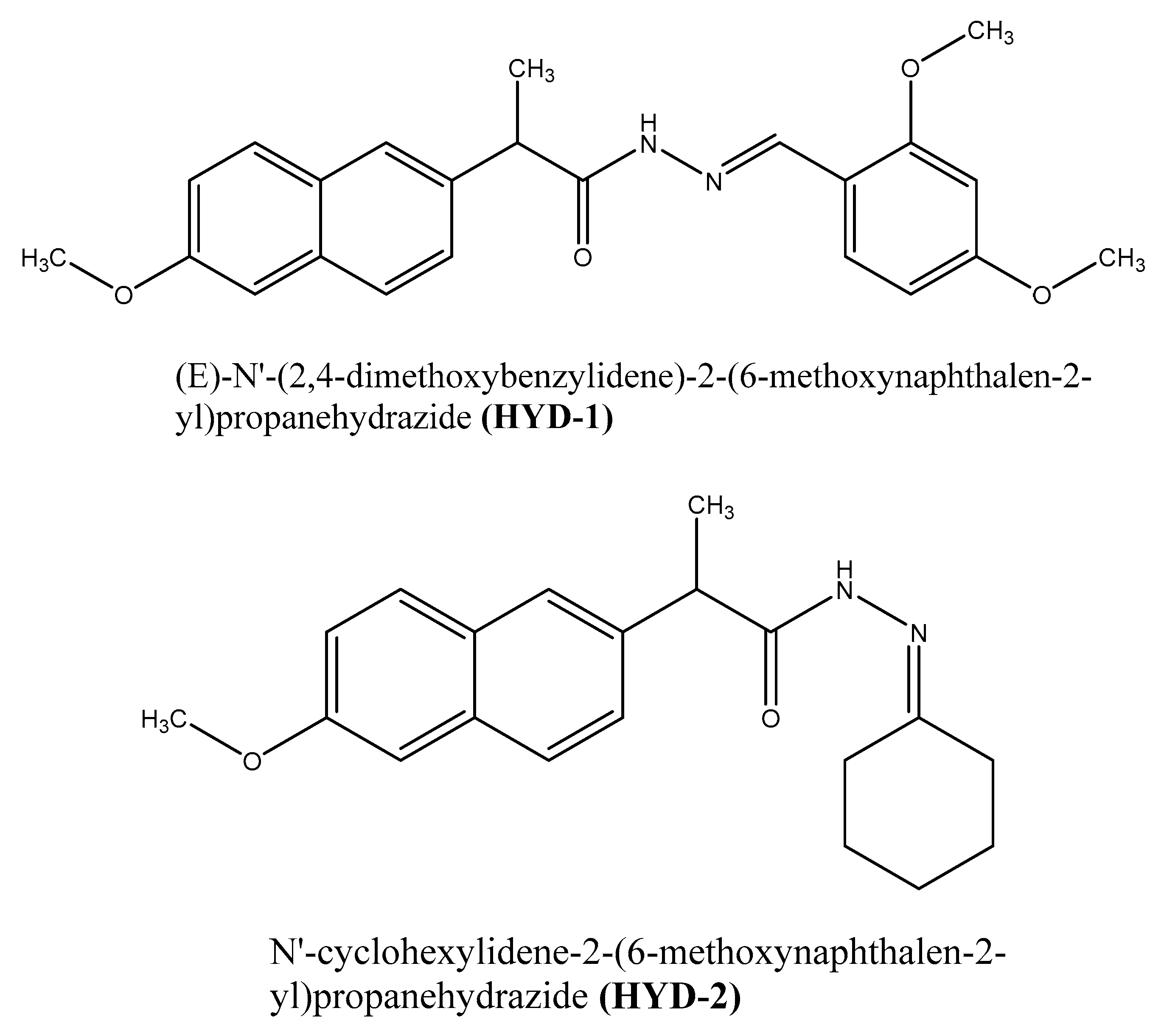
Green Corrosion Inhibition of Mild Steel by Hydrazone Derivatives in 1.0 M HCl
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials and Electrolytes
2.2. Weight Loss (WL) Study
2.3. Electrochemical Measurements for Corrosion Inhibition
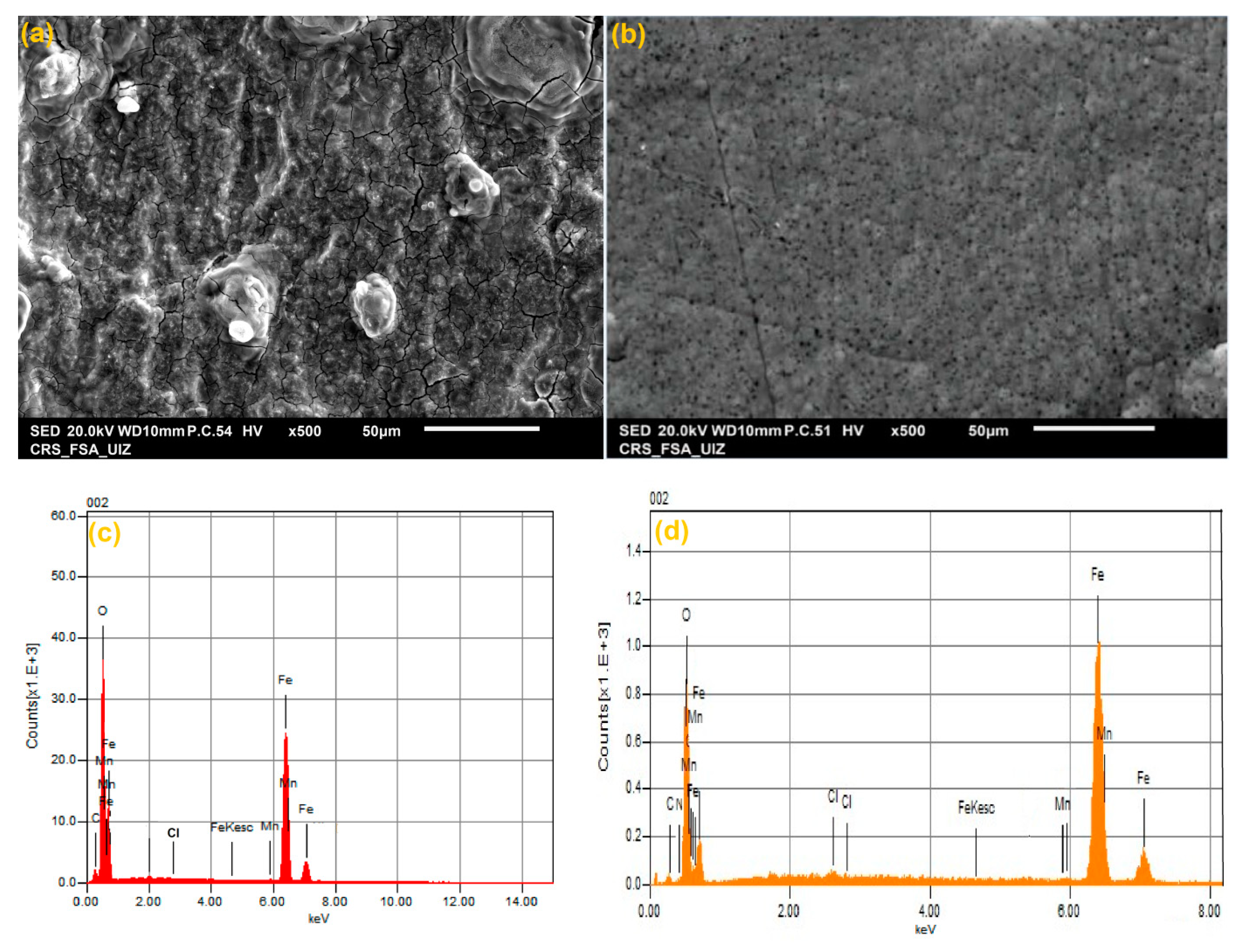
2.4. Surface Characterization (SEM/EDX)
3. Results and Discussion
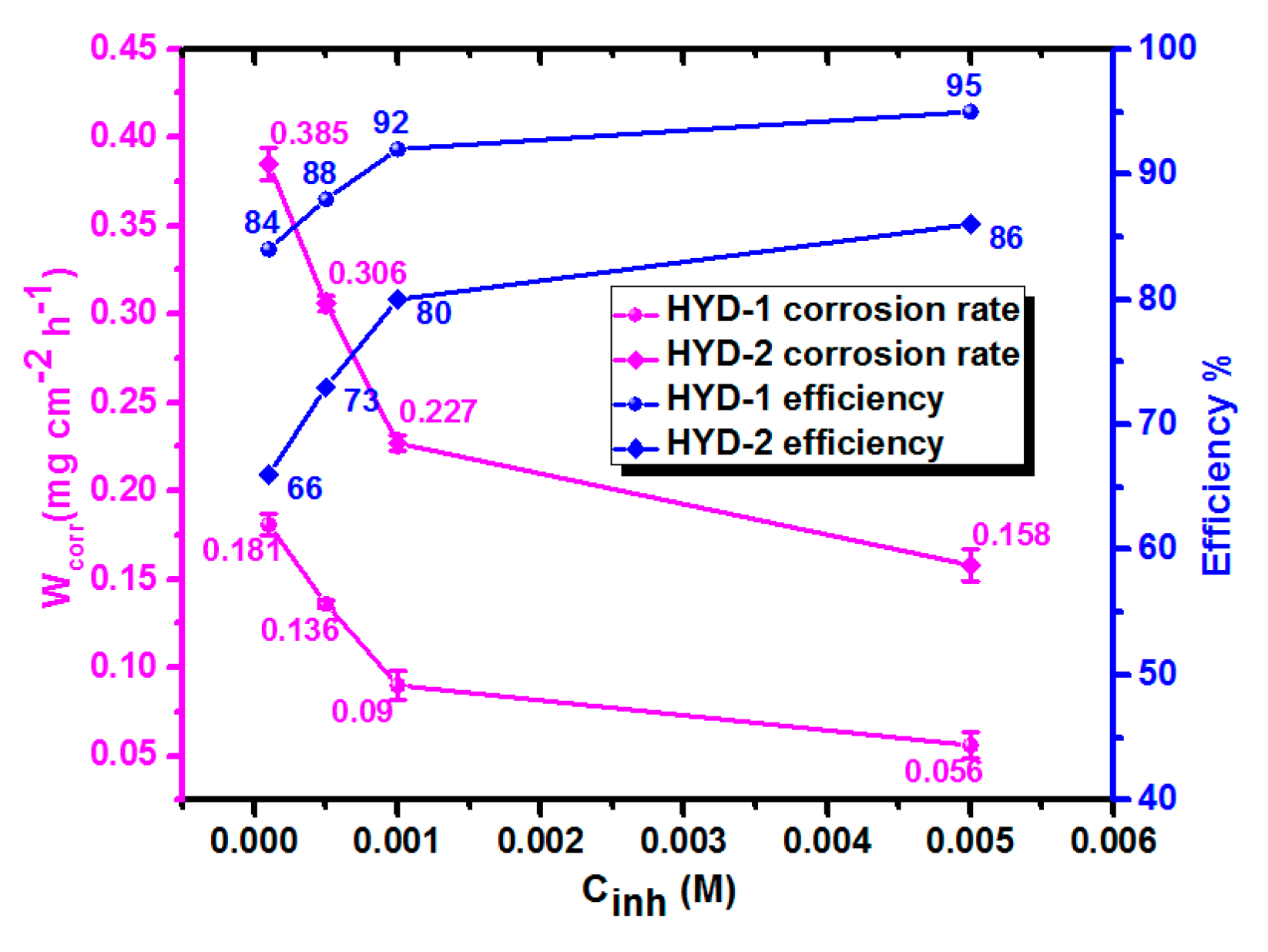
3.1. Comparison of Inhibition Performances Using Weight Loss Strategy
3.1.1. Influence of Inhibitors Concentrations
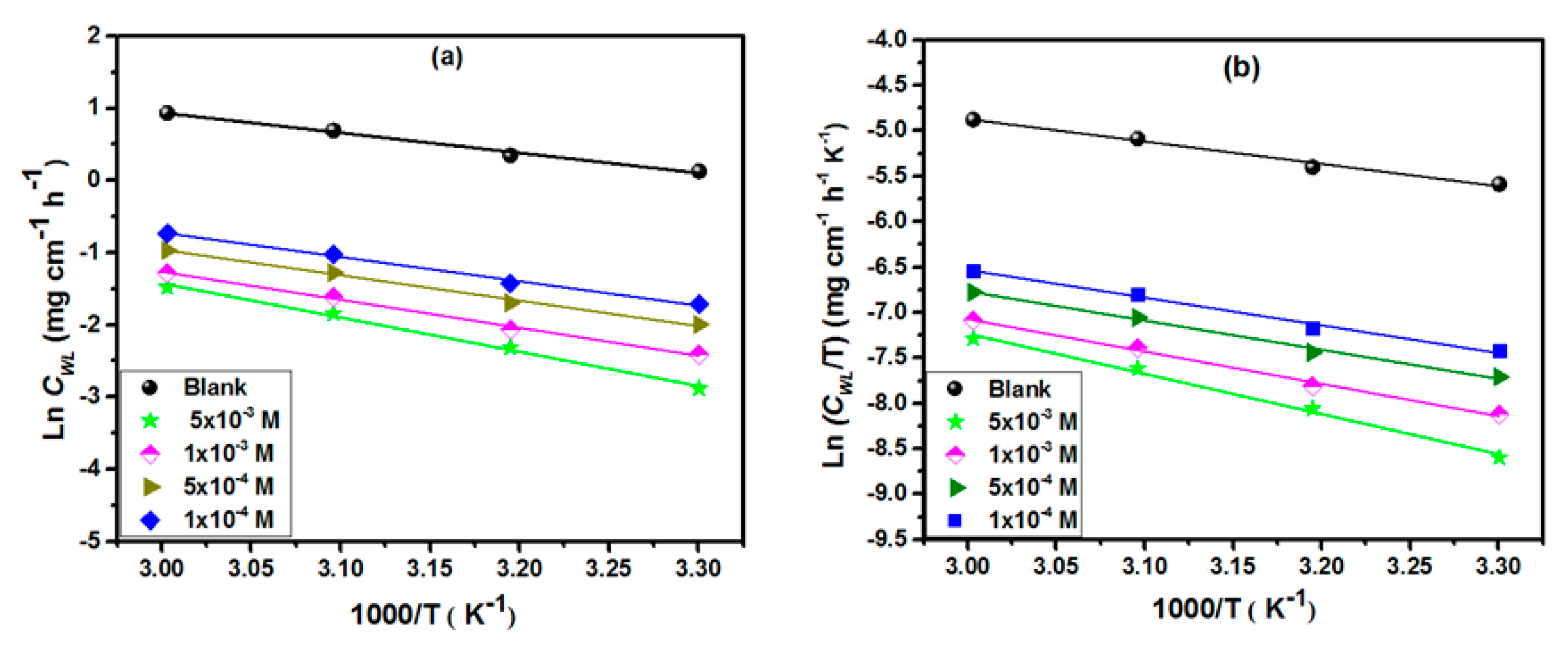
3.1.2. Influence of the Temperature on Inhibitor Performances
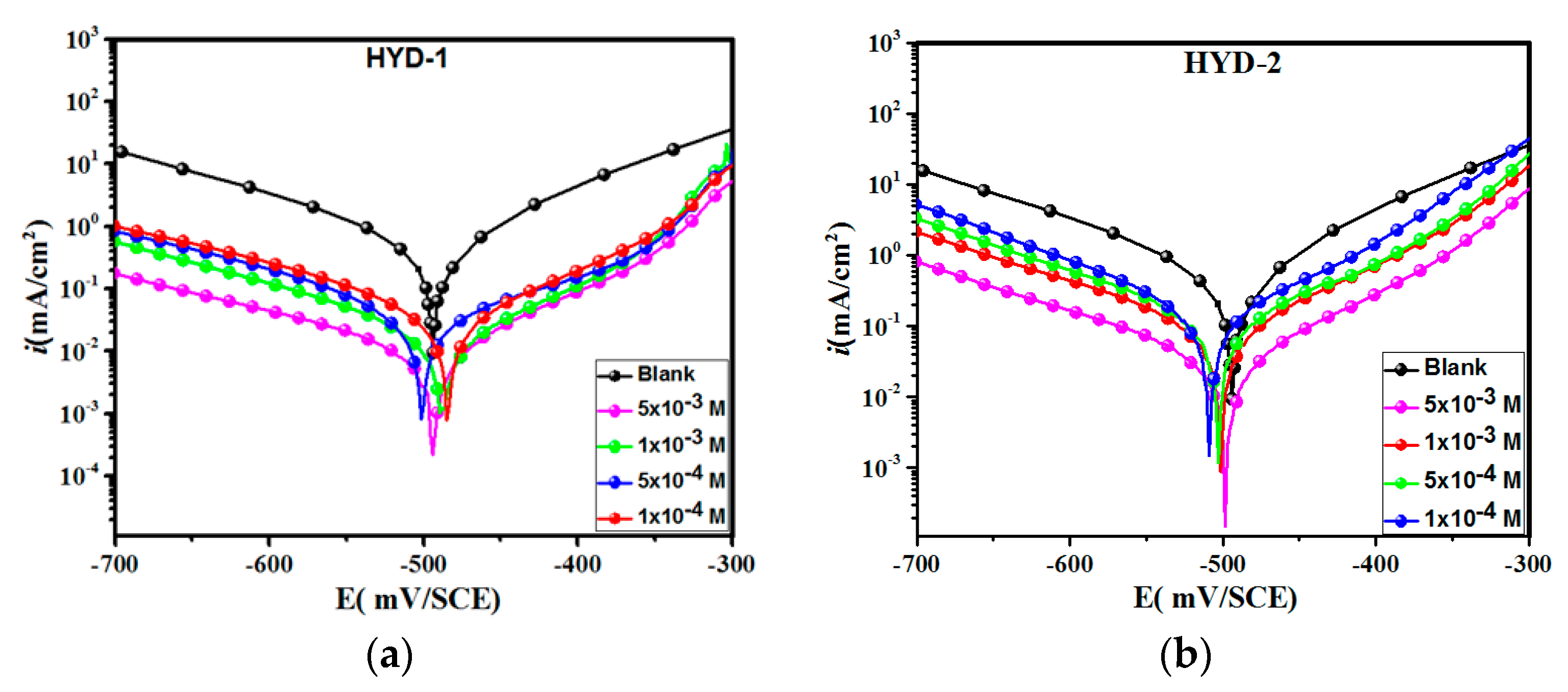
3.2. Potentiodynamic Polarization (PDP) Measurements
3.3. Electrochemical Impedance Spectroscopy (EIS) Study
3.3.1. Concentration Effect
3.3.2. Immersion Time Effect on the Anti-Corrosive Activity of HYD-1
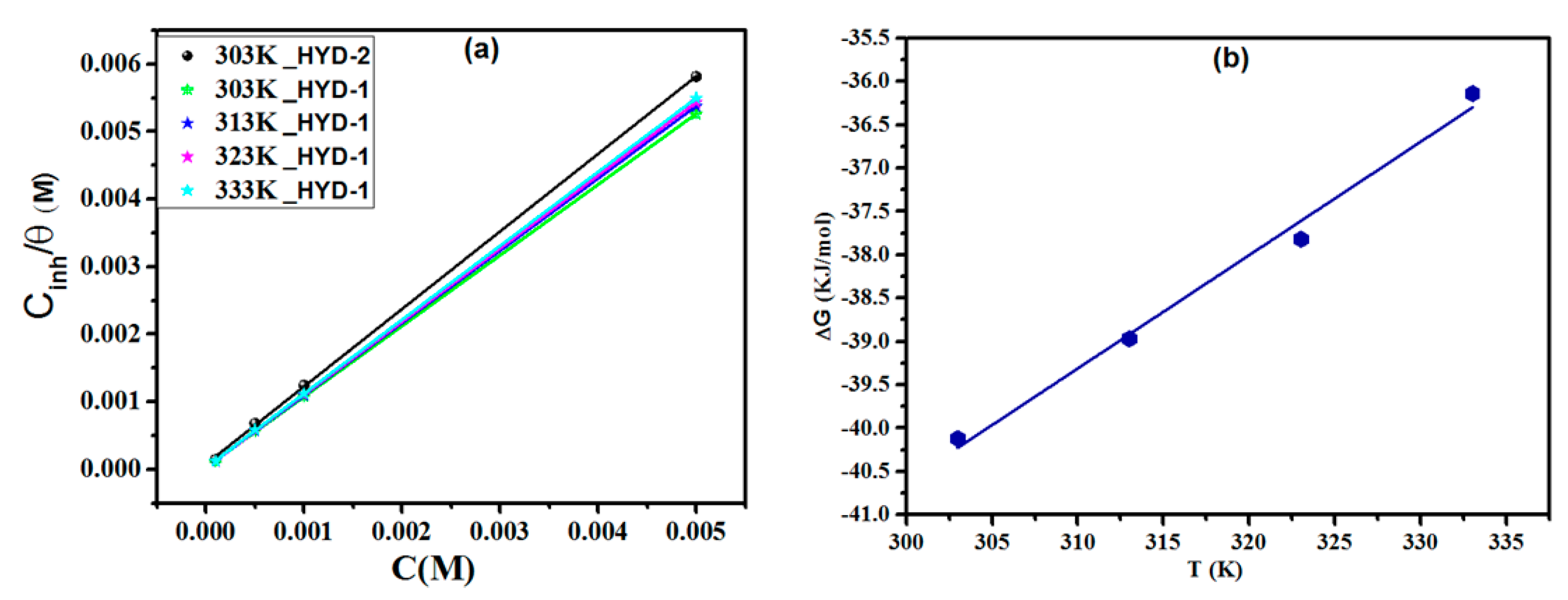
3.4. Adsorption Isotherm
3.5. Surface Morphological Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bentiss, F.; Gassama, F.; Barbry, D.; Gengembre, L.; Vezin, H.; Lagrenée, M.; Traisnel, M. Enhanced corrosion resistance of mild steel in molar hydrochloric acid solution by 1, 4-bis (2-pyridyl)-5H-pyridazino [4, 5-b] indole: Electrochemical, theoretical and XPS studies. Appl. Surf. Sci. 2006, 252, 2684–2691. [Google Scholar] [CrossRef]
