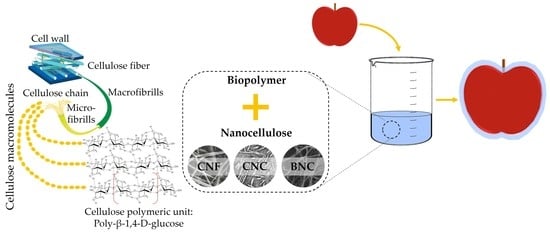
The Use of Nanocellulose in Edible Coatings for the Preservation of Perishable Fruits and Vegetables
Abstract
:1. Introduction
2. Edible Coating Deposition and Optimization
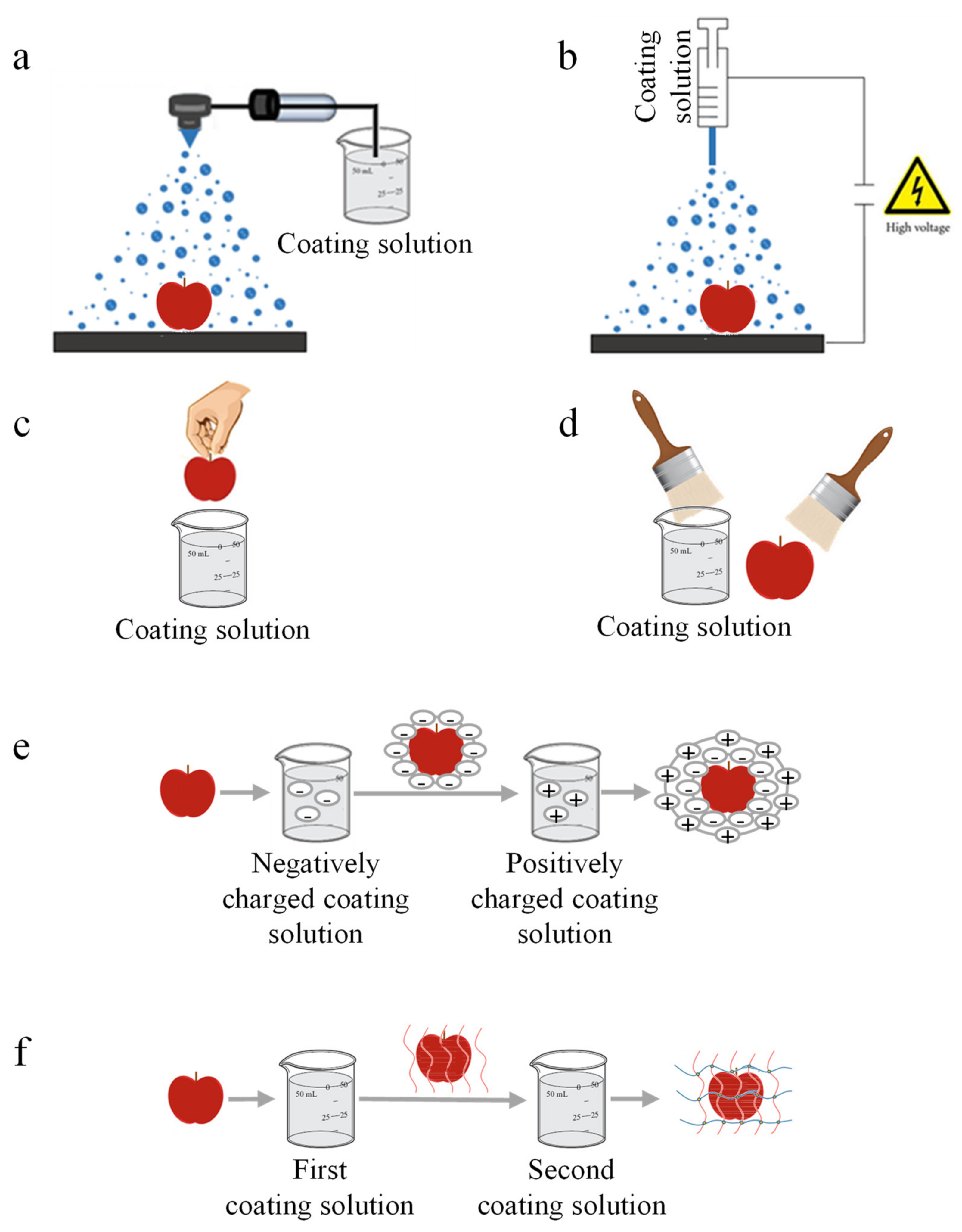
2.1. Methods of Coating Application to Food Products
2.1.1. Spraying Method
2.1.2. Electrospraying Method
2.1.3. Dipping Technique
- Immersion and holding (dwell time). The substrate is immersed into the coating solution, followed by a holding time to allow the substrate to interact for a sufficient dwell time with the coating solution to complete wetting.
- Deposition and drainage. By pulling the substrate upward, a thin layer of the coating solution is entrained, causing film deposition. In this stage, excess liquid drains from the surface of the substrate.
- Evaporation and/or drying. The excess diluent leaves the food surface by evaporation at room temperature or drying with heated air, thus achieving a thin film of the coating solution.
2.1.4. Spreading Method
2.1.5. Layer-by-Layer Deposition
2.1.6. Cross-Linking Technique
2.2. Optimization of Film-Forming Formulation
3. Classification and Properties of Nanocellulose
3.1. Cellulose Nanocrystals (CNC)
3.2. Cellulose Nanofibers (CNF)
3.3. Bacterial Nanocellulose (BNC)
4. Characterization of Nanocellulose (NC)-Reinforced Coatings
4.1. Physical-Chemical Properties
4.1.1. Thickness Determination
4.1.2. Mechanical Properties
4.1.3. Surface Wettability
4.1.4. Barrier Properties
- Diffusion. It is the rate of movement of a permeant molecule through the tangled polymer matrix, based, for example, on the size of the permeant molecule and the structure of the polymer matrix. Molecular diffusion through a film generally obeys Fick’s first law in one dimension, as described by Equation (2):where J is the molecular diffusion of the permeant molecule, D is its diffusion coefficient and C its concentration, l is the thickness of the edible film, and subscripts 1 and 2 refer to the internal and external sides of the coating.
- Solubility. This is the partitioning behavior of a permeant molecule between the surface of the polymer and the surrounding headspace. The solubility coefficient C can be defined by Henry’s law, as shown in Equation (3):where S is the solubility coefficient of the permeant molecule, and P is the environmental pressure.
- Permeability. This is the rate of transport of a permeant molecule through the polymeric layer as a result of the combined effects of diffusion (D) and solubility (S). Therefore, the permeability coefficient (Π), which characterizes the intrinsic permeability of the edible film, can be described as shown in Equation (4):
4.1.5. Optical Properties
- A translucent colorant layer on the top of an opaque background;
- Within the colorant layer, both absorption and scattering occur;
- The light within the colorant layer is completely diffuse.
- Color difference ΔE (Equation (8)):
- Chrome C (Equation (9)):
- Hue angle H (Equation (10)):
- Whiteness index WI (Equation (11)):where ΔL, Δa, and Δb are the differences of L*, a*, and b* with the standard color of a white disk (L*0, a*0, and b*0, respectively).
