ZnO Nano-Flowers Assembled on Carbon Fiber Textile for High-Performance Supercapacitor’s Electrode
Abstract
:1. Introduction
2. Experimental
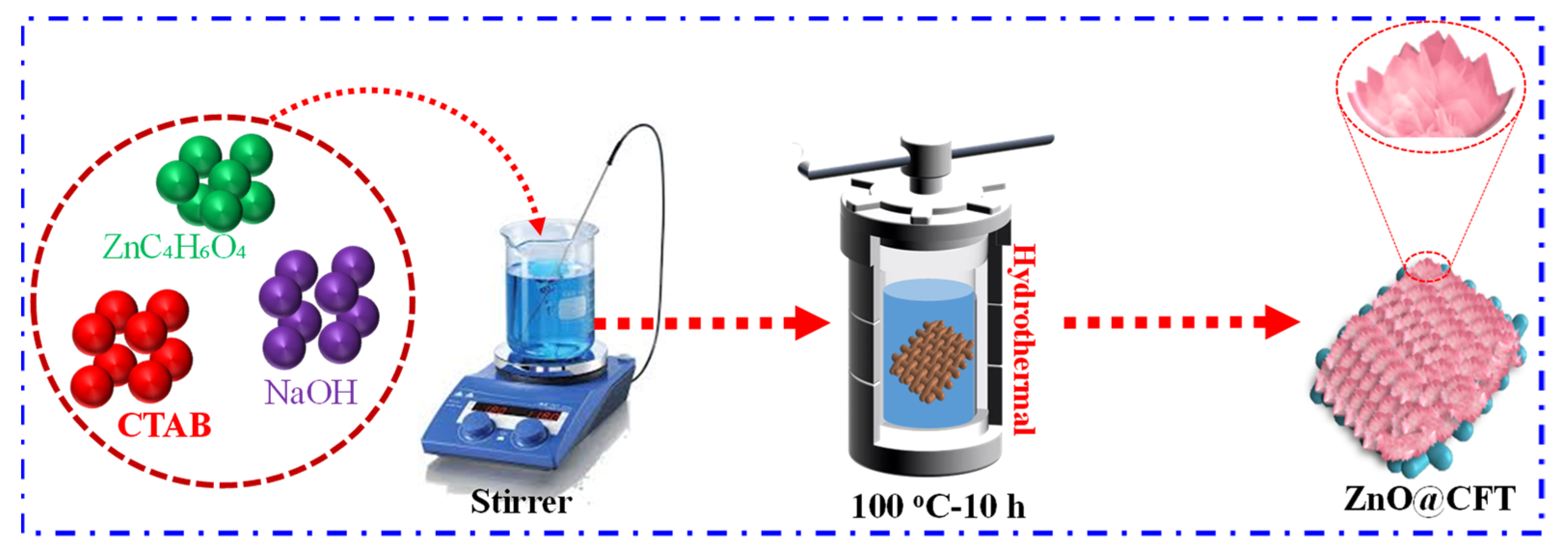
2.1. The Preparation of ZnO Nano-Flowers on Carbon Fabric Cloth for a Binder-Free Electrode
2.2. ZnO@CFT Electrode Fabrication
2.3. Materials Characterization
2.4. Measurements of Electrochemistry
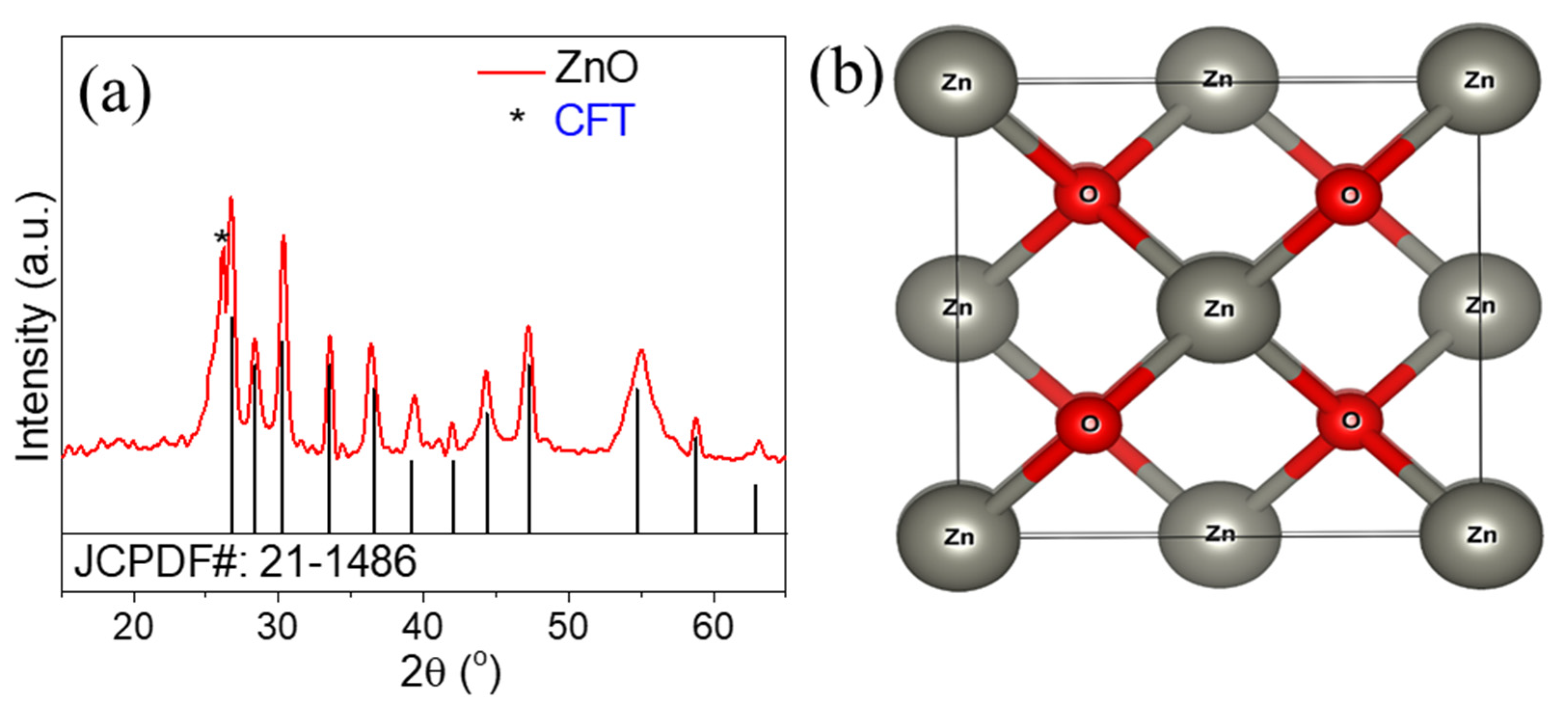
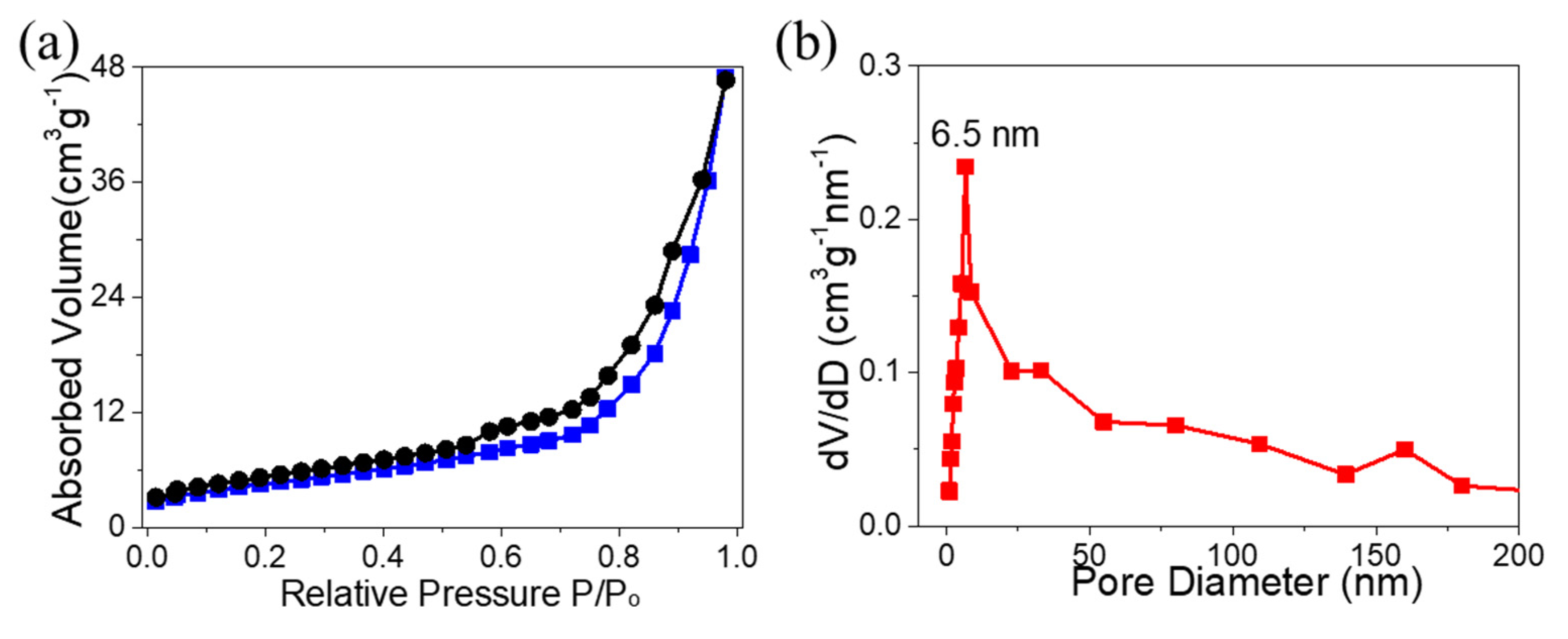
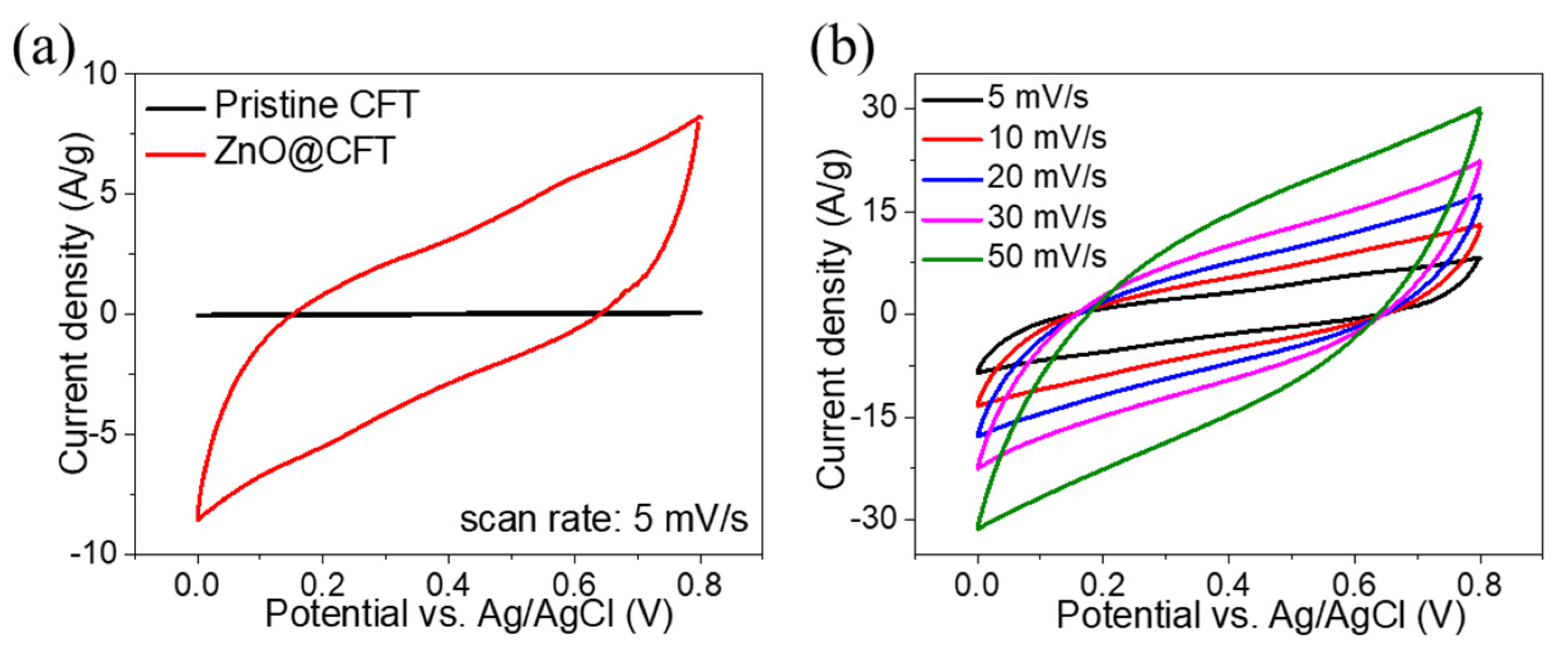
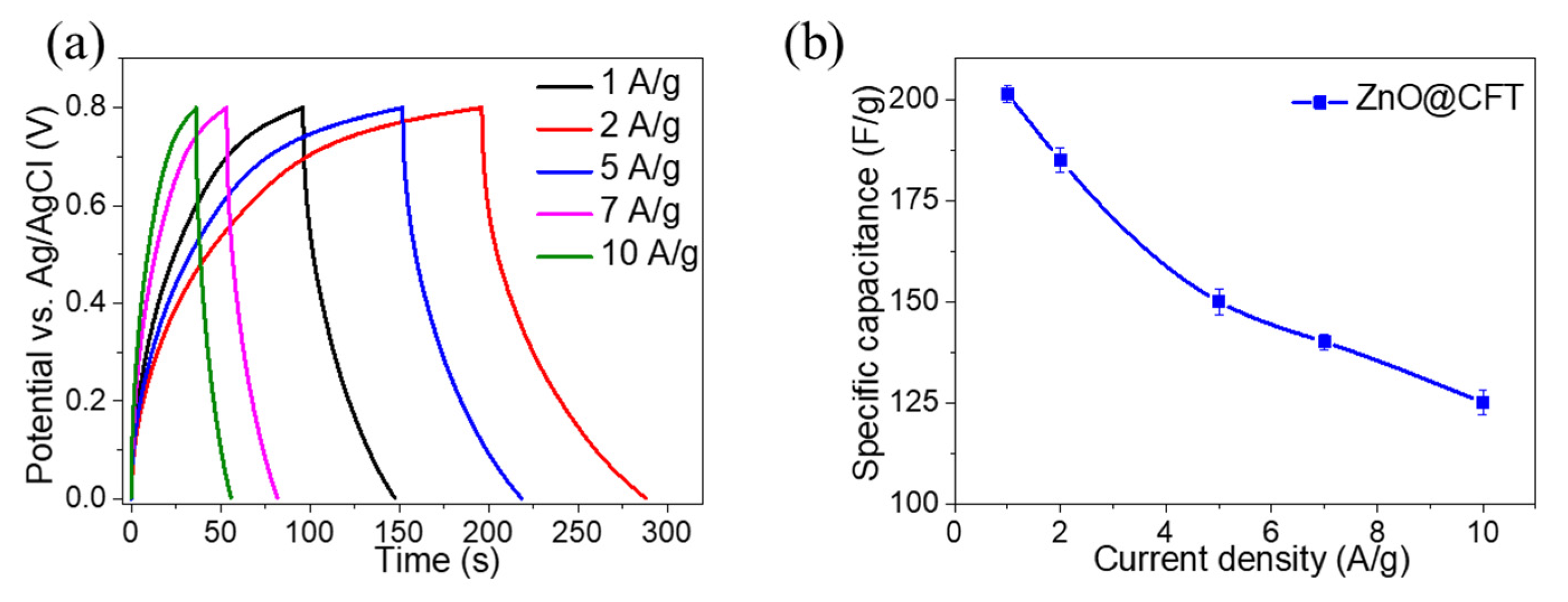
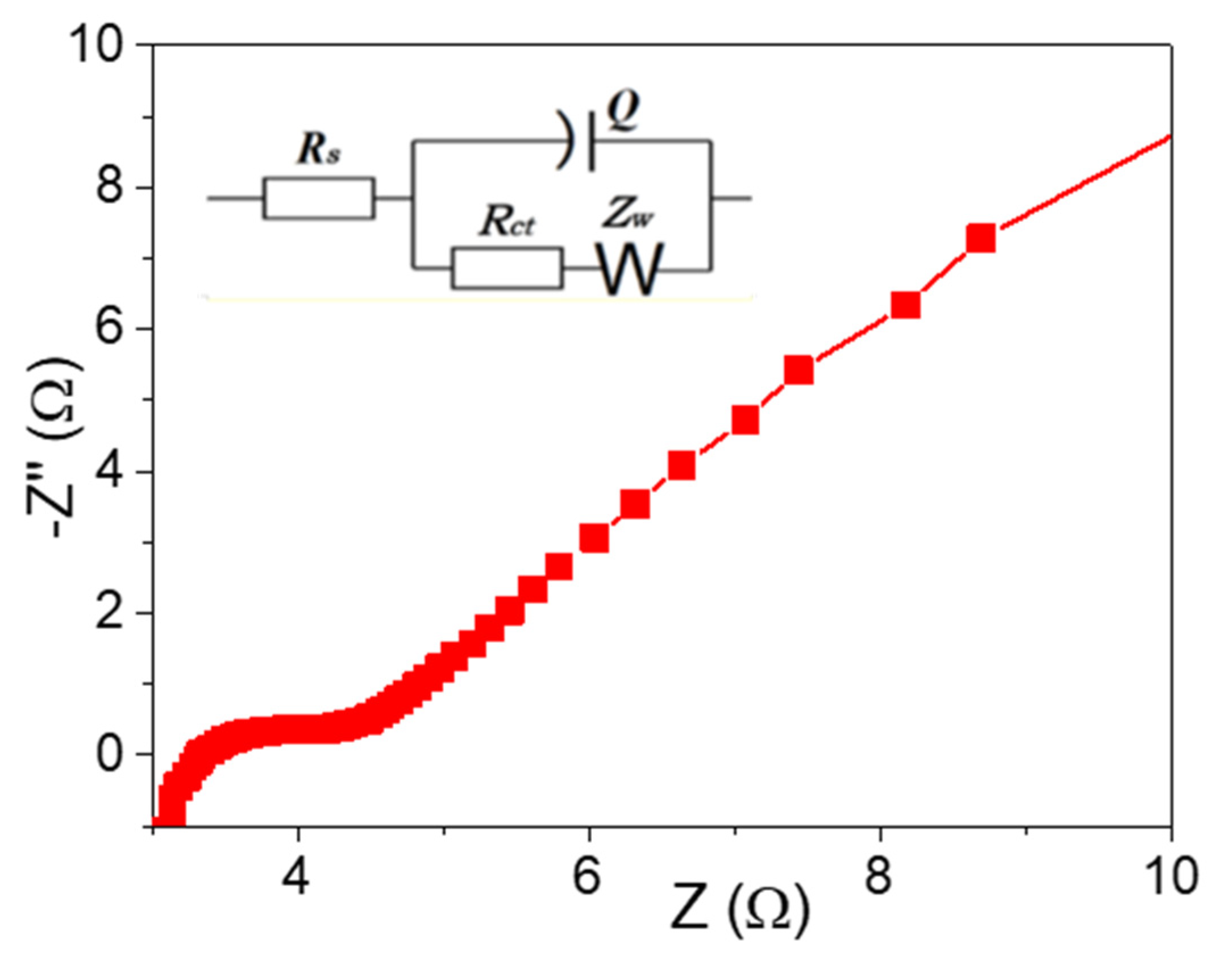
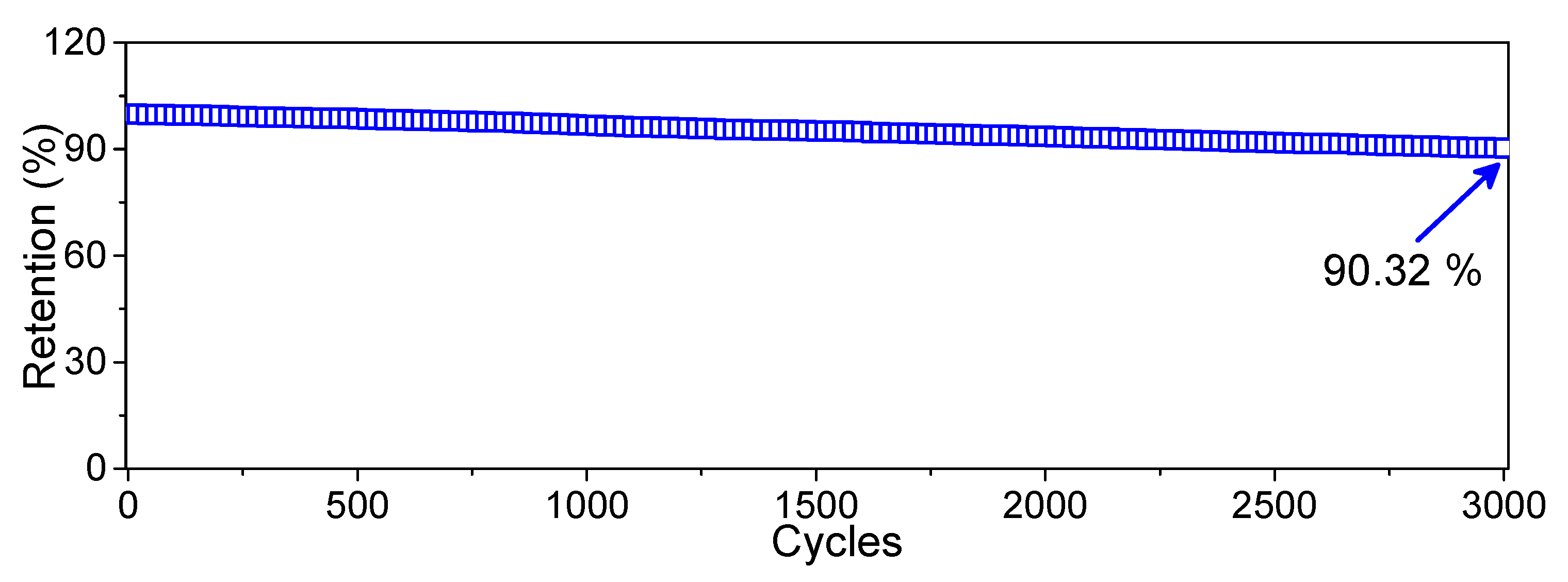
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaheen, I.; Ahmad, K.S.; Zequine, C.; Gupta, R.K.; Thomas, A.G.; Malik, M.A. Green synthesis of ZnO–Co3O4 nanocomposite using facile foliar fuel and investigation of its electro-chemical behaviour for supercapacitors. New J. Chem. 2020, 44, 18281–18292. [Google Scholar] [CrossRef]
- Javed, M.S.; Khan, A.J.; Ahmad, A.; Siyal, S.H.; Akram, S.; Zhao, G.; Bahajjaj, A.A.A.; Ouladsmane, M.; Alfakeer, M. Design and fabrication of bimetallic oxide nanonest-like structure/carbon cloth composite electrode for supercapacitors. Ceram. Int. 2021, 47, 30747–30755. [Google Scholar] [CrossRef]
- Syah, R.; Ahmad, A.; Davarpanah, A.; Elveny, M.; Ramdan, D.; Albaqami, M.D.; Ouladsmane, M. Incorporation of Bi2O3 residuals with metallic bi as high performance electrocatalyst toward hydrogen evolution reaction. Catalysts 2021, 11, 1099. [Google Scholar] [CrossRef]