- Solmaz, R. Investigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-Dimethylaminobenzylidene)rhodanine. Corros. Sci. 2014, 79, 169–176. [Google Scholar] [CrossRef]
- Lgaz, H.; Saha, S.K.; Chaouiki, A.; Bhat, K.S.; Salghi, R.; Banerjee, P.; Ali, I.H.; Khan, M.I.; Chung, I.-M. Exploring the potential role of pyrazoline derivatives in corrosion inhibition of mild steel in hydrochloric acid solution: Insights from experimental and computational studies. Constr. Build. Mater. 2020, 233, 117320. [Google Scholar] [CrossRef]
- Ouici, H.B.; Benali, O.; Guendouzi, A. Experimental and quantum chemical studies on the corrosion inhibition effect of synthesized pyrazole derivatives on mild steel in hydrochloric acid. Res. Chem. Intermed. 2016, 42, 7085–7109. [Google Scholar] [CrossRef]
- Bentiss, F.; Lagrenee, M.; Traisnel, M.; Hornez, J. The corrosion inhibition of mild steel in acidic media by a new triazole derivative. Corros. Sci. 1999, 41, 789–803. [Google Scholar] [CrossRef]
- Chugh, B.; Singh, A.K.; Chaouiki, A.; Salghi, R.; Thakur, S.; Pani, B. A comprehensive study about anti-corrosion behaviour of pyrazine carbohydrazide: Gravimetric, electrochemical, surface and theoretical study. J. Mol. Liq. 2020, 299, 112160. