Plasma Etching Behavior of SF6 Plasma Pre-Treatment Sputter-Deposited Yttrium Oxide Films
Abstract
1. Introduction
2. Materials and Methods
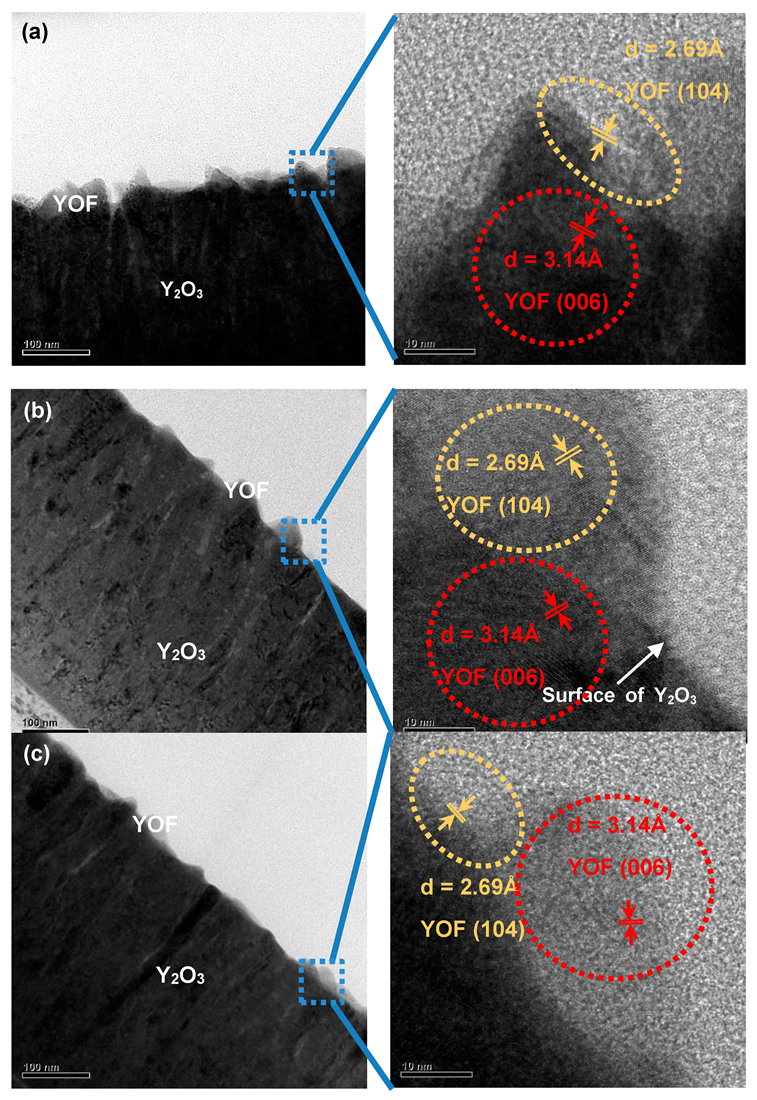
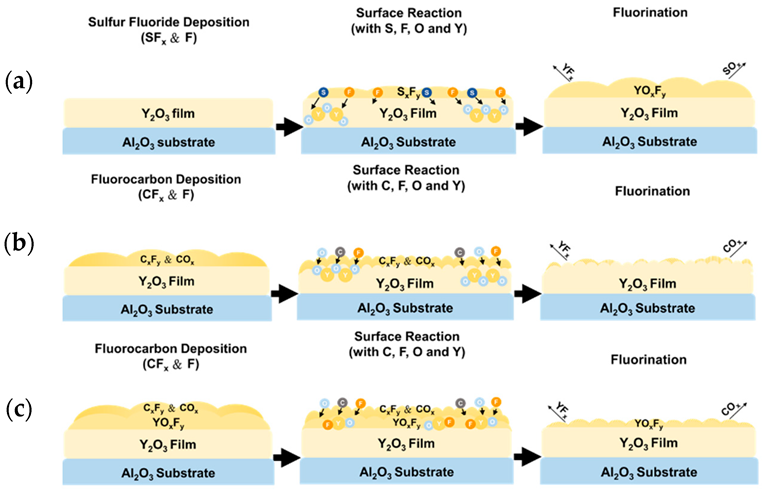
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ito, N.; Moriya, T.; Uesugi, F.; Matsumoto, M.; Liu, S.; Kitayama, Y. Reduction of particle contamination in plasma-etching equipment by dehydration of chamber wall. Jpn. J. Appl. Phys. 2008, 47, 3630–3634. [Google Scholar] [CrossRef]
- Yoo, S.W.; Hwang, N.M.; You, S.J.; Kim, J.H.; Seong, D.J. Control of nanoparticle size and amount by using the mesh grid and applying DC-bias to the substrate in silane ICP-CVD process. J. Nanopart. Res. 2017, 19, 374. [Google Scholar] [CrossRef]
- Cardinaud, C.; Peignon, M.C.; Tessier, P.Y. Plasma etching: Principles, mechanisms, application to micro- and nano-technologies. Appl. Surf. Sci. 2000, 164, 72–83. [Google Scholar] [CrossRef]
- Kasashima, Y.; Natsuko, N.; Uesugi, F. Instantaneous generation of many flaked particles by impulsive force of electric field stress acting on inner wall of mass-production plasma etching equipment. Jpn. J. Appl. Phys. 2013, 52, 066201. [Google Scholar] [CrossRef]
- Kim, D.M.; Jang, M.R.; Oh, Y.S.; Kim, S.; Lee, S.M.; Lee, S.H. Relative sputtering rates of oxides and fluorides of aluminum and yttrium. Surf. Coat. Technol. 2017, 309, 694–697. [Google Scholar] [CrossRef]