- Siyal, S.H.; Javed, M.S.; Ahmad, A.; Sajjad, M.; Batool, S.; Khan, A.J.; Akram, S.; Alothman, A.A.; Alshgari, R.A.; Najam, T. Free-standing 3D Co3O4@NF micro-flowers composed of porous ultra-long nanowires as an advanced cathode material for supercapacitor. Curr. Appl. Phys. 2021, 31, 221–227. [Google Scholar] [CrossRef]
- Saleem, M.; Irfan, M.; Tabassum, S.; Albaqami, M.D.; Javed, M.S.; Hussain, S.; Pervaiz, M.; Ahmad, I.; Ahmad, A.; Zuber, M. Experimental and theoretical study of highly porous lignocellulose assisted metal oxide photoelectrodes for dye-sensitized solar cells. Arab. J. Chem. 2021, 14, 102937. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Liang, J.; Chen, Y. Hydrous RuO 2 -decorated MXene coordinating with silver nanowire inks enabling fully printed micro-supercapacitors with extraordinary volumetric performance. Adv. Energy Mater. 2019, 9, 1803987. [Google Scholar] [CrossRef]
- Asim, S.; Javed, M.S.; Hussain, S.; Rana, M.; Iram, F.; Lv, D.; Hashim, M.; Saleem, M.; Khalid, M.; Jawaria, R.; et al. RuO2 nanorods decorated CNTs grown carbon cloth as a free standing electrode for supercapacitor and lithium ion batteries. Electrochim. Acta 2019, 326, 135009. [Google Scholar] [CrossRef]