4.1.6. Microstructure
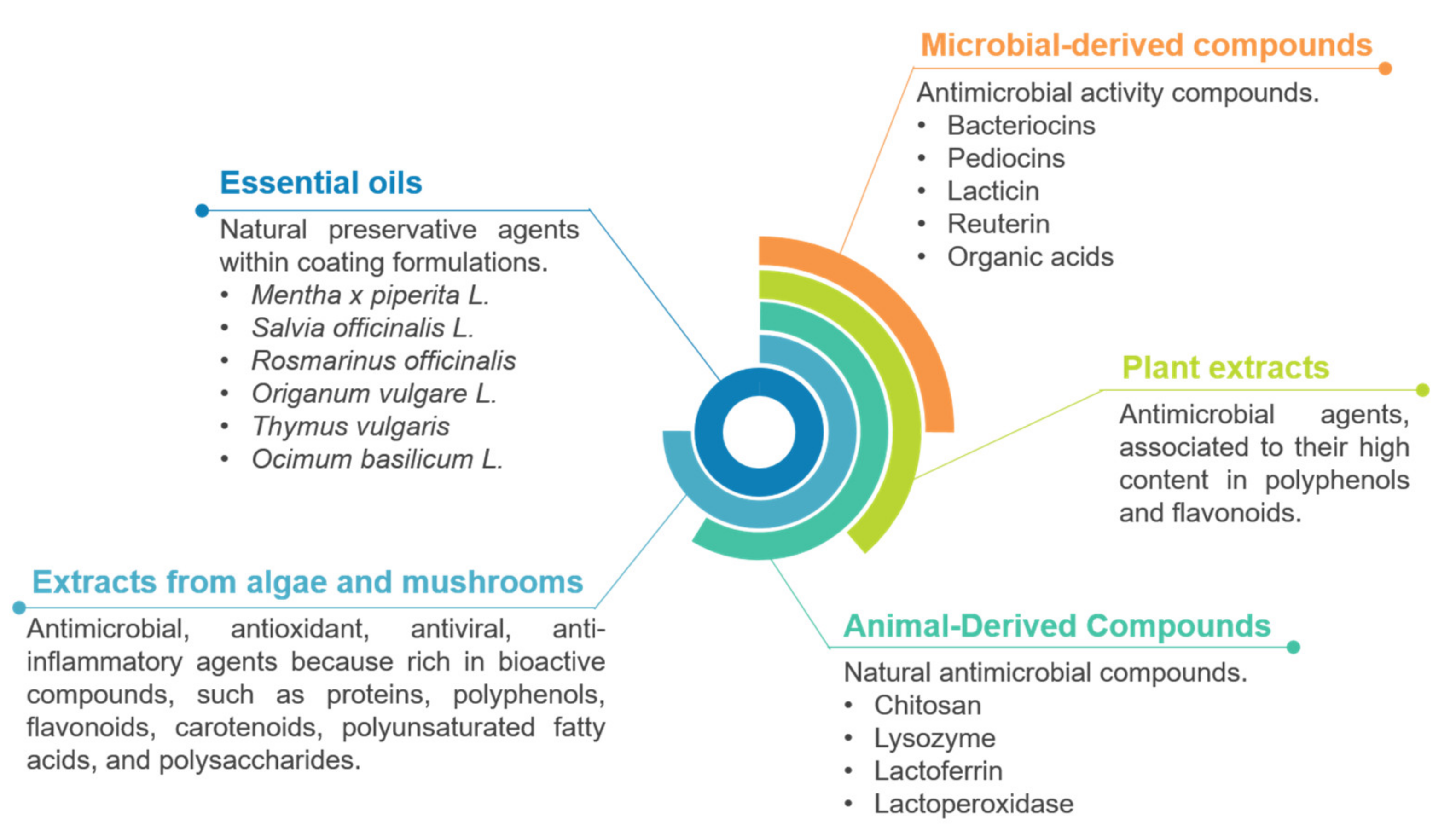
4.2. Antimicrobial Properties and Shelf-Life Extension
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Pirozzi, A.; Del Grosso, V.; Ferrari, G.; Pataro, G.; Donsì, F. Combination of edible coatings containing oregano essential oil nanoemulsion and pulsed light treatments for improving the shelf life of tomatoes. Chem. Eng. Trans. 2021, 87, 61–66. [Google Scholar]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Krochta, J.M. Edible packaging materials. Annu. Rev. Food Sci. Technol. 2010, 1, 415–448. [Google Scholar] [CrossRef] [PubMed]
- Bourtoom, T. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Krochta, J.M. Edible Coatings to Improve Food Quality and Food Safety and Minimize Packaging Cost; Springer: New York, NY, USA, 2009. [Google Scholar]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2021, 166, 289–296. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; O’Connell, C.; Viaux, A.S.; Bou-Maroun, E.; Seuvre, A.M.; Brachais, C.H.; Debeaufort, F. Sorption kinetic of aroma compounds by edible bio-based films from marine-by product macromolecules: Effect of relative humidity conditions. Food Chem. 2019, 298, 125064. [Google Scholar] [CrossRef]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Mohammadi Nafchi, A.; Salehabadi, A.; Oladzad-abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing Spray Systems for Application of Edible Coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Dhanapal, A.; Rajamani, L.; Banu, M. Edible films from Polysaccharides. Food Sci. Qual. 2012, 3, 9–18. [Google Scholar]
- Bergeron, V. Designing intelligent fluids for controlling spray applications. C. R. Phys. 2003, 4, 211–219. [Google Scholar] [CrossRef]
- Skurtys, O.; Acevedo, C.; Pedreschi, F.; Enronoe, J.; Osorio, F.; Aguilera, J.M. Food Hydrocolloid Edible Films and Coatings; Nova Science Publishers: Hauppauge, NY, USA, 2010; ISBN 9781616682699. [Google Scholar]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, K.; Nadeem, H.; Browne, C.; Garnier, G.; Batchelor, W. Engineering surface roughness of nanocellulose film via spraying to produce smooth substrates. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124396. [Google Scholar] [CrossRef]
- Shanmugam, K.; Varanasi, S.; Garnier, G.; Batchelor, W. Rapid preparation of smooth nanocellulose films using spray coating. Cellulose 2017, 24, 2669–2676. [Google Scholar] [CrossRef]
- Nadeem, H.; Naseri, M.; Shanmugam, K.; Dehghani, M.; Browne, C.; Miri, S.; Garnier, G.; Batchelor, W. An energy efficient production of high moisture barrier nanocellulose/carboxymethyl cellulose films via spray-deposition technique. Carbohydr. Polym. 2020, 250, 116911. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.I.; Schutyser, M.; Schroën, K.; Boom, R. Barrier properties and storage stability of edible coatings prepared with electrospraying. Innov. Food Sci. Emerg. Technol. 2014, 23, 182–187. [Google Scholar] [CrossRef]
- Jaworek, A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Jaworek, A. Electrostatic micro- and nanoencapsulation and electroemulsification: A brief review. J. Microencapsul. 2008, 25, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Kim, K.; Kim, S. Characterization of deposition patterns produced by twin-nozzle electrospray. J. Aerosol Sci. 2008, 39, 801–813. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T. Electrospraying route to nanotechnology: An overview. J. Electrostat. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Schutyser, M.A.I.; Schroën, K.; Boom, R. The potential of electrospraying for hydrophobic film coating on foods. J. Food Eng. 2012, 108, 410–416. [Google Scholar] [CrossRef]
- Katiyar, V.; Dhar, P. Processing techniques for nanocellulose-based value-added products. In Cellulose Nanocrystals: An Emerging Nanocellulose for Numerous Chemical; Walter De Gruyter GmbH: Berlin, Germany, 2020. [Google Scholar]
- Brinker, C.J. Dip Coating. In Chemical Solution Deposition of Functional Oxide Thin Films; Schneller, T., Waser, R., Kosec, M., Payne, D., Eds.; Springer: Wien, Austria, 2013; Volume 10, pp. 233–261. ISBN 9783211993118. [Google Scholar]
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Grant, L.A.; Burns, J. Application of coatings. In Edible Coatings and Films to Improve Food Quality; Krochta, J.M., Baldwin, E.A., Nisperos-Carriedo, M.O., Eds.; Technomic Publ. Co: Lancaster, PA, USA, 1994; pp. 189–200. ISBN 9781566761130. [Google Scholar]
- Martín-Belloso, O.; Rojas-Graü, M.A.; Soliva-Fortuny, R. Delivery of Flavor and Active Ingredients Using Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 295–313. [Google Scholar]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Herrera, M.A.; Sirviö, J.A.; Mathew, A.P.; Oksman, K. Environmental friendly and sustainable gas barrier on porous materials: Nanocellulose coatings prepared using spin- and dip-coating. Mater. Des. 2016, 93, 19–25. [Google Scholar] [CrossRef]
- Khan, M.I.; Nasef, M.M. Spreading behaviour of silicone oil and glycerol drops on coated papers. Leonardo J. Sci. 2009, 8, 18–30. [Google Scholar]
- Kumar, G.; Prabhu, K.N. Review of non-reactive and reactive wetting of liquids on surfaces. Adv. Colloid Interface Sci. 2007, 133, 61–89. [Google Scholar] [CrossRef]
- Cao, L.; Ge, T.; Meng, F.; Xu, S.; Li, J.; Wang, L. An edible oil packaging film with improved barrier properties and heat sealability from cassia gum incorporating carboxylated cellulose nano crystal whisker. Food Hydrocoll. 2020, 98, 105251. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Wang, L.F.; Shankar, S.; Rhim, J.W. Properties of alginate-based films reinforced with cellulose fibers and cellulose nanowhiskers isolated from mulberry pulp. Food Hydrocoll. 2017, 63, 201–208. [Google Scholar] [CrossRef]
- Chen, Q.J.; Zhou, L.L.; Zou, J.Q.; Gao, X. The preparation and characterization of nanocomposite film reinforced by modified cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 132, 1155–1162. [Google Scholar] [CrossRef]
- Kassab, Z.; Aziz, F.; Hannache, H.; Ben Youcef, H.; El Achaby, M. Improved mechanical properties of k-carrageenan-based nanocomposite films reinforced with cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 123, 1248–1256. [Google Scholar] [CrossRef]
- Jiang, S.J.; Zhang, T.; Song, Y.; Qian, F.; Tuo, Y.; Mu, G. Mechanical properties of whey protein concentrate based film improved by the coexistence of nanocrystalline cellulose and transglutaminase. Int. J. Biol. Macromol. 2019, 126, 1266–1272. [Google Scholar] [CrossRef]
- Mujtaba, M.; Salaberria, A.M.; Andres, M.A.; Kaya, M.; Gunyakti, A.; Labidi, J. Utilization of flax (Linum usitatissimum) cellulose nanocrystals as reinforcing material for chitosan films. Int. J. Biol. Macromol. 2017, 104, 944–952. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; González-Martínez, C. Recent advances in edible coatings for fresh and minimally processed fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional materials in food nanotechnology. J. Food Sci. 2006, 71, R107. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Hong, T.; Tan, L.; Jeon, H.; Oh, D.X. Biorenewable, transparent, and oxygen/moisture barrier nanocellulose/nanochitin-based coating on polypropylene for food packaging applications. Carbohydr. Polym. 2021, 271, 118421. [Google Scholar] [CrossRef]
- Martin, C.; Jean, B. Nanocellulose/polymer multilayered thin films: Tunable architectures towards tailored physical properties. Nord. Pulp Pap. Res. J. 2014, 29, 19–30. [Google Scholar] [CrossRef]
- Li, F.; Biagioni, P.; Finazzi, M.; Tavazzi, S.; Piergiovanni, L. Tunable green oxygen barrier through layer-by-layer self-assembly of chitosan and cellulose nanocrystals. Carbohydr. Polym. 2013, 92, 2128–2134. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Sui, L.; Elkasabi, Y.; Burgardt, P.; Lee, J.; Miryala, A.; Kusumaatmaja, W.; Carman, M.R.; Shtein, M.; Kieffer, J.; et al. Layer-by-layer assembled films of cellulose nanowires with antireflective properties. Langmuir 2007, 23, 7901–7906. [Google Scholar] [CrossRef]