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.; Obot, I.; Ebenso, E.E.; Quraishi, M. 5-Arylpyrimido-[4, 5-b] quinoline-diones as new and sustainable corrosion inhibitors for mild steel in 1 M HCl: A combined experimental and theoretical approach. RSC Adv. 2016, 6, 15639–15654. [Google Scholar] [CrossRef]
- Rani, B.; Basu, B.B.J. Green inhibitors for corrosion protection of metals and alloys: An overview. Int. J. Corros. 2012, 2012. [Google Scholar] [CrossRef]
- Lgaz, H.; Zehra, S.; Albayati, M.; Toumiat, K.; El Aoufir, Y.; Chaouiki, A.; Salghi, R.; Ali, I.H.; Khan, M.; Chung, I. Corrosion Inhibition of Mild Steel in 1.0 M HCl by two Hydrazone Derivatives. Int. J. Electrochem. Sci. 2019, 14, 6667–6681. [Google Scholar]
- Chaouiki, A.; Lgaz, H.; Zehra, S.; Salghi, R.; Chung, I.-M.; El Aoufir, Y.; Bhat, K.S.; Ali, I.H.; Gaonkar, S.L.; Khan, M.I. Exploring deep insights into the interaction mechanism of a quinazoline derivative with mild steel in HCl: Electrochemical, DFT, and molecular dynamic simulation studies. J. Adhes. Sci. Technol. 2019, 33, 921–944. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Lgaz, H.; Salghi, R.; Bhaskar, K.V.; Marzouki, R.; Bhat, K.S.; Ali, I.H.; Khan, M.I.; Chung, I.-M. Inhibition performances of spirocyclopropane derivatives for mild steel protection in HCl. Mater. Chem. Phys. 2020, 243, 122582. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Lgaz, H.; Salghi, R.; Bhaskar, K.V.; Thakur, P.S.; Bhat, K.S.; Ali, I.H.; Khan, M.; Chung, I.M. New spirocyclopropane derivatives: Synthesis and evaluation of their performances toward corrosion inhibition of mild steel in acidic media. Res. Chem. Intermed. 2020, 46, 2881–2918. [Google Scholar] [CrossRef]
- Chaouiki, A.; Lgaz, H.; Salghi, R.; Chafiq, M.; Oudda, H.; Bhat, K.; Cretescu, I.; Ali, I.; Marzouki, R.; Chung, I. Assessing the impact of electron-donating-substituted chalcones on inhibition of mild steel corrosion in HCl solution: Experimental results and molecular-level insights. Colloids Surf. Physicochem. Eng. Asp. 2020, 588, 124366. [Google Scholar] [CrossRef]
- Mamatha, N.; Babu, N.S.; Mukkanti, K.; Pal, S. 2-(6-Methoxynaphthalen-2-yl) propionic acid (1, 3-dimethylbutylidene) hydrazide. Molbank 2011, 2011, M741. [Google Scholar] [CrossRef]
- Almasirad, A.; Tajik, M.; Bakhtiari, D.; Shafiee, A.; Abdollahi, M.; Zamani, M.J.; Khorasani, R.; Esmaily, H. Synthesis and analgesic activity of N-arylhydrazone derivatives of mefenamic acid. J. Pharm. Pharm. Sci. 2005, 8, 419–425. [Google Scholar] [PubMed]
- Lgaz, H.; Chaouiki, A.; Albayati, M.R.; Salghi, R.; El Aoufir, Y.; Ali, I.H.; Khan, M.I.; Mohamed, S.K.; Chung, I.-M. Synthesis and evaluation of some new hydrazones as corrosion inhibitors for mild steel in acidic media. Res. Chem. Intermed. 2019, 45, 2269–2286. [Google Scholar] [CrossRef]