- Javed, M.S.; Imran, M.; Assiri, M.A.; Hussain, I.; Hussain, S.; Siyal, S.H.; Saleem, M.; Shah, S.S.A. One-step synthesis of carbon incorporated 3D MnO2 nanorods as a highly efficient electrode material for pseudocapacitors. Mater. Lett. 2021, 295, 129838. [Google Scholar] [CrossRef]
- Khan, A.J.; Khan, A.; Javed, M.S.; Arshad, M.; Asim, S.; Khalid, M.; Siyal, S.H.; Hussain, S.; Hanif, M.; Liu, Z. Surface assembly of Fe3O4 nanodiscs embedded in reduced graphene oxide as a high-performance negative electrode for supercapacitors. Ceram. Int. 2020, 46, 19499–19505. [Google Scholar] [CrossRef]
- Samuel, E.; Londhe, P.U.; Joshi, B.; Kim, M.-W.; Kim, K.; Swihart, M.; Chaure, N.B.; Yoon, S.S. Electrosprayed graphene decorated with ZnO nanoparticles for supercapacitors. J. Alloy. Compd. 2018, 741, 781–791. [Google Scholar] [CrossRef]
- Laureti, S.; Peddis, D.; Del Bianco, L.; Testa, A.; Varvaro, G.; Agostinelli, E.; Binns, C.; Baker, S.; Qureshi, M.; Fiorani, D. Exchange bias and magnetothermal properties in Fe@Mn nanocomposites. J. Magn. Magn. Mater. 2012, 324, 3503–3507. [Google Scholar] [CrossRef]
- Golubeva, E.V.; Stepanova, E.A.; Balymov, K.G.; Volchkov, S.O.; Kurlyandskaya, G.V. Magnetic properties and the giant magnetoimpedance of amorphous co-based wires with a carbon coating. Phys. Met. Met. 2018, 119, 324–331. [Google Scholar] [CrossRef]