- Liu, X.; Han, W.; Zhu, Y.; Xuan, H.; Ren, J.; Zhang, J.; Ge, L. Anti-Oxidative and Antibacterial Self-Healing Edible Polyelectrolyte Multilayer Film in Fresh-Cut Fruits. J. Nanosci. Nanotechnol. 2018, 18, 2592–2600. [Google Scholar] [CrossRef]
- Arnon, H.; Granit, R.; Porat, R.; Poverenov, E. Development of polysaccharides-based edible coatings for citrus fruits: A layer-by-layer approach. Food Chem. 2015, 166, 465–472. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Cerqueira, M.A.; Texeira, J.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Effect of an Edible Nanomultilayer Coating by Electrostatic Self-Assembly on the Shelf Life of Fresh-Cut Mangoes. Food Bioprocess Technol. 2015, 8, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Arnon, H.; Zaitsev, Y.; Porat, R.; Poverenov, E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol. Technol. 2014, 87, 21–26. [Google Scholar] [CrossRef]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-Layer Electrostatic Deposition of Edible Coating on Fresh Cut Melon Model: Anticipated and Unexpected Effects of Alginate-Chitosan Combination. Food Bioprocess Technol. 2014, 7, 1424–1432. [Google Scholar] [CrossRef]
- Kittitheeranun, P.; Dubas, S.T.; Dubas, L. Layer-by-layer suface modification of fruits with edible nano-coatings. Appl. Mech. Mater. 2012, 229–231, 2745–2748. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Ye, Z.; Sun, L.; Jin, Y.; Xu, Q.; Yang, M.; Wang, Y.; Zhou, Y.; Ji, J.; Chen, H.; et al. Bacterial infection microenvironment-responsive enzymatically degradable multilayer films for multifunctional antibacterial properties. J. Mater. Chem. B 2017, 5, 8532–8541. [Google Scholar] [CrossRef]
- Wu, G.M.; Deng, H.B.; Jiang, T.; Tu, H.; Chen, J.J.; Zhan, Y.F.; Wang, Y.N.; Ma, X. Regulating the gaps between folds on the surface of silk fibro in membranes via LBL deposition for improving their biomedical properties. Colloids Surf. B Biointerfaces 2017, 154, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, L.; Zhang, X.; Chen, S.; Zhang, M.; Zhao, W.; Sun, S.; Zhao, C. Integrating zwitterionic polymer and Ag nanoparticles on polymeric membrane surface to prepare antifouling and bactericidal surface via Schiff-based layer-by-layer assembly. J. Colloid Interface Sci. 2018, 510, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, R. Size Separations in Capillary Gels and Polymer Networks. In Practical Capillary Electrophoresis; Weinberger, R., Ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 245–292. ISBN 9780127423562. [Google Scholar]
- Linnhoff, B.; Hindmarsh, E. The pinch design method for heat exchanger networks. Chem. Eng. Sci. 1983, 38, 745–763. [Google Scholar] [CrossRef]
- Guo, Q.; Paliy, M.; Kobe, B.; Trebicky, T.; Suhan, N.; Arsenault, G.; Ferrari, L.; Yang, J. Characterization of cross-linking depth for thin polymeric films using atomic force microscopy. J. Appl. Polym. Sci. 2015, 132, 6–11. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Gómez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Functional properties of bioplastics made from wheat gliadins with cinnamaldehyde. J. Agric. Food Chem. 2011, 59, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Rimdusit, S.; Jingjid, S.; Damrongsakkul, S.; Tiptipakorn, S.; Takeichi, T. Biodegradability and property characterizations of Methyl Cellulose: Effect of nanocompositing and chemical crosslinking. Carbohydr. Polym. 2008, 72, 444–455. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Physicochemical and bioactivity of cross-linked chitosan-PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef]
- Yeng, C.M.; Husseinsyah, S.; Ting, S.S. Chitosan/corn cob biocomposite films by cross-linking with glutaraldehyde. BioResources 2013, 8, 2910–2923. [Google Scholar]
- Garg, S.; Jana, A.K. Studies on the properties and characteristics of starch-LDPE blend films using cross-linked, glycerol modified, cross-linked and glycerol modified starch. Eur. Polym. J. 2007, 43, 3976–3987. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.J. Characteristics of crosslinked potato starch and starch-filled linear low-density polyethylene films. Carbohydr. Polym. 2002, 50, 331–337. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; da Silva, M.A.; Braga, M.E.M.; Sousa, H.J.C.; Kieckbusch, T.G. Effect of calcium and/or barium crosslinking on the physical and antimicrobial properties of natamycin-loaded alginate films. LWT Food Sci. Technol. 2014, 57, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Galietta, G.; Di Gioia, L.; Guilbert, S.; Cuq, B. Mechanical and Thermomechanical Properties of Films Based on Whey Proteins as Affected by Plasticizer and Crosslinking Agents. J. Dairy Sci. 1998, 81, 3123–3130. [Google Scholar] [CrossRef]
- Kumar, A.P.; Singh, R.P. Biocomposites of cellulose reinforced starch: Improvement of properties by photo-induced crosslinking. Bioresour. Technol. 2008, 99, 8803–8809. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Coma, V.; Sebti, I.; Pardon, P.; Pichavant, F.H.; Deschamps, A. Film properties from crosslinking of cellulosic derivatives with a polyfunctional carboxylic acid. Carbohydr. Polym. 2002, 51, 265–271. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Kontou-Vrettou, C.; Moates, G.K.; Wellner, N.; Cross, K.; Pereira, P.H.F.; Waldron, K.W. Wheat straw hemicellulose films as affected by citric acid. Food Hydrocoll. 2015, 50, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rouhi, M.; Razavi, S.H.; Mousavi, S.M. Optimization of crosslinked poly(vinyl alcohol) nanocomposite films for mechanical properties. Mater. Sci. Eng. C 2017, 71, 1052–1063. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohydr. Polym. 2010, 82, 270–276. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll. 2007, 21, 575–584. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Amylose-lipid complex as a measure of variations in physical, mechanical and barrier attributes of rice starch-ι-carrageenan biodegradable edible film. Food Packag. Shelf Life 2017, 14, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Nandane, A.S.; Dave, R.K.; Rao, T.V.R. Optimization of edible coating formulations for improving postharvest quality and shelf life of pear fruit using response surface methodology. J. Food Sci. Technol. 2017, 54, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Handayasari, F.; Suyatma, N.E.; Nurjanah, S. Physiochemical and antibacterial analysis of gelatin–chitosan edible film with the addition of nitrite and garlic essential oil by response surface methodology. J. Food Process. Preserv. 2019, 43, e14265. [Google Scholar] [CrossRef]
- Pirozzi, A.; Del Grosso, V.; Ferrari, G.; Donsì, F. Edible coatings containing oregano essential oil nanoemulsion for improving postharvest quality and shelf life of tomatoes. Foods 2020, 9, 1605. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Biol. Macromol. 2020, 143, 704–713. [Google Scholar] [CrossRef]
- Rangel-Marrónab, L.; Mani-Lópeza, E.; Paloua, E.; López-Malo, A. Effects of alginate-glycerol-citric acid concentrations on selected physical, mechanical, and barrier properties of papaya puree-based edible films and coatings, as evaluated by response surface methodology. LWT 2019, 101, 83–91. [Google Scholar] [CrossRef]
- Mustapha, F.A.; Jai, J.; Nik Raikhan, N.H.; Sharif, Z.I.M.; Yusof, N.M. Response surface methodology analysis towards biodegradability and antimicrobial activity of biopolymer film containing turmeric oil against Aspergillus niger. Food Control 2019, 99, 106–113. [Google Scholar] [CrossRef]
- Noorbakhsh-Soltani, S.M.; Zerafat, M.M.; Sabbaghi, S. A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications. Carbohydr. Polym. 2018, 189, 48–55. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Thomas, S.; Dufresne, A. Handbook of Nanocellulose and Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Mondal, S. Review on Nanocellulose Polymer Nanocomposites. Polym.-Plast. Technol. Eng. 2018, 57, 1377–1391. [Google Scholar] [CrossRef]