- Lgaz, H.; Chung, I.-M.; Albayati, M.R.; Chaouiki, A.; Salghi, R.; Mohamed, S.K. Improved corrosion resistance of mild steel in acidic solution by hydrazone derivatives: An experimental and computational study. Arab. J. Chem. 2020, 13, 2934–2954. [Google Scholar] [CrossRef]
- ASTM G31-72 Standard Practice for Laboratory Immersion Corrosion Testing of Metals; ASTM: Philadelphia, PA, USA, 1995.
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 2019, 279, 603–624. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Singh, A.; Singh, V.K.; Yadav, D.K.; Singh, A.K. Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater. Chem. Phys. 2010, 122, 114–122. [Google Scholar] [CrossRef]
- Verma, C.B.; Quraishi, M.; Singh, A. 2-Aminobenzene-1, 3-dicarbonitriles as green corrosion inhibitor for mild steel in 1 M HCl: Electrochemical, thermodynamic, surface and quantum chemical investigation. J. Taiwan Inst. Chem. Eng. 2015, 49, 229–239. [Google Scholar] [CrossRef]
- Wadhwani, P.M.; Ladha, D.G.; Panchal, V.K.; Shah, N.K. Enhanced corrosion inhibitive effect of p-methoxybenzylidene-4, 4′-dimorpholine assembled on nickel oxide nanoparticles for mild steel in acid medium. RSC Adv. 2015, 5, 7098–7111. [Google Scholar] [CrossRef]