- Spizzo, F.; Sgarbossa, P.; Sieni, E.; Semenzato, A.; Dughiero, F.; Forzan, M.; Bertani, R.; Del Bianco, L. Synthesis of ferrofluids made of iron oxide nanoflowers: Interplay between carrier fluid and magnetic properties. Nanomaterials 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Zhang, X.T.; Zhang, M.Y.; Gao, Y.; Gao, H.; Liu, X.C.; Lui, H.; Wong, K.W.; Lau, W.M. Rationally designed hierarchical MnO2-shell/ZnO-nanowire/carbon-fabric for high-performance supercapacitor electrodes. J. Power Sources 2014, 272, 654–660. [Google Scholar] [CrossRef]
- Cao, F.; Pan, G.; Xia, X.; Tang, P.; Chen, H. Synthesis of hierarchical porous NiO nanotube arrays for supercapacitor application. J. Power Sources 2014, 264, 161–167. [Google Scholar] [CrossRef]
- Javed, M.S.; Lei, H.; Li, J.; Wang, Z.; Mai, W. Construction of highly dispersed mesoporous bimetallic-sulfide nanoparticles locked in N-doped graphitic carbon nanosheets for high energy density hybrid flexible pseudocapacitors. J. Mater. Chem. A 2019, 7, 17435–17445. [Google Scholar] [CrossRef]
- Javed, M.S.; Lei, H.; Shah, H.U.; Asim, S.; Raza, R.; Mai, W. Achieving high rate and high energy density in an all-solid-state flexible asymmetric pseudocapacitor through the synergistic design of binder-free 3D ZnCo2O4 nano polyhedra and 2D layered Ti3C2Tx-MXenes. J. Mater. Chem. A 2019, 7, 24543–24556. [Google Scholar] [CrossRef]
- Chen, H.-C.; Lyu, Y.-R.; Fang, A.; Lee, G.-J.; Karuppasamy, L.; Wu, J.J.; Lin, C.-K.; Anandan, S.; Chen, C.-Y. The design of ZnO nanorod arrays coated with MnOx for high electrochemical stability of a pseudocapacitor electrode. Nanomaterials 2020, 10, 475. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gao, Z.; Li, Z.; Wang, B.; Yan, Y.; Liu, Q.; Mann, T.; Zhang, M.; Jiang, Z. Green synthesis of graphene nanosheets/ZnO composites and electrochemical properties. J. Solid State Chem. 2011, 184, 1421–1427. [Google Scholar] [CrossRef]
- Lu, T.; Pan, L.; Li, H.; Zhu, G.; Lv, T.; Liu, X.; Sun, Z.; Chen, T.; Chua, D.H. Microwave-assisted synthesis of graphene–ZnO nanocomposite for electrochemical supercapacitors. J. Alloy. Compd. 2011, 509, 5488–5492. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Pan, L.; Lu, T.; Sun, Z. Capacitive behavior of graphene–ZnO composite film for supercapacitors. J. Electroanal. Chem. 2009, 634, 68–71. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Palaniappa, M.; Balasubramanian, K. Balasubramanian, single step solution combustion synthesis of ZnO/carbon composite and its electrochemical characterization for supercapacitor application. Int. J. Electrochem. Sci. 2008, 3, 96–103. [Google Scholar]
- He, X.; Yoo, J.E.; Lee, M.H.; Bae, J. Morphology engineering of ZnO nanostructures for high performance supercapacitors: Enhanced electrochemistry of ZnO nanocones compared to ZnO nanowires. Nanotechnology 2017, 28, 245402. [Google Scholar] [CrossRef]
- Alver, Ü.M.İ.T.; Tanrıverdi, A.; Akgül, Ö. Hydrothermal preparation of ZnO electrodes synthesized from different pre-cursors for electrochemical supercapacitors. Synth. Met. 2016, 211, 30–34. [Google Scholar] [CrossRef]