- Ma, T.; List, T.; Donnelly, V.M. Comparisons of NF3 plasma-cleaned Y2O3, YOF, and YF3 chamber coatings during silicon etching in Cl2 plasmas. J. Vac. Sci. Technol. A 2018, 36, 031305. [Google Scholar] [CrossRef]
- Song, J.B.; Kim, J.T.; Oh, S.G.; Shin, J.S.; Chun, J.R.; Yun, J.Y. Effect of sealing time of anodic aluminum oxide (AAO) film for preventing plasma damage. Sci. Adv. Mater. 2015, 7, 127–132. [Google Scholar] [CrossRef]
- Fukumoto, H.; Fujikake, I.; Takao, Y.; Eriguchi, K.; Ono, K. Plasma chemical behavior of reactants and reaction products during inductively coupled CF4 plasma etching of SiO2. Plasma Sources Sci. Technol. 2009, 18, 045027. [Google Scholar] [CrossRef]
- Duc, L.M.; Tan, C.M.; Luo, M.; Leng, I.C.H. Maintenance scheduling of plasma etching chamber in wafer fabrication for high-yield etching process. IEEE Trans. Semicond. Manuf. 2014, 27, 204–211. [Google Scholar] [CrossRef]
- Ashizawa, H.; Yoshida, K. Effect of the microstructures of yttrium ceramics on their plasma corrosion behavior. Ceram. Int. 2019, 45, 21162–21167. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, M.K.; Cho, H.; Park, T.E.; Yun, Y.H. Fabrication and plasma resistance of Y2O3 ceramics. Ceram. Int. 2015, 41, 12757–12762. [Google Scholar] [CrossRef]
- Kitamura, J.; Ibe, H.; Yuasa, F.; Mizuno, H. Plasma sprayed coatings of high-purity ceramics for semiconductor and flat-panel-display production equipment. J. Therm. Spray Technol. 2008, 17, 878–886. [Google Scholar] [CrossRef]
