 ± 1.25 |
| OLE film (10%) | (3.97 ± 0.32) × 10−11 | 25.13 ± 1.62 | 18.7 ± 1.33 |
| OLE film (20%) | (2.90 ± 0.20) × 10−11 | 18.12 ± 3.45 | 12.4 ± 3.04 |
| OLE film (30%) | (2.72 ± 0.30) × 10−11 | 21.97 ± 3.14 | 11.6 ± 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musella, E.; Ouazzani, I.C.e.; Mendes, A.R.; Rovera, C.; Farris, S.; Mena, C.; Teixeira, P.; Poças, F. Preparation and Characterization of Bioactive Chitosan-Based Films Incorporated with Olive Leaves Extract for Food Packaging Applications. Coatings 2021, 11, 1339. https://doi.org/10.3390/coatings11111339
Musella E, Ouazzani ICe, Mendes AR, Rovera C, Farris S, Mena C, Teixeira P, Poças F. Preparation and Characterization of Bioactive Chitosan-Based Films Incorporated with Olive Leaves Extract for Food Packaging Applications. Coatings. 2021; 11(11):1339. https://doi.org/10.3390/coatings11111339
Chicago/Turabian StyleMusella, Enrica, Ismael Chahed el Ouazzani, Ana Rita Mendes, Cesare Rovera, Stefano Farris, Cristina Mena, Paula Teixeira, and Fátima Poças. 2021. "Preparation and Characterization of Bioactive Chitosan-Based Films Incorporated with Olive Leaves Extract for Food Packaging Applications" Coatings 11, no. 11: 1339. https://doi.org/10.3390/coatings11111339
APA StyleMusella, E., Ouazzani, I. C. e., Mendes, A. R., Rovera, C., Farris, S., Mena, C., Teixeira, P., & Poças, F. (2021). Preparation and Characterization of Bioactive Chitosan-Based Films Incorporated with Olive Leaves Extract for Food Packaging Applications. Coatings, 11(11), 1339. https://doi.org/10.3390/coatings11111339