- Luo, Q.; Xu, P.; Qiu, Y.; Cheng, Z.; Chang, X.; Fan, H. Synthesis of ZnO tetrapods for high-performance supercapacitor applications. Mater. Lett. 2017, 198, 192–195. [Google Scholar] [CrossRef]
- Javed, M.S.; Lei, H.; Wang, Z.; Liu, B.-T.; Cai, X.; Mai, W. 2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries. Nano Energy 2020, 70, 104573. [Google Scholar] [CrossRef]
- Abbas, Y.; Yun, S.; Javed, M.S.; Chen, J.; Tahir, M.F.; Wang, Z.; Yang, C.; Arshad, A.; Hussain, S. Anchoring 2D NiMoO4 nano-plates on flexible carbon cloth as a binder-free electrode for efficient energy storage devices. Ceram. Int. 2020, 46, 4470–4476. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, Q.Y.; Wang, H.Q.; Huang, Y.G.; Zhang, X.H.; Wu, Q.; Gao, H.-Q.; Yang, J.H. Synthesis and electrochemical properties of nickel–manganese oxide on MWCNTs/CFP substrate as a super-capacitor electrode. Appl. Energy 2015, 153, 78–86. [Google Scholar] [CrossRef]
- Javed, M.S.; Chen, J.; Chen, L.; Xi, Y.; Zhang, C.; Wan, B.; Hu, C. Flexible full-solid state supercapacitors based on zinc sulfide spheres growing on carbon textile with superior charge storage. J. Mater. Chem. A 2016, 4, 667–674. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials–Capacitive, Pseudocapacitive, or Battery-Like? ACS Publications: Washington, DC, USA, 2018. [Google Scholar]