- Yadav, M.; Gope, L.; Kumari, N.; Yadav, P. Corrosion inhibition performance of pyranopyrazole derivatives for mild steel in HCl solution: Gravimetric, electrochemical and DFT studies. J. Mol. Liq. 2016, 216, 78–86. [Google Scholar] [CrossRef]
- Yadav, D.K.; Chauhan, D.; Ahamad, I.; Quraishi, M. Electrochemical behavior of steel/acid interface: Adsorption and inhibition effect of oligomeric aniline. RSC Adv. 2013, 3, 632–646. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H. Three pyrazine derivatives as corrosion inhibitors for steel in 1.0 M H2SO4 solution. Corros. Sci. 2011, 53, 3241–3247. [Google Scholar] [CrossRef]
- Chaouiki, A.; Lgaz, H.; Chung, I.-M.; Ali, I.; Gaonkar, S.L.; Bhat, K.; Salghi, R.; Oudda, H.; Khan, M. Understanding corrosion inhibition of mild steel in acid medium by new benzonitriles: Insights from experimental and computational studies. J. Mol. Liq. 2018, 266, 603–616. [Google Scholar] [CrossRef]
- Kowsari, E.; Arman, S.; Shahini, M.; Zandi, H.; Ehsani, A.; Naderi, R.; PourghasemiHanza, A.; Mehdipour, M. In situ synthesis, electrochemical and quantum chemical analysis of an amino acid-derived ionic liquid inhibitor for corrosion protection of mild steel in 1M HCl solution. Corros. Sci. 2016, 112, 73–85. [Google Scholar] [CrossRef]
- Yüce, A.O.; Mert, B.D.; Kardaş, G.; Yazıcı, B. Electrochemical and quantum chemical studies of 2-amino-4-methyl-thiazole as corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2014, 83, 310–316. [Google Scholar] [CrossRef]