- Amara, C.; El Mahdi, A.; Medimagh, R.; Khwaldia, K. Nanocellulose-based composites for packaging applications. Curr. Opin. Green Sustain. Chem. 2021, 31, 100512. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials-binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Bin Johan, M.R.; Fen, L.B. Comprehensive review on nanocellulose: Recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2020, 1–14. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The potential of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Lavoine, N.; Jiang, F.; Tang, J.; Lin, N. Reducing end modification on cellulose nanocrystals: Strategy, characterization, applications and challenges. Nanoscale Horizons 2020, 5, 607–627. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Verma, C.; Chhajed, M.; Gupta, P.; Roy, S.; Maji, P.K. Isolation of cellulose nanocrystals from different waste bio-mass collating their liquid crystal ordering with morphological exploration. Int. J. Biol. Macromol. 2021, 175, 242–253. [Google Scholar] [CrossRef]
- Yang, G.; Xia, Y.; Lin, Z.; Zhang, K.; Fatehi, P.; Chen, J. Physicochemical impact of cellulose nanocrystal on oxidation of starch and starch based composite films. Int. J. Biol. Macromol. 2021, 184, 42–49. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Chen, H.; Hu, J.; Cui, L. Preparation of different morphologies cellulose nanocrystals from waste cotton fibers and its effect on PLLA/PDLA composites films. Compos. Part B Eng. 2021, 217, 108934. [Google Scholar] [CrossRef]
- Dong, H.; Ding, Q.; Jiang, Y.; Li, X.; Han, W. Pickering emulsions stabilized by spherical cellulose nanocrystals. Carbohydr. Polym. 2021, 265, 118101. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Basturk, S.B.; Dancer, C.E.J.; McNally, T. Cellulose nanocrystals produced using recyclable sulfuric acid as hydrolysis media and their wetting molecular dynamics simulation. Int. J. Biol. Macromol. 2021, 184, 405–414. [Google Scholar]
- Mariia, K.; Arif, M.; Shi, J.; Song, F.; Chi, Z.; Liu, C. Novel chitosan-ulvan hydrogel reinforcement by cellulose nanocrystals with epidermal growth factor for enhanced wound healing: In vitro and in vivo analysis. Int. J. Biol. Macromol. 2021, 183, 435–446. [Google Scholar] [CrossRef]
- Catori, D.M.; Fragal, E.H.; Messias, I.; Garcia, F.P.; Nakamura, C.V.; Rubira, A.F. Development of composite hydrogel based on hydroxyapatite mineralization over pectin reinforced with cellulose nanocrystal. Int. J. Biol. Macromol. 2021, 167, 726–735. [Google Scholar] [CrossRef]
- Pandi, N.; Sonawane, S.H.; Anand Kishore, K. Synthesis of cellulose nanocrystals (CNCs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2021, 70, 105353. [Google Scholar] [CrossRef]
- Alonso-Lerma, B.; Larraza, I.; Barandiaran, L.; Ugarte, L.; Saralegi, A.; Corcuera, M.A.; Perez-Jimenez, R.; Eceiza, A. Enzymatically produced cellulose nanocrystals as reinforcement for waterborne polyurethane and its applications. Carbohydr. Polym. 2021, 254, 117478. [Google Scholar] [CrossRef] [PubMed]
- Kamtsikakis, A.; Delepierre, G.; Weder, C. Cellulose nanocrystals as a tunable nanomaterial for pervaporation membranes with asymmetric transport properties. J. Membr. Sci. 2021, 635, 119473. [Google Scholar] [CrossRef]
- Shahrousvand, E.; Shahrousvand, M. Preparation of polyurethane/poly(2-hydroxyethyl methacrylate) semi-IPNs containing cellulose nanocrystals for biomedical applications. Mater. Today Commun. 2021, 27, 102421. [Google Scholar] [CrossRef]
- Adeniyi, A.; Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Oyewo, O.; Sithole, B.; Onyango, M. Incorporation of Cellulose Nanocrystals (CNC) derived from sawdust into polyamide thin-film composite membranes for enhanced water recovery. Alex. Eng. J. 2020, 59, 4201–4210. [Google Scholar] [CrossRef]
- Hossain, S.; Shahruzzaman, M.; Kabir, S.F.; Rahman, M.S.; Sultana, S.; Mallik, A.K.; Haque, P.; Takafuji, M.; Rahman, M.M. Jute cellulose nanocrystal/poly(N,N-dimethylacrylamide-co-3-methacryloxypropyltrimethoxysilane) hybrid hydrogels for removing methylene blue dye from aqueous solution. J. Sci. Adv. Mater. Devices 2021, 6, 254–263. [Google Scholar] [CrossRef]
- Alothman, O.Y.; Kian, L.K.; Saba, N.; Jawaid, M.; Khiari, R. Cellulose nanocrystal extracted from date palm fibre: Morphological, structural and thermal properties. Ind. Crops Prod. 2021, 159, 113075. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Liu, J.; Qin, S.; Pei, Y.; Zheng, X.; Tang, K. High mechanical strength gelatin composite hydrogels reinforced by cellulose nanofibrils with unique beads-on-a-string morphology. Int. J. Biol. Macromol. 2020, 164, 1776–1784. [Google Scholar] [CrossRef]
- Messa, L.L.; Faez, R.; Hsieh, Y.-L. Phosphorylated cellulose nanofibrils from sugarcane bagasse with pH tunable gelation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100085. [Google Scholar]
- Arancibia, F.; Izquierdo, E.; Pereira, M. Stabilization of the emulsion of Alkenyl Succinic Anhydride (ASA) in water using cellulose nanofibrils. Chem. Eng. Sci. 2021, 233, 116407. [Google Scholar] [CrossRef]
- Baniasadi, H.; Ajdary, R.; Trifol, J.; Rojas, O.J.; Seppälä, J. Direct ink writing of aloe vera/cellulose nanofibrils bio-hydrogels. Carbohydr. Polym. 2021, 266, 118114. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, J.; Lampimäki, M.; Laitinen, O.; Vainio, T.; Kangasluoma, J.; Siivola, E.; Petäjä, T.; Liimatainen, H. High-performance and sustainable aerosol filters based on hierarchical and crosslinked nanofoams of cellulose nanofibers. J. Clean. Prod. 2021, 310, 127498. [Google Scholar] [CrossRef]
- Islam, M.A.; Ong, H.L.; Villagracia, A.R.; Halim, K.A.A.; Ganganboina, A.B.; Doong, R.-A. Biomass–derived cellulose nanofibrils membrane from rice straw as sustainable separator for high performance supercapacitor. Ind. Crops Prod. 2021, 170, 113694. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.N.; Wu, Q.; Li, Q.; Huang, J.; Li, W.; Zhang, W.; Wang, S. Phosphorus containing group and lignin toward intrinsically flame retardant cellulose nanofibril-based film with enhanced mechanical properties. Compos. Part B Eng. 2021, 212, 108699. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Wang, S.; Fang, F.; Wang, X.; Hou, Q. Nanocomposites derived from licorice residues cellulose nanofibril and chitosan nanofibril: Effects of chitosan nanofibril dosage on resultant properties. Int. J. Biol. Macromol. 2020, 165, 2404–2411. [Google Scholar] [CrossRef]
- Naidu, D.S.; John, M.J. Cellulose nanofibrils reinforced xylan-alginate composites: Mechanical, thermal and barrier properties. Int. J. Biol. Macromol. 2021, 179, 448–456. [Google Scholar] [CrossRef]
- Shoda, M.; Sugano, Y. Recent advances in bacterial cellulose production. Biotechnol. Bioprocess Eng. 2005, 10, 1. [Google Scholar] [CrossRef]
- Seo, C.; Lee, H.W.; Suresh, A.; Yang, J.W.; Jung, J.K.; Kim, Y.C. Improvement of fermentative production of exopolysaccharides from Aureobasidium pullulans under various conditions. Korean J. Chem. Eng. 2014, 31, 1433–1437. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef]
- Arserim-Uçar, D.K.; Korel, F.; Liu, L.S.; Yam, K.L. Characterization of bacterial cellulose nanocrystals: Effect of acid treatments and neutralization. Food Chem. 2021, 336, 127597. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Antimicrobial efficacy of nisin-loaded bacterial cellulose nanocrystals against selected meat spoilage lactic acid bacteria. Carbohydr. Polym. 2021, 251, 117096. [Google Scholar] [CrossRef]
- Nam, J.; Hyun, Y.; Oh, S.; Park, J.; Jin, H.J.; Kwak, H.W. Effect of cross-linkable bacterial cellulose nanocrystals on the physicochemical properties of silk sericin films. Polym. Test. 2021, 97, 107161. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Qiu, D.; Pei, Y.; Li, Y.; Li, B.; Liu, S. Effect of surface charge density of bacterial cellulose nanofibrils on the rheology property of O/W Pickering emulsions. Food Hydrocoll. 2021, 120, 106944. [Google Scholar] [CrossRef]