- Javed, M.S.; Shaheen, N.; Hussain, S.; Li, J.; Shah, S.S.A.; Abbas, Y.; Ahmad, M.A.; Raza, R.; Mai, W. An ultra-high energy density flexible asymmetric supercapacitor based on hierarchical fabric decorated with 2D bimetallic oxide nanosheets and MOF-derived porous carbon polyhedra. J. Mater. Chem. A 2019, 7, 946–957. [Google Scholar] [CrossRef]
- Lin, J.C.; Peng, K.C.; Liao, H.L.; Lee, S.L. Transparent conducting Sc-codoped AZO film prepared from ZnO: Al–Sc by RF-DC sputtering. Thin Solid Film. 2008, 516, 5349–5354. [Google Scholar] [CrossRef]
- Chang, F.-M.; Brahma, S.; Huang, J.-H.; Wu, Z.-Z.; Lo, K.-Y. Strong correlation between optical properties and mechanism in deficiency of normalized self-assembly ZnO nanorods. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- György, E.; del Pino, A.P.; Logofatu, C.; Duta, A.; Isac, L. Effect of nitrogen doping on wetting and photoactive properties of laser processed zinc oxide-graphene oxide nanocomposite layers. J. Appl. Phys. 2014, 116, 024906. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A. Characterization of nanoporous materials from adsorption and desorption isotherms. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 187–188, 11–21. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, L.-B.; Cai, J.-J.; Luo, Y.-C.; Kang, L. Nanoflake-like cobalt hydroxide/ordered mesoporous carbon composite for electrochemical capacitors. J. Solid State Electrochem. 2010, 14, 2065–2075. [Google Scholar] [CrossRef]