- Kim, D.M.; Oh, Y.S.; Kim, S.; Kim, H.T.; Lim, D.S.; Lee, S.M. The erosion behaviors of Y2O3 and YF3 coatings under fluorocarbon plasma. Thin Solid Film. 2011, 519, 6698–6702. [Google Scholar] [CrossRef]
- Akatsu, R.; Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Densification behavior of yttrium oxyfluoride ceramics by rate controlled sintering and their mechanical properties. Jpn. J. Appl. Phys. 2019, 58, SEEG02. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, H.; Prak, H.; Lee, C. Correlation of plasma erosion resistance and the microstructure of YF3 coatings prepared by vacuum kinetic spray. J. Therm. Spray Technol. 2020, 29, 1016–1026. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, Y.; Park, H.; Lee, C. The importance of intimate inter-crystallite bonding for the plasma erosion resistance of vacuum kinetic sprayed Y2O3 coating. Surf. Coat. Technol. 2019, 374, 493–499. [Google Scholar] [CrossRef]
- Lei, P.; Zhu, J.Q.; Zhu, Y.K.; Han, J.C. Preparation and optical properties of sputtered-deposition yttrium fluoride film. Nucl. Instrum. Methods Phys. Res. Sect. B 2013, 307, 429–433. [Google Scholar]
- Su, W.T.; Li, B.; Liu, D.Q.; Zhang, F.S. The determination of infrared optical constants of rare earth fluorides by classical Lorentz oscillator model. J. Phys. D Appl. Phys. 2007, 40, 3343–3347. [Google Scholar] [CrossRef]
- Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication, characterization, and fluorine-plasma exposure behavior of dense yttrium oxyfluoride ceramics. Jpn. J. Appl. Phys. 2017, 56, 06HC02. [Google Scholar] [CrossRef]
- Shiba, Y.; Teramoto, A.; Goto, T.; Kishi, Y.; Shirai, Y.; Sugawa, S. Stable yttrium oxyfluoride used in plasma process chamber. J. Vac. Sci. Technol. A 2017, 35, 021405. [Google Scholar] [CrossRef]
- Lin, T.K.; Wang, W.K.; Huang, S.Y.; Tasi, C.T.; Wuu, D.S. Comparison of erosion behavior and particle contamination in mass-production CF4/O2 plasma chambers using Y2O3 and YF3 protective coatings. Nanomaterials 2017, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Wuu, D.S.; Huang, S.Y.; Wang, W.K. Characteristics of yttrium fluoride and yttrium oxide coatings for plasma process equipment prepared by atmospheric plasma spraying. Jpn. J. App. Phys. 2016, 55, 126201. [Google Scholar] [CrossRef]
- Lin, T.K.; Wuu, D.S.; Huang, S.Y.; Wang, W.K. Preparation and characterization of sprayed-yttrium oxyfluoride corrosion protective coating for plasma process chambers. Coatings 2018, 8, 373. [Google Scholar] [CrossRef]
- Song, J.B.; Kim, J.T.; Oh, S.G.; Yun, J.Y. Contamination particles and plasma etching behavior of atmospheric plasma sprayed Y2O3 and YF3 coatings under NF3 plasma. Coatings 2019, 9, 102. [Google Scholar] [CrossRef]
- Wang, W.K.; Lin, X.Y.; Xu, Y.J. Structural and fluorine plasma etching behavior of sputter-deposition yttrium fluoride film. Nanomaterials 2018, 8, 936. [Google Scholar] [CrossRef]