- Gupte, Y.; Kulkarni, A.; Raut, B.; Sarkar, P.; Choudhury, R.; Chawande, A.; Kumar, G.R.K.; Bhadra, B.; Satapathy, A.; Das, G.; et al. Characterization of nanocellulose production by strains of Komagataeibacter sp. isolated from organic waste and Kombucha. Carbohydr. Polym. 2021, 266, 118176. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.M.; Seo, M.; Shin, K.; Choi, S.; Kim, J.W. Bacterial cellulose nanofibrils-armored Pickering emulsions with limited influx of metal ions. Carbohydr. Polym. 2021, 258, 117730. [Google Scholar] [CrossRef] [PubMed]
- Barría, J.C.; Vázquez, A.; Pereira, J.M.; Manzanal, D. Effect of bacterial nanocellulose on the fresh and hardened states of oil well cement. J. Pet. Sci. Eng. 2021, 199, 108259. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Y.; Shabbir, M.; Pei, Y.; Liang, H.; Li, J.; Chen, Y.; Li, Y.; Li, B.; Luo, X.; et al. Coalescence behavior of eco-friendly Pickering-MIPES and HIPEs stabilized by using bacterial cellulose nanofibrils. Food Chem. 2021, 349, 129163. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K.; Pathak, P. Static intermittent fed-batch production of bacterial nanocellulose from black tea and its modification using chitosan to develop antibacterial green packaging material. J. Clean. Prod. 2021, 279, 123608. [Google Scholar] [CrossRef]
- Gaoquan, H.U.; Chen, L.; Zhao, S.; Hong, F.F. Mercerization of tubular bacterial nanocellulose for control of the size and performance of small-caliber vascular grafts. Chem. Eng. J. 2021, 428, 131104. [Google Scholar]
- Kousheh, S.A.; Moradi, M.; Tajik, H.; Molaei, R. Preparation of antimicrobial/ultraviolet protective bacterial nanocellulose film with carbon dots synthesized from lactic acid bacteria. Int. J. Biol. Macromol. 2020, 155, 216–225. [Google Scholar] [CrossRef]
- Fancey, K.S. A coating thickness uniformity model for physical vapour deposition systems: Overview. Surf. Coat. Technol. 1995, 71, 16–29. [Google Scholar] [CrossRef]
- Hagenmaier, R.D.; Baker, R.A. Layered coatings to control weight loss and preserve gloss of citrus fruit. HortScience 1995, 30, 296–298. [Google Scholar] [CrossRef] [Green Version]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Løkke, M.M.; Micklander, E.; Engelsen, S.B. Vibrational microspectroscopy of food. Raman vs. FT-IR. Trends Food Sci. Technol. 2003, 14, 50–57. [Google Scholar] [CrossRef]
- Mcanally, G.D.; Everall, N.J.; Chalmers, J.M.; Smith, W.E. Analysis of thin film coatings on poly(ethylene terephthalate) by confocal Raman microscopy and surface-enhanced raman scattering. Appl. Spectrosc. 2003, 57, 44–50. [Google Scholar] [CrossRef]
- Arham, R.; Mulyati, M.T.; Metusalach, M.; Salengke, S. Physical and mechanical properties of agar based edible film with glycerol plasticizer. Int. Food Res. J. 2016, 23, 1669–1675. [Google Scholar]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Díaz, D.A.; Amaya, E. Biodegradability and mechanical properties of starch films from Andean crops. Int. J. Biol. Macromol. 2011, 48, 603–606. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and aloe vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Kuzmina, O.; Heinze, T.; Wawro, D. Blending of Cellulose and Chitosan in Alkyl Imidazolium Ionic Liquids. ISRN Polym. Sci. 2012, 2012, 251950. [Google Scholar] [CrossRef] [Green Version]
- Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Physical and mechanical properties of chitosan films as affected by drying methods and addition of antimicrobial agent. J. Food Eng. 2013, 119, 140–149. [Google Scholar] [CrossRef]
- Rhim, J.W. Physical-Mechanical Properties of Agar/κ-Carrageenan Blend Film and Derived Clay Nanocomposite Film. J. Food Sci. 2012, 77, N66–N73. [Google Scholar] [CrossRef]
- Tahsiri, Z.; Mirzaei, H.; Hosseini, S.M.H.; Khalesi, M. Gum arabic improves the mechanical properties of wild almond protein film. Carbohydr. Polym. 2019, 222, 114994. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of composite edible films based on pectin/alginate/whey protein concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, M.; Vu, K.D. Edible Coating and Film Materials: Proteins. In Innovations in Food Packaging; Han, J.H., Ed.; Bio-Green Elsevier (Exc): Plano, TX, USA, 2014; pp. 277–304. ISBN 9780123946010. [Google Scholar]
- Atef, M.; Rezaei, M.; Behrooz, R. Preparation and characterization agar-based nanocomposite film reinforced by nanocrystalline cellulose. Int. J. Biol. Macromol. 2014, 70, 537–544. [Google Scholar] [CrossRef]
- Ghosh, T.; Nakano, K.; Katiyar, V. Curcumin doped functionalized cellulose nanofibers based edible chitosan coating on kiwifruits. Int. J. Biol. Macromol. 2021, 184, 936–945. [Google Scholar] [CrossRef]
- Shih, Y.T.; Zhao, Y. Development, characterization and validation of starch based biocomposite films reinforced by cellulose nanofiber as edible muffin liner. Food Packag. Shelf Life 2021, 28, 100655. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Nilsson, K.; Trifol, J.; Langton, M.; Gomez-Caturla, J.; Balart, R.; Garcia-Garcia, D.; Moriana, R. Faba bean protein films reinforced with cellulose nanocrystals as edible food packaging material. Food Hydrocoll. 2021, 107019. [Google Scholar] [CrossRef]
- Yao Désiré, A.; Charlemagne, N.; Degbeu Claver, K.; Fabrice Achille, T.; Marianne, S. Starch-based edible films of improved cassava varieties Yavo and TMS reinforced with microcrystalline cellulose. Heliyon 2021, 7, e06804. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zheng, Y.; Wang, X.; Huang, Y.; Ni, L.; Chen, X.; Wu, Z.; Huang, C.; Yi, Q.; Li, J.; et al. Study on physicochemical properties, antioxidant and antimicrobial activity of okara soluble dietary fiber/sodium carboxymethyl cellulose/thyme essential oil active edible composite films incorporated with pectin. Int. J. Biol. Macromol. 2020, 165, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan. Int. J. Biol. Macromol. 2020, 145, 634–645. [Google Scholar] [CrossRef]
- Jancy, S.; Shruthy, R.; Preetha, R. Fabrication of packaging film reinforced with cellulose nanoparticles synthesised from jack fruit non-edible part using response surface methodology. Int. J. Biol. Macromol. 2020, 142, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Chiu, F.C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, C.; Hao, W.; Ullah, N.; Chen, L.; Li, Z.; Feng, X. Development and characterization of agar-based edible films reinforced with nano-bacterial cellulose. Int. J. Biol. Macromol. 2018, 118, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Gontard, N.; Guilbert, S. Bio-packaging: Technology and properties of edible and/or biodegradable material of agricultural origin. In Food Packaging and Preservation; Mathlouthi, M., Ed.; Springer: Boston, MA, USA, 1994; pp. 159–181. ISBN 9780834213494. [Google Scholar]
- Chi, K.; Catchmark, J.M. Improved eco-friendly barrier materials based on crystalline nanocellulose/chitosan/carboxymethyl cellulose polyelectrolyte complexes. Food Hydrocoll. 2018, 80, 195–205. [Google Scholar] [CrossRef]
- Abdollahi, M.; Alboofetileh, M.; Behrooz, R.; Rezaei, M.; Miraki, R. Reducing water sensitivity of alginate bio-nanocomposite film using cellulose nanoparticles. Int. J. Biol. Macromol. 2013, 54, 166–173. [Google Scholar] [CrossRef]
- Krochta, J.M. Edible protein films and coatings. In Food Proteins and Their Applications; Damodaran, S., Ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9780367401047. [Google Scholar]
- Rulon, E.J.; Rorbert, H.D. Wetting of low-energy surfaces. In Wettability; John, C.B., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1993; pp. 4–73. [Google Scholar]
- Mustafa, W.; Pataro, G.; Ferrari, G.; Donsì, F. Novel approaches to oil structuring via the addition of high-pressure homogenized agri-food residues and water forming capillary bridges. J. Food Eng. 2018, 236, 9–18. [Google Scholar] [CrossRef]