- Saini, N.; Kumar, R.; Lgaz, H.; Salghi, R.; Chung, I.-M.; Kumar, S.; Lata, S. Minified dose of urispas drug as better corrosion constraint for soft steel in sulphuric acid solution. J. Mol. Liq. 2018, 269, 371–380. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, N.; Huang, X.; Shang, W.; Wu, L. Synergistic inhibition of carbon steel corrosion in 0.5 M HCl solution by indigo carmine and some cationic organic compounds: Experimental and theoretical studies. RSC Adv. 2016, 6, 22250–22268. [Google Scholar]
- Quraishi, M.; Sardar, R.; Jamal, D. Corrosion inhibition of mild steel in hydrochloric acid by some aromatic hydrazides. Mater. Chem. Phys. 2001, 71, 309–313. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Chaibi, N.; Mernari, B.; Vezin, H.; Lagrenee, M. 2, 5-Bis (n-methoxyphenyl)-1, 3, 4-oxadiazoles used as corrosion inhibitors in acidic media: Correlation between inhibition efficiency and chemical structure. Corros. Sci. 2002, 44, 2271–2289. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Wang, F. A cationic gemini-surfactant as effective inhibitor for mild steel in HCl solutions. Corros. Sci. 2010, 52, 1268–1276. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardaş, G.; Çulha, M.; Yazıcı, B.; Erbil, M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochimica Acta 2008, 53, 5941–5952. [Google Scholar] [CrossRef]