- Hong, H.X.; Hong, J.M.; Cao, X.F.; Chen, X.; Xue, T. Ionic-liquid-assisted synthesis of YF3 with different crystalline phases and morphologies. Mater. Res. Bull. 2009, 44, 623–628. [Google Scholar]
- Kim, D.M.; Lee, S.H.; Alexander, W.B.; Kim, K.B.; Oh, Y.S.; Lee, S.M. X-ray photoelectron spectroscopy study on the interaction of yttrium-aluminum oxide with fluorine-based plasma. J. Am. Ceram. Soc. 2011, 94, 3455–3459. [Google Scholar] [CrossRef]
- Xia, J.J.; Liang, W.P.; Maio, Q.; Depla, D. The effect of energy and momentum transfer during magnetron sputter deposition of yttrium oxide thin films. Appl. Surf. Sci. 2018, 439, 545–551. [Google Scholar] [CrossRef]
- Tahara, R.; Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication of dense yttrium oxyfluoride ceramics by hot pressing and their mechanical, thermal, and electrical properties. Jpn. J. Appl. Phys. 2018, 57, 06JF04. [Google Scholar] [CrossRef]
- Miwa, K.; Takada, N. Fluorination mechanisms of Al2O3 and Y2O3 surfaces irradiated by high-density CF4/O2 and SF6/O2 plasmas. J. Vac. Sci. Technol. A 2009, 27, 831–839. [Google Scholar] [CrossRef]
- Miyashita, K.; Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fluorine and oxygen plasma exposure behavior of yttrium oxyfluoride ceramics. Jpn. J. Appl. Phys. 2019, 58, EEC01. [Google Scholar] [CrossRef]

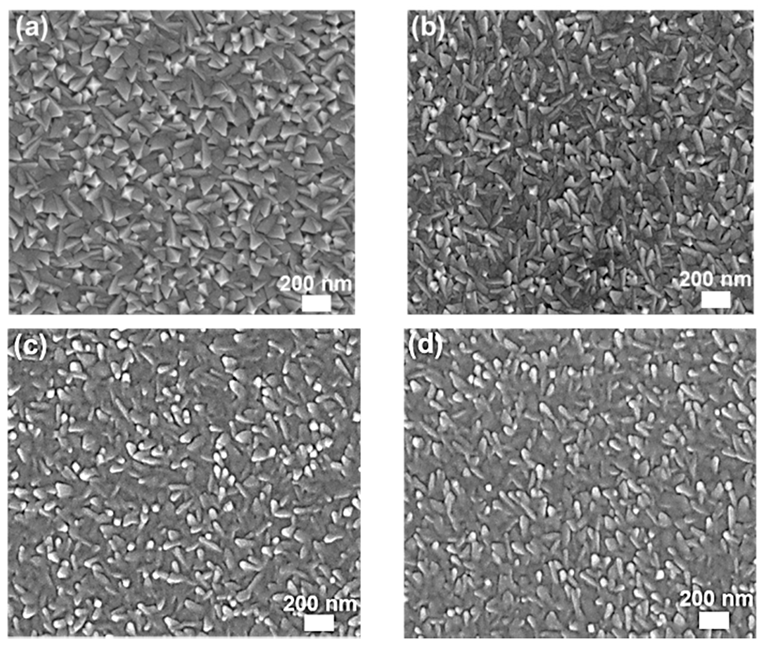
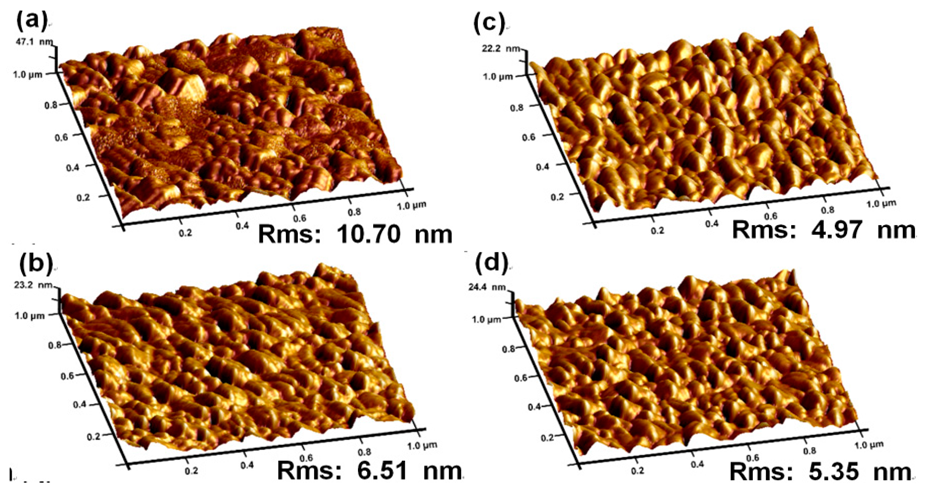