- Taştan, Ö.; Ferrari, G.; Baysal, T.; Donsì, F. Understanding the effect of formulation on functionality of modified chitosan films containing carvacrol nanoemulsions. Food Hydrocoll. 2016, 61, 756–771. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Lu, H.; Wang, H.; Shi, Y. Direct measurement of the contact angle of water droplet on quartz in a reservoir rock with atomic force microscopy. Chem. Eng. Sci. 2018, 177, 445–454. [Google Scholar] [CrossRef]
- Miller, K.S.; Krochta, J.M. Oxygen and aroma barrier properties of edible films: A review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Park, H.J.; Chinnan, M.S. Gas and water vapor barrier properties of edible films from protein and cellulosic materials. J. Food Eng. 1995, 25, 497–507. [Google Scholar] [CrossRef]
- Jasse, B.; Seuvre, A.M.; Mathlouthi, M. Permeability and structure in polymeric packaging materials. In Food Packaging and Preservation; Mathlouthi, M., Ed.; Springer: Boston, MA, USA, 1994; pp. 1–22. ISBN 978-1-4615-2173-0. [Google Scholar]
- Villalobos, R.; Chanona, J.; Hernández, P.; Gutiérrez, G.; Chiralt, A. Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocoll. 2005, 19, 53–61. [Google Scholar] [CrossRef]
- De Souza Coelho, C.C.; Silva, R.B.S.; Carvalho, C.W.P.; Rossi, A.L.; Teixeira, J.A.; Freitas-Silva, O.; Cabral, L.M.C. Cellulose nanocrystals from grape pomace and their use for the development of starch-based nanocomposite films. Int. J. Biol. Macromol. 2020, 159, 1048–1061. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food Color and Appearance; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9781441951939. [Google Scholar]
- Gupta, M.; Brennan, C.; Tiwari, B.K. Starch-based Edible Films and Coatings. In Starch-Based Polymeric Materials and Nanocomposites: Chemistry, Processing, and Applications; Ahmed, J., Tiwari, B.K., Imam, S.H., Rao, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 239–254. ISBN 9781138198623. [Google Scholar]
- Trezza, T.A.; Krochta, J.M. Color stability of edible coatings during prolonged storage. J. Food Sci. 2000, 65, 1166–1169. [Google Scholar] [CrossRef]
- Nikolova, K.; Panchev, I.; Sainov, S. Optical characteristics of biopolymer films from pectin and gelatin. J. Optoelectron. Adv. Mater. 2005, 7, 1439–1444. [Google Scholar]
- Fabra, M.J.; Talens, P.; Chiralt, A. Microstructure and optical properties of sodium caseinate films containing oleic acid-beeswax mixtures. Food Hydrocoll. 2009, 23, 676–683. [Google Scholar] [CrossRef]
- Trezza, T.A.; Krochta, J.M. Specular reflection, gloss, roughness and surface heterogeneity of biopolymer coatings. J. Appl. Polym. Sci. 2001, 79, 2221–2229. [Google Scholar] [CrossRef]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Nur Hanani, Z.A.; Lasik-Kurdyś, M. Combination of alginate coating and repetitive pulsed light for shelf life extension of fresh-cut cantaloupe (Cucumis melo L. reticulatus cv. Glamour). J. Food Process. Preserv. 2018, 42, e13786. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Huber, K.C.; Pavlath, A.E.; Orts, W.; Dangaran, K.; Tomasula, P.M.; Qi, P.; Nieto, M.B.; Kramer, M.E.; Debeaufort, F.; et al. Edible Films and Coatings for Food Applications; Embuscado, M.E., Huber, K.C., Eds.; Springer: New York, NY, USA, 2009; ISBN 978-0-387-92823-4. [Google Scholar]
- Abu Salha, B.; Gedanken, A. Extending the Shelf Life of Strawberries by the Sonochemical Coating of their Surface with Nanoparticles of an Edible Anti-Bacterial Compound. Appl. Nano 2021, 2, 14–24. [Google Scholar] [CrossRef]
- Sharif, Z.I.M.; Jai, J.; Subuki, I.; Zaki, N.A.M.; Mustapha, F.A.; Mohd Yusof, N.; Idris, S.A. Optimization of Starch Composite Edible Coating Formulation on Fresh-Cut “Fuji” Apple through Surface Tension, Wettability and FTIR Spectroscopy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 507, 012009. [Google Scholar] [CrossRef]
- Rai, S.; Suman Rai, C.; Poonia, A. Formulation and characterization of edible films from pea starch and casein. J. Pharmacogn. Phytochem. 2019, 8, 317–321. [Google Scholar]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Martínez, I.C.; Saavedra-Leos, M.Z. Application of differential scanning calorimetry (DSC) and modulated differential scanning calorimetry (MDSC) in food and drug industries. Polymers 2020, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Oun, A.A.; Rhim, J.W. Effect of post-treatments and concentration of cotton linter cellulose nanocrystals on the properties of agar-based nanocomposite films. Carbohydr. Polym. 2015, 134, 20–29. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crops Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, H.; Luan, Q.; Zheng, M.; Tang, H.; Huang, F. Fabrication of cellulose nanowhiskers reinforced chitosan-xylan nanocomposite films with antibacterial and antioxidant activities. Carbohydr. Polym. 2018, 184, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, X.; Zhao, R.; Guo, D.; Zhang, J. Preparation and properties of chitosan/guar gum/nanocrystalline cellulose nanocomposite films. Carbohydr. Polym. 2018, 197, 128–136. [Google Scholar] [CrossRef]
- Silva, A.P.M.; Oliveira, A.V.; Pontes, S.M.A.; Pereira, A.L.S.; Rosa, M.F.; Azeredo, H.M.C. Mango kernel starch films as affected by starch nanocrystals and cellulose nanocrystals. Carbohydr. Polym. 2019, 211, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirozzi, A.; Pataro, G.; Donsì, F.; Ferrari, G. Edible Coating and Pulsed Light to Increase the Shelf Life of Food Products. Food Eng. Rev. 2020. [Google Scholar] [CrossRef]
- Vojdani, F.; Torres, J.A. Potassium Sorbate Permeability of Methylcellulose and Hydroxypropyl Methylcellulose Coatings: Effect of Fatty Acids. J. Food Sci. 1990, 55, 841–846. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Zactiti, E.M.; Kieckbusch, T.G. Release of potassium sorbate from active films of sodium alginate crosslinked with calcium chloride. Packag. Technol. Sci. 2009, 22, 349–358. [Google Scholar] [CrossRef]
- Quintavalla, S.; Vicini, L. Antimicrobial food packaging in meat industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K. Natural Antimicrobial Edible Coatings for Microbial Safety and Food Quality Enhancement. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef]
- Shahidi, F. Antioxidants in food and food antioxidants. Food/Nahrung 2000, 44, 158–163. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.V.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Dong, X.; Wrolstad, R.E.; Sugar, D. Extending shelf life of fresh-cut pears. J. Food Sci. 2000, 65, 181–186. [Google Scholar] [CrossRef]
- Rocha, A.M.C.N.; Morais, A.M.M.B. Polyphenoloxidase activity and total phenolic content as related to browning of minimally processed “Jonagored” apple. J. Sci. Food Agric. 2002, 82, 120–126. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Tapia, M.S.; Martín-Belloso, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT Food Sci. Technol. 2008, 41, 139–147. [Google Scholar] [CrossRef]
- Mujtaba, M.; Akyuz, L.; Koc, B.; Kaya, M.; Ilk, S.; Cansaran-Duman, D.; Martinez, A.S.; Cakmak, Y.S.; Labidi, J.; Boufi, S. Novel, multifunctional mucilage composite films incorporated with cellulose nanofibers. Food Hydrocoll. 2019, 89, 20–28. [Google Scholar] [CrossRef]
- Ranjbaryan, S.; Pourfathi, B.; Almasi, H. Reinforcing and release controlling effect of cellulose nanofiber in sodium caseinate films activated by nanoemulsified cinnamon essential oil. Food Packag. Shelf Life 2019, 21, 100341. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Salgado, P.R.; Dufresne, A.; Mauri, A.N. Microfibrillated cellulose addition improved the physicochemical and bioactive properties of biodegradable films based on soy protein and clove essential oil. Food Hydrocoll. 2018, 79, 416–427. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Gelatin/agar-based functional film integrated with Pickering emulsion of clove essential oil stabilized with nanocellulose for active packaging applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127220. [Google Scholar] [CrossRef]