- Shi, S.; Zhuang, X.; Cheng, B.; Wang, X. Solution blowing of ZnO nanoflake-encapsulated carbon nanofibers as electrodes for supercapacitors. J. Mater. Chem. A 2013, 1, 13779–13788. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, Q.; Javed, M.S.; Ahmad, A.; Siyal, S.H.; Asim, I.; Luque, R.; Albaqami, M.D.; Tighezza, A.M. ZnO Nano-Flowers Assembled on Carbon Fiber Textile for High-Performance Supercapacitor’s Electrode. Coatings 2021, 11, 1337. https://doi.org/10.3390/coatings11111337
Abbas Q, Javed MS, Ahmad A, Siyal SH, Asim I, Luque R, Albaqami MD, Tighezza AM. ZnO Nano-Flowers Assembled on Carbon Fiber Textile for High-Performance Supercapacitor’s Electrode. Coatings. 2021; 11(11):1337. https://doi.org/10.3390/coatings11111337
Chicago/Turabian StyleAbbas, Qasim, Muhammad Sufyan Javed, Awais Ahmad, Sajid Hussain Siyal, Idrees Asim, Rafael Luque, Munirah D. Albaqami, and Ammar Mohamed Tighezza. 2021. "ZnO Nano-Flowers Assembled on Carbon Fiber Textile for High-Performance Supercapacitor’s Electrode" Coatings 11, no. 11: 1337. https://doi.org/10.3390/coatings11111337
APA StyleAbbas, Q., Javed, M. S., Ahmad, A., Siyal, S. H., Asim, I., Luque, R., Albaqami, M. D., & Tighezza, A. M. (2021). ZnO Nano-Flowers Assembled on Carbon Fiber Textile for High-Performance Supercapacitor’s Electrode. Coatings, 11(11), 1337. https://doi.org/10.3390/coatings11111337