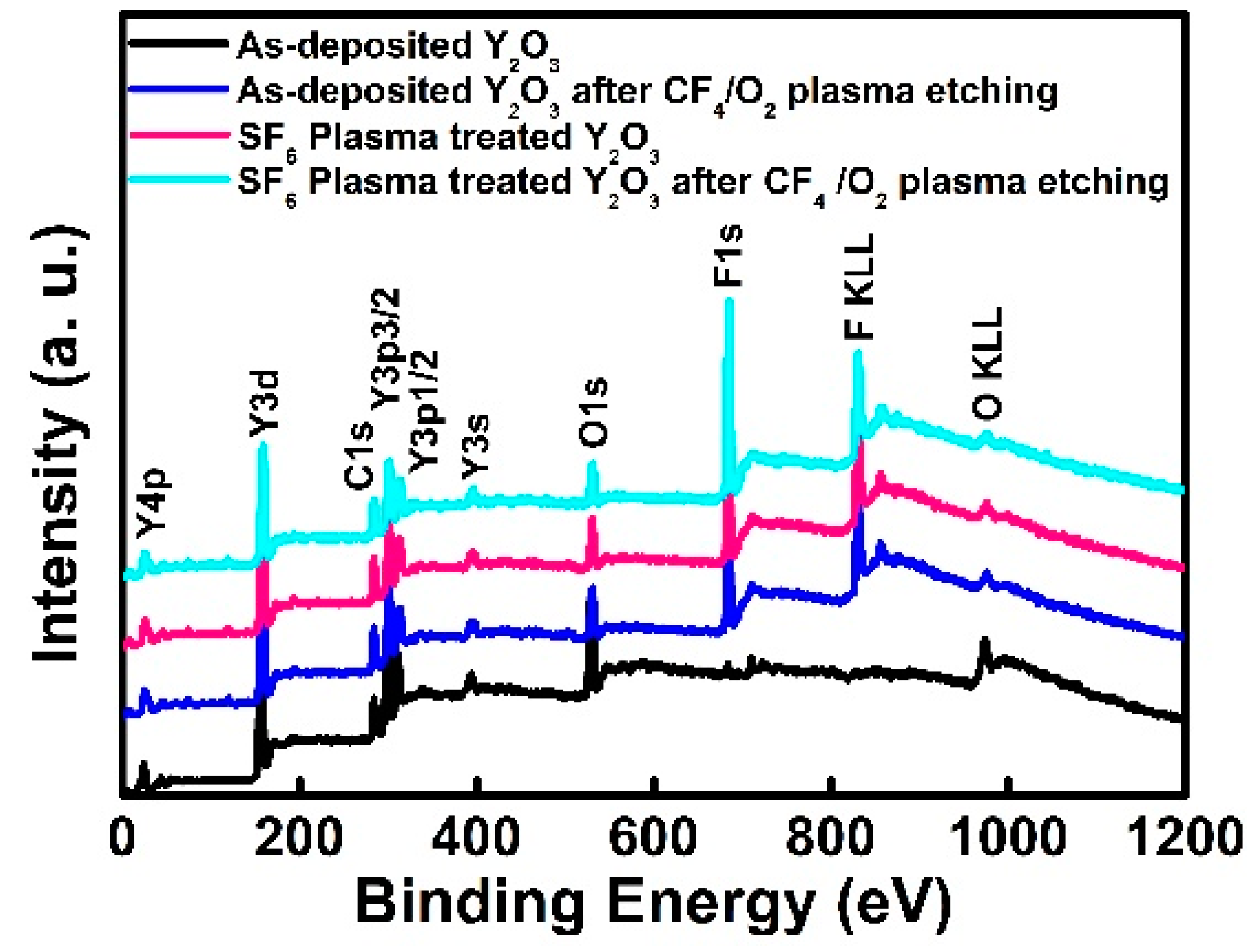
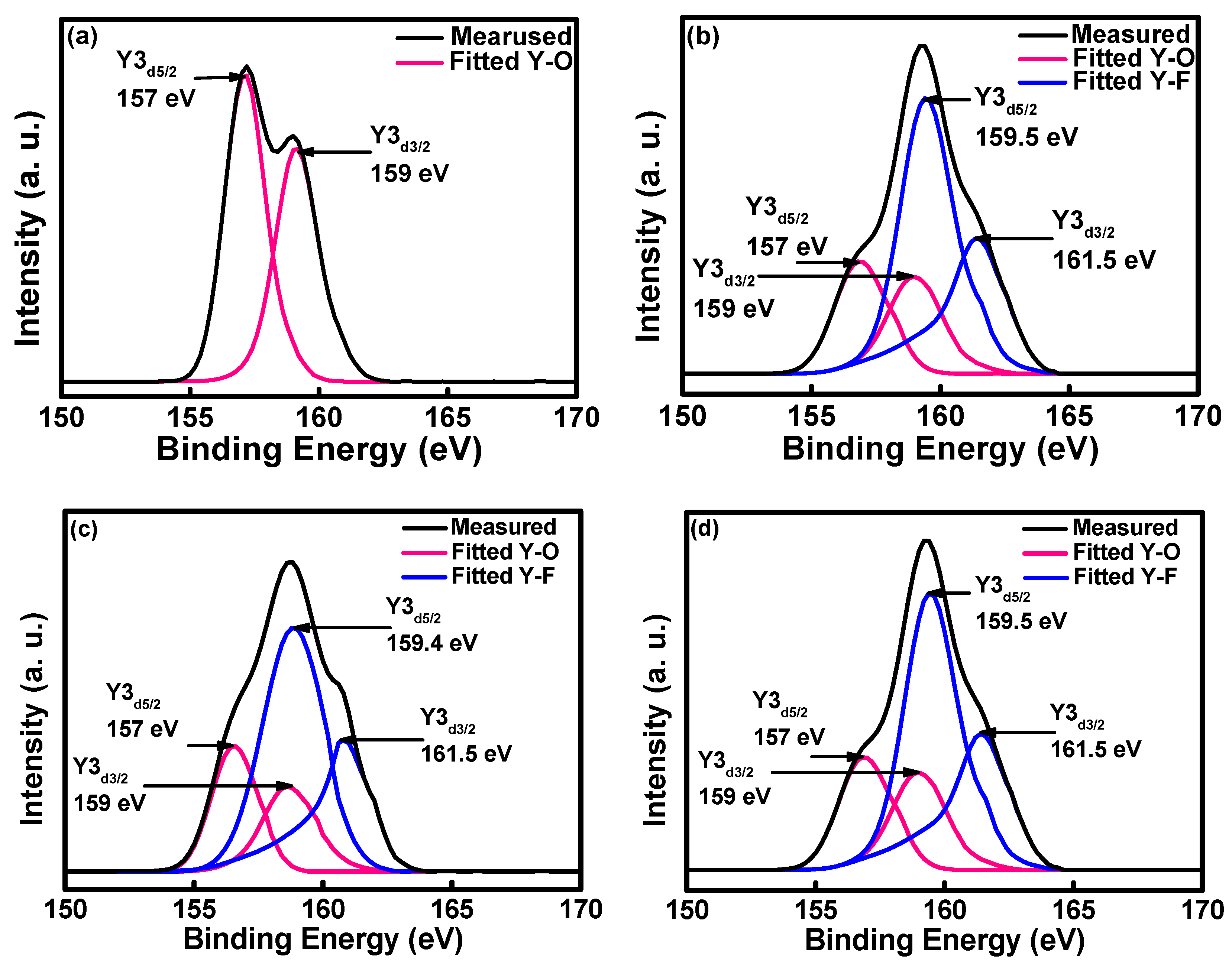
| Type | Elemental Composition (at%) | Atomic Ratio | |||
|---|---|---|---|---|---|
| Y3d | F 1s | O 1s | C 1s | F/Y | |
| As-deposited | 28.38 | 3.33 | 54.22 | 14.07 | 0.12 |
| CF4/O2 plasma etching | 21.82 | 55.09 | 16.05 | 7.04 | 2.58 |
| SF6 plasma-treated | 24.82 | 40.27 | 25.52 | 9.39 | 1.62 |
| CF4/O2 plasma etching | 23.20 | 54.75 | 17.58 | 4.47 | 2.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-K.; Wang, S.-Y.; Liu, K.-F.; Tsai, P.-C.; Zhang, Y.-H.; Huang, S.-Y. Plasma Etching Behavior of SF6 Plasma Pre-Treatment Sputter-Deposited Yttrium Oxide Films. Coatings 2020, 10, 637. https://doi.org/10.3390/coatings10070637
Wang W-K, Wang S-Y, Liu K-F, Tsai P-C, Zhang Y-H, Huang S-Y. Plasma Etching Behavior of SF6 Plasma Pre-Treatment Sputter-Deposited Yttrium Oxide Films. Coatings. 2020; 10(7):637. https://doi.org/10.3390/coatings10070637
Chicago/Turabian StyleWang, Wei-Kai, Sung-Yu Wang, Kuo-Feng Liu, Pi-Chuen Tsai, Yu-Hao Zhang, and Shih-Yung Huang. 2020. "Plasma Etching Behavior of SF6 Plasma Pre-Treatment Sputter-Deposited Yttrium Oxide Films" Coatings 10, no. 7: 637. https://doi.org/10.3390/coatings10070637
APA StyleWang, W.-K., Wang, S.-Y., Liu, K.-F., Tsai, P.-C., Zhang, Y.-H., & Huang, S.-Y. (2020). Plasma Etching Behavior of SF6 Plasma Pre-Treatment Sputter-Deposited Yttrium Oxide Films. Coatings, 10(7), 637. https://doi.org/10.3390/coatings10070637