- Montero, Y.; Souza, A.G.; Oliveira, É.R.; dos Santos Rosa, D. Nanocellulose functionalized with cinnamon essential oil: A potential application in active biodegradable packaging for strawberry. Sustain. Mater. Technol. 2021, 29, e00289. [Google Scholar]
| Product | Polyelectrolytes | Results | References | |
|---|---|---|---|---|
| Anionic | Cationic | |||
| - | Nanocellulose | Nanochitin | Reinforcing film agent with excellent gas-barrier properties, highly transparent, unfavorable to bacterial adhesion and thermally recyclable, thus promising for advanced food packaging applications. | [45] |
| - | Nanocellulose | Chitosan, cationic starch and collagen | Ability to finely tailor the nanoarchitecture of the film providing ways high performance free-standing films or coatings with advanced properties. | [46] |
| - | Nanocellulose | Chitosan | Promising nanocomposite film with high oxygen barrier in transparent flexible packaging materials and semi rigid tridimensional objects. | [47] |
| - | Nanocellulose | Polyethyleneimine | Thin films with unique mechanical properties and the morphology of a “porous matchsticks pile”, which brings about strong antireflective properties. | [48] |
| Fresh-cut apples | Carboxymethylcellulose sodium salt (NaCMC) | Chitosan | Polyelectrolyte multilayer (PEM) film shown good browning, weight loss, and metabolic activity inhibition ability. | [49] |
| Mandarin fruits | Carboxymethylcellulose (CMC) | Chitosan | The LbL polysaccharides-based coating notably improved the physiological quality of mandarins and their firmness. | [50] |
| Fresh-cut mangoes | Sodium alginate | Chitosan | Nanomultilayer coating by electrostatic self-assembly improved the microbiological and physicochemical quality of during storage time. | [51] |
| Citrus fruit | Carboxymethyl cellulose (CMC) | Chitosan | CMC/chitosan electrostatic bilayer EC greatly enhanced fruit glossiness and appearance but was not very effective in preventing weight loss. | [52] |
| Fresh-cut melons | Sodium alginate | Chitosan | LbL electrostatic deposition of ECs had benefits on food firmness, gas exchange, and microbiological protection | [53] |
| Mango fruits | Polystyrene sulfonate sodium salt (PSS) | Poly diallyl dimethylammonium chloride (PDADMAC) | PDADMAC/PSS films based-coated fruit shown significantly improved the hydrophilicity of the outer surface. | [54] |
| Cross-Linking Agent | Biopolymers | References |
|---|---|---|
| Glutaraldehyde | Gelatin Cellulosic derivatives Chitosan | [64] [65] [66,67] |
| Epichlorohydrin | Starch | [68,69] |
| Ca2+ ions | Alginate Pectin Whey protein | [70,71,72] [70] [73] |
| Sodium benzoate | Starch | [74] |
| Citric acid | Starch Cellulosic derivatives | [75,76] [77,78] |
| Boric acid | Cellulose | [79] |
| Tannic acid | Chitosan Gelatin | [80] [81] |
| Ferulic acid | Gelatin | [81] |
| Source | Production Process | Morphology/Shape | Dimensions | Crystallinity | Applications | References |
|---|---|---|---|---|---|---|
| Pine | Acid hydrolysis | Spherical morphology | 50–100 nm diameter | 55% | – | [111] |
| Teak | Rod-like surface topographies | 50–60 nm diameter | 52% | |||
| Sugarcane bagasse | Rod-like structure | 20–60 nm in diameter | 45% | |||
| Eucalyptus pulp | Acid hydrolysis | Rod-like structure | 130–250 nm in length and 15–30 nm in diameter | – | Starch based composite film | [112] |
| Waste cotton fibers | Ultrasound-assisted acid hydrolysis | Short rod shape | 200–500 nm length and 10–15 nm diameter | 86% | PLLA/PDLA composites films | [113] |
| Commercial microcrystalline cellulose | Alkali hydrolysis followed by ultrasound-assisted acid hydrolysis | Spherical shape | 30–60 nm in diameter | 81% | Stabilizer for Pickering emulsions | [114] |
| Water hyacinth stem fiber | Acid hydrolysis | Spherical-like particles | 20–50 nm in diameter | 72% | Reinforcement for polyvinyl alcohol (PVA)-gelatin nanocomposite | [115] |
| Commercial microcrystalline cellulose | Acid hydrolysis | Spherical shape | 126–134 nm length and 3–11 nm diameter | 77%–83% | Pickering emulsion stabilizers and surface cleaning agents | [116] |
| Enteromorpha Ulva prolifera green seaweed | Acid hydrolysis | – | – | – | Reinforcement for chitosan-ulvan hydrogel | [117] |
| Cellulose-rich cotton fibers | Alkali hydrolysis followed by acid hydrolysis | Bundles of rod-like particles | 60 nm in lenght | 89% | Reinforcement for chitosan-ulvan hydrogel | [118] |
| Cotton | Ultrasound-assisted acid hydrolysis | Spherical rod-like shape | 50 nm in diameter | 81% | – | [119] |
| Commercial cellulose | Acid hydrolysis | Ribbon-like structure | 173 ± 6.3 nm in length and 10 ± 0.4 nm in diameter | 81% | Reinforcement for waterborne polyurethanes | [120] |
| Commercial cellulose | Acid hydrolysis | Rod-like particles | 128 ± 55 nm in length and 14 ± 4 nm in diameter | 84% | Tunable nanomaterial for pervaporation membranes based on a hydrophobic poly(styrene)-poly(butadiene)-poly(styrene) (SBS) matrix | [121] |
| Paper powders | Acid hydrolysis | Rod-like particles | 100 nm in length and 7 nm in diameter | 65% | Reinforcement for polyurethane (PU) nanocomposites for medical applications | [122] |
| Sawdust | Ultrasound pre-treatment followed by aid hydrolysis | Dot-like shape | 6 nm in diameter | – | Polyamide thin-film composite membranes for enhanced water recovery | [123] |
| Jute fibers | Acid hydrolysis followed by alkali hydrolysis | Rod-like structure | 400–1200 nm length and 40–90 nm diameter | – | Reinforcement for pSiDm hydrogel to treat waste effluent | [124] |
| Palm fibre | Acid hydrolysis | Rod-like shapes | – | 84% | Potential filling agent | [125] |
| Source | Production Process | Morphology/Shape | Dimensions | Crystallinity | Applications | References |
|---|---|---|---|---|---|---|
| Waste cotton fibers | Ultrasound-assisted acid hydrolysis | Fibrous | 15–20 nm in width and 1000–3000 nm in length | 79% | PLLA/PDLA composites films | [113] |
| Sugarcane bagasse | (NH4)2HPO4 phosphorylation and mechanical high-speed blending | Fiber bundles | 18 ± 9 μm in width and 458 ± 130 μm in length | 69% | Gel | [128] |
| Bleached pulp paper | Enzymatic pre-treatment and then a high-pressure homogenization step | Fiber bundles | 28.1 nm in diameter and 4.9 µm length | – | Stabilization of the emulsion of Alkenyl Succinic Anhydride in water | [129] |
| Birch fibers | Microfluidizer assisted TEMPO-mediated oxidation | – | – | – | Reinforcement for hydrogels | [130] |
| Recycled milk-container board | Deep eutectic solvent treated and mechanical grinding | – | 2–80 nm in diameter | – | Filter material for aerosol filtration | [131] |
| Rice straw | Alkaline hydrolysis, bleaching and TEMPO-mediated oxidation | Homogeneous fibril structure | 5–10 μm diameter and 10–40 nm width | – | Composite membrane to increase electrochemical performance of supercapacitor | [132] |
| Wood pulp sheets | (NH4)2HPO4 phosphorylation and mechanical ultra-fine grinder | Soft fiber structure | 10–20 μm in diameter | – | Cellulose-based film for flame-retardant packaging materials | [133] |
| Bamboo pulp sheets | ||||||
| Low lignin-containing bamboo pulp sheets | ||||||
| Bamboo powder | Rod-like structure | |||||
| Commercial microcrystalline cellulose | Ultrasonic treatment following sulfuric acid hydrolysis | Beads-on-a-string cellulose nanofibril | 10–30 μm width and 40–50 μm length | 77% | Gelatin composite hydrogels | [127] |
| Licorice residues | Alkali and enzymatic hydrolysis followed by high-pressure homogenization | Nanofiber structure | 130 nm in diameter and 8 µm in lenght | – | Nanocomposite film | [134] |
| Commercial chitosan powder | High-pressure homogenization assisted TEMPO-mediated oxidation | 204 nm in diameter and 13 µm in lenght | ||||
| Maize stalk waste residues | Mechanical grinding assisted chemical treatments | Highly entangled fibres network and web like structure | 35.48 ± 12.60 nm in diameter | 71% | Reinforcement material for biopolymer films for food packaging applications | [135] |