- Ganjali, M.; Norouzi, P.; Alizadeh, T.; Adib, M. Application of 8-amino-N-(2-hydroxybenzylidene) naphthyl amine as a neutral ionophore in the construction of a lanthanum ion-selective sensor. Anal. Chim. Acta 2006, 576, 275–282. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Lin, T.; Xie, X.; Du, G. 2-Mercaptopyrimidine as an effective inhibitor for the corrosion of cold rolled steel in HNO3 solution. Corros. Sci. 2017, 118, 202–216. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M.; Solomon, M.M.; Udoh, A.P. Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab. J. Chem. 2016, 9, S209–S224. [Google Scholar] [CrossRef]
- Aljourani, J.; Raeissi, K.; Golozar, M. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Gurudatt, D.M.; Mohana, K.N. Synthesis of new pyridine based 1, 3, 4-oxadiazole derivatives and their corrosion inhibition performance on mild steel in 0.5 M hydrochloric acid. Ind. Eng. Chem. Res. 2014, 53, 2092–2105. [Google Scholar] [CrossRef]
- Mahdavian, M.; Ashhari, S. Corrosion inhibition performance of 2-mercaptobenzimidazole and 2-mercaptobenzoxazole compounds for protection of mild steel in hydrochloric acid solution. Electrochimica Acta 2010, 55, 1720–1724. [Google Scholar] [CrossRef]
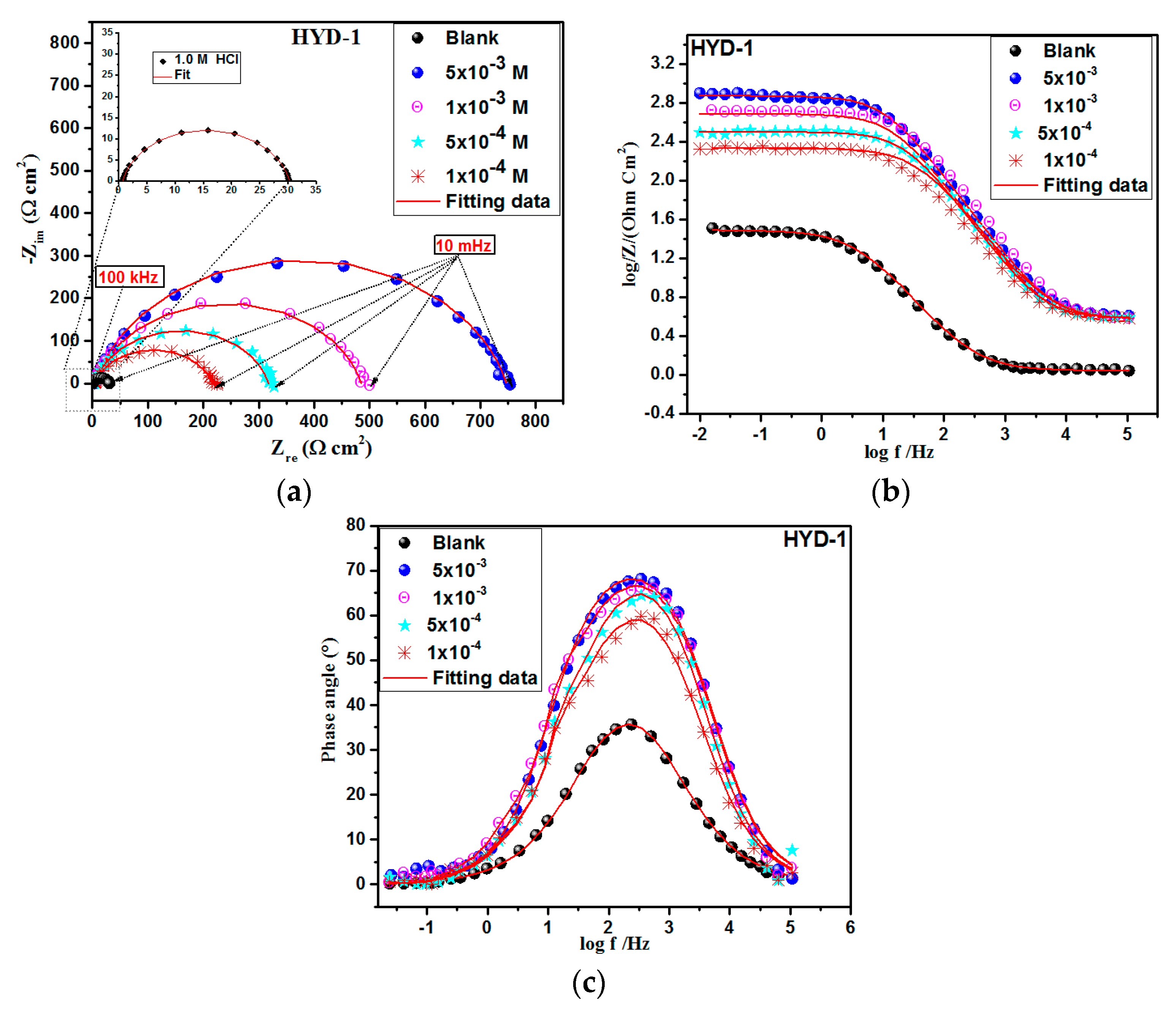
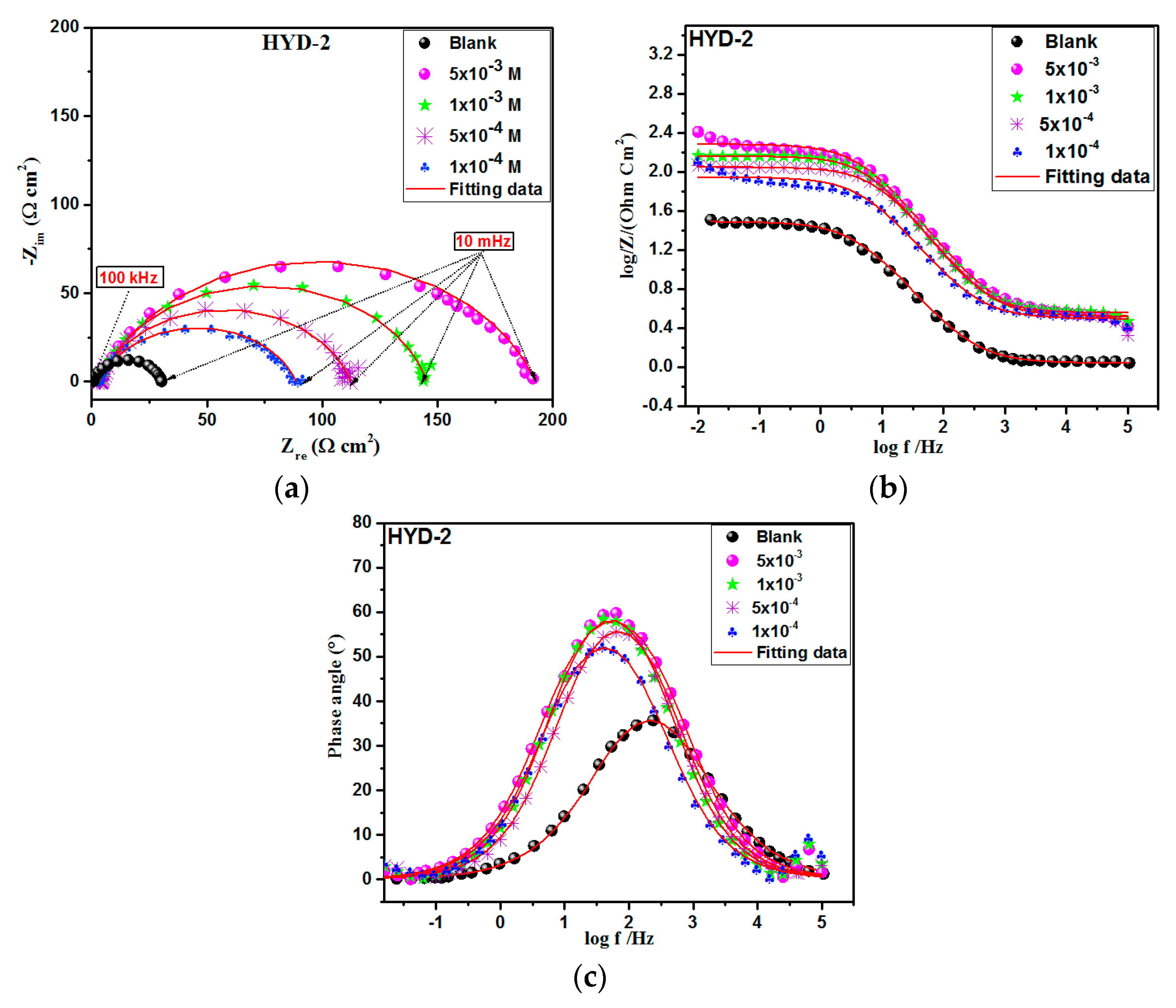