| Source | Production Process | Morphology/Shape | Dimensions | Crystallinity | Applications | References |
|---|---|---|---|---|---|---|
| Bacterial cellulose pellicles | Acid hydrolysis and ultrasonic treatment | Rod or needle-shaped nanocrystals | 15–56 nm in width and 259–1142 nm in length | 83% | Nisin-loaded BCNs as antimicrobial agents in active food packaging | [140] |
| Pellicle-shaped bacterial cellulose | Mechanically defibrillation and acid hydrolysis | Rod-type crystal morphology | 20–30 nm in diameter | - | Reinforcement for sericin film | [141] |
| Bacterial cellulose | 2,2,6,6-tetramethylpiperidine-nitrogen-oxide (TEMPO) oxidation | Fibrils bundles | 70–100 nm in width | - | O/W Pickering emulsion stabilizer | [142] |
| Bacterial cellulose pellicles from organic waste and kombucha | Fermentation using glycerol as carbon source | 3D structure of cellulose fibrils | 100–2000 nm in length and 5 nm in width | 64%–80% | Composites | [143] |
| Bacterial cellulose | 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) oxidation | Nanofibrils | 5–10 nm in width | - | Pickering emulsion system stabilizer | [144] |
| Bacterial cellulose pellicles from grape pomace | Fermentation using carbon and nitrogen source | Ribbon-shaped cellulose nanofibers and nanofiber aggregates | 18–57 nm in width and micrometers in length | 68%–85% | Nanoadditives for oil well cement cement | [145] |
| Bacterial cellulose | High-pressure homogenization treatment | Nanofibrils | 97 nm in width and 6 nm in height | - | Pickering emulsion stabilizer | [146] |
| SCOBY, black tea | Fermentation | Nanofibers | 20–100 nm in diameter | 73%–79% | Reinforcement for chitosan nano-biocomposite films | [147] |
| Bacterial cellulose | Alkaline treatment | Tangled fibers | 50.73–140.25 nm in diameter | 84%–88% | Small-caliber vascular grafts | [148] |
| Bacterial cellulose | Fermentation in static culture | Ribbon-shaped fibrils | 70–80 nm in width | - | Reinforcement for film with carbon dots | [149] |
| Film-Forming Material | Concentration (% w/w) | Thickness (µm) | Mechanical Properties | References | |
|---|---|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | ||||
| Agar | 1–3 | 31.2–70.2 | 14.3–37.4 | 12.4–31.8 | [155] |
| Starch | 5 | 200 | 1.41–8.03 | 12.97–56.25 | [156] |
| Alginate | 1.5 | 26.2–38.9 | 44–52 | 12.1–16.4 | [157] |
| Cellulose | 5 | 500 | 25 | 7 | [158] |
| Chitosan | 1.5 | 14.4–16.2 | 47.8–58.2 | 27.7–36.1 | [159] |
| Carrageenan | 2.5 | 51.6–64.8 | 40 | 20 | [160] |
| Gums | 10 | - | 3.5 | 60–80 | [161] |
| Pectin | 3 | 36 | 42–82 | 12–28 | [162] |
| Proteins | - | - | 3.3–3.9 | 160-213 | [163] |
| Film-Forming Material | Cellulose | Thickness (µm) | Mechanical Properties | References | ||
|---|---|---|---|---|---|---|
| Type | Concentration (% w/w) | Tensile Strength (MPa) | Elongation at Break (%) | |||
| Chitosan | CNF | 1.5 | 14.5–21.2 | - | - | [165] |
| Tapioca, potato, corn | CNF | 0 | 2.99 | 0.047 | 6.67 | [166] |
| 10 | 6.33 | 0.055 | 22.67 | |||
| 20 | 5.71 | 0.056 | 30.51 | |||
| Faba bean protein isolate | CNC | 0 | - | 4.3 | 105.0 | [167] |
| 1 | 4.2 | 61.3 | ||||
| 3 | 3.8 | 48.1 | ||||
| 5 | 5.3 | 48.2 | ||||
| 7 | 6.5 | 46.3 | ||||
| Cassava starch | Microcrystalline cellulose | 0 | - | 7.15 ± 0.6 | 22.75 ± 2.34 | [168] |
| 0.14 | 8.19 ± 0.9 | 19.23 ± 2.25 | ||||
| 0.3 | 9.91 ± 0.7 | 5.85 ± 1.43 | ||||
| 0.6 | 10.99 ± 0.5 | 1.31 ± 0.25 | ||||
| Okara soluble dietary fiber and pectin | Sodium carboxymethyl cellulose | 0.5 | 123 ± 70 | 6.567 ± 0.33 | 16.67 ± 0.35 | [169] |
| Konjac glucomannan | BNC | 0 | 39 ± 6 | 46.43 | 6.34 | [170] |
| 1 | 40 ± 12 | 50.36 | 8.58 | |||
| 2 | 41 ± 0 | 69.29 | 9.44 | |||
| 3 | 41 ± 15 | 74.05 | 8.18 | |||
| 4 | 42 ± 10 | 82.01 | 5.70 | |||
| Cassia-gum | Carboxylated CNC | 0 | 89 ± 5 | 18.53 | 28.87 | [36] |
| 2 | 90 ± 3 | 24.77 | 31.88 | |||
| 4 | 93 ± 2 | 32.85 | 34.75 | |||
| 6 | 98 ± 4 | 28.75 | 36.51 | |||
| Polyvinyl alcohol | NC | 1 | - | 6.42 ± 0.59 | 89.99 ± 11.77 | [171] |
| 3 | 9.47 ± 1.62 | 106.94 ± 7.04 | ||||
| 5 | 11.17 ± 1.08 | 117.52 ± 10.28 | ||||
| κ-carrageenan | CNC | 0 | 20 | 38.33 ± 3.79 | 21.50 ± 3.72 | [172] |
| 1 | 30 | 38.43 ± 5.94 | 22.93 ± 1.50 | |||
| 3 | 40 | 39.83 ± 0.38 | 23.83 ± 2.71 | |||
| 5 | 25 | 40.07 ± 2.80 | 24.33 ± 3.00 | |||
| 7 | 25 | 52.73 ± 0.70 | 28.27 ± 2.39 | |||
| 9 | 35 | 39.10 ± 1.04 | 25.83 ± 2.61 | |||
| k-CA biopolymer | CNC | 0 | 80 | 49.0 | 27.5 | [40] |
| 1 | 59.2 | 23.1 | ||||
| 3 | 66.6 | 20.7 | ||||
| 5 | 80.9 | 18.9 | ||||
| 8 | 85.1 | 15.4 | ||||
| Whey protein | CNC | 0 | - | 1.30 | 47 | [41] |
| 1 | 1.65 | 35 | ||||
| 2 | 2.04 | 33 | ||||
| 3 | 2.10 | 34 | ||||
| 4 | 2.29 | 35 | ||||
| 5 | 2.30 | 35 | ||||
| 10 | 2.70 | 25 | ||||
| 15 | 3.15 | 24 | ||||
| Corn nanostarch | CNC | 0 | 300 | 3.41 ± 0.17 | - | [39] |
| 0.2 | 5.99 ± 0.30 | |||||
| 0.4 | 7.28 ± 0.36 | |||||
| 0.6 | 8.61 ± 0.43 | |||||
| 0.8 | 11.25 ± 0.56 | |||||
| 1 | 7.78 ± 0.39 | |||||
| Agar | BNC | 0 | - | 22.10 ± 0.64 | 10.76 ± 2.30 | [173] |
| 0.045 | 27.95 ± 1.42 | 14.50 ± 0.88 | ||||
| 0.075 | 31.26 ± 2.26 | 27.47 ± 1.08 | ||||
| 0.12 | 34.20 ± 1.35 | 21.53 ± 1.62 | ||||
| 0.15 | 44.51 ± 1.86 | 13.02 ± 1.70 | ||||
| Whey protein | CNC | 0 | - | 2.30 ± 0.35 | 46.07 ± 23.25 | [174] |
| 2 | 3.41 ± 0.87 | 20.82 ± 9.85 | ||||
| 5 | 3.49 ± 0.91 | 26.54 ± 9.12 | ||||
| 8 | 4.93 ± 0.49 | 17.63 ± 3.93 | ||||
| Chitosan | BNC | 0 | 90 | 21.07 ± 1.64 | 33.84 ± 2.51 | [37] |
| 2 | 100 | 27.03 ± 1.46 | 29.71 ± 2.15 | |||
| 4 | 100 | 41.32 ± 2.20 | 23.76 ± 1.52 | |||
| 6 | 110 | 34.75 ± 1.02 | 25.11 ± 2.93 | |||
| Film-Forming Material | Additives | Effect of NC on Active Film | References | |
|---|---|---|---|---|
| Reinforcing Agent | Active Agent | |||
| Sodium caseinate (4% w/w) | Cellulose nanofibers (2.5%–5% w/w) | Cinnamon bark essential oil-nanoemulsion (5% w/w) | NC decreases the release rate of the essential oil from sodium caseinate matrix and also improves the antioxidant properties of the film. | [218] |
| Soy protein (5% w/v) | Microfibrillated cellulose (0%–0.6% w/v) | Clove essential oil (2.5% w/v) | MFC’s presence favors the release of the active compounds of CEO. A higher concentration of MFC increases the antioxidant properties as well as the antimicrobial activity. | [219] |
| Mucilage (50% v/v) | Cellulose nanofibers (3%–6% w/v) | - | NCs incorporation successfully enhances the mechanical, hydrophobic, antioxidant and antimicrobial properties of the mucilage composite films. | [217] |
| Gelatin/agar (2% w/v) | Cellulose nanofibers (0.75% w/v) | Clove essential oil-based Pickering emulsion (0, 0.02, 0.1, 0.2% w/v) | Composite film is transparent and shows high UV-light barrier properties and water-resistant properties, and improved antioxidant activity. | [220] |
| Poly (butylene adipate-co-terephthalate) (PBAT) (15% w/w) | Cellulose nanofibers (0.5, 1, 3% w/w) | Cinnamon essential oil | Films showed good thermal stability, higher oil release, decreasing water vapor permeability values and preventing microbial attack through the release of the essential oil. | [221] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirozzi, A.; Ferrari, G.; Donsì, F. The Use of Nanocellulose in Edible Coatings for the Preservation of Perishable Fruits and Vegetables. Coatings 2021, 11, 990. https://doi.org/10.3390/coatings11080990
Pirozzi A, Ferrari G, Donsì F. The Use of Nanocellulose in Edible Coatings for the Preservation of Perishable Fruits and Vegetables. Coatings. 2021; 11(8):990. https://doi.org/10.3390/coatings11080990
Chicago/Turabian StylePirozzi, Annachiara, Giovanna Ferrari, and Francesco Donsì. 2021. "The Use of Nanocellulose in Edible Coatings for the Preservation of Perishable Fruits and Vegetables" Coatings 11, no. 8: 990. https://doi.org/10.3390/coatings11080990
APA StylePirozzi, A., Ferrari, G., & Donsì, F. (2021). The Use of Nanocellulose in Edible Coatings for the Preservation of Perishable Fruits and Vegetables. Coatings, 11(8), 990. https://doi.org/10.3390/coatings11080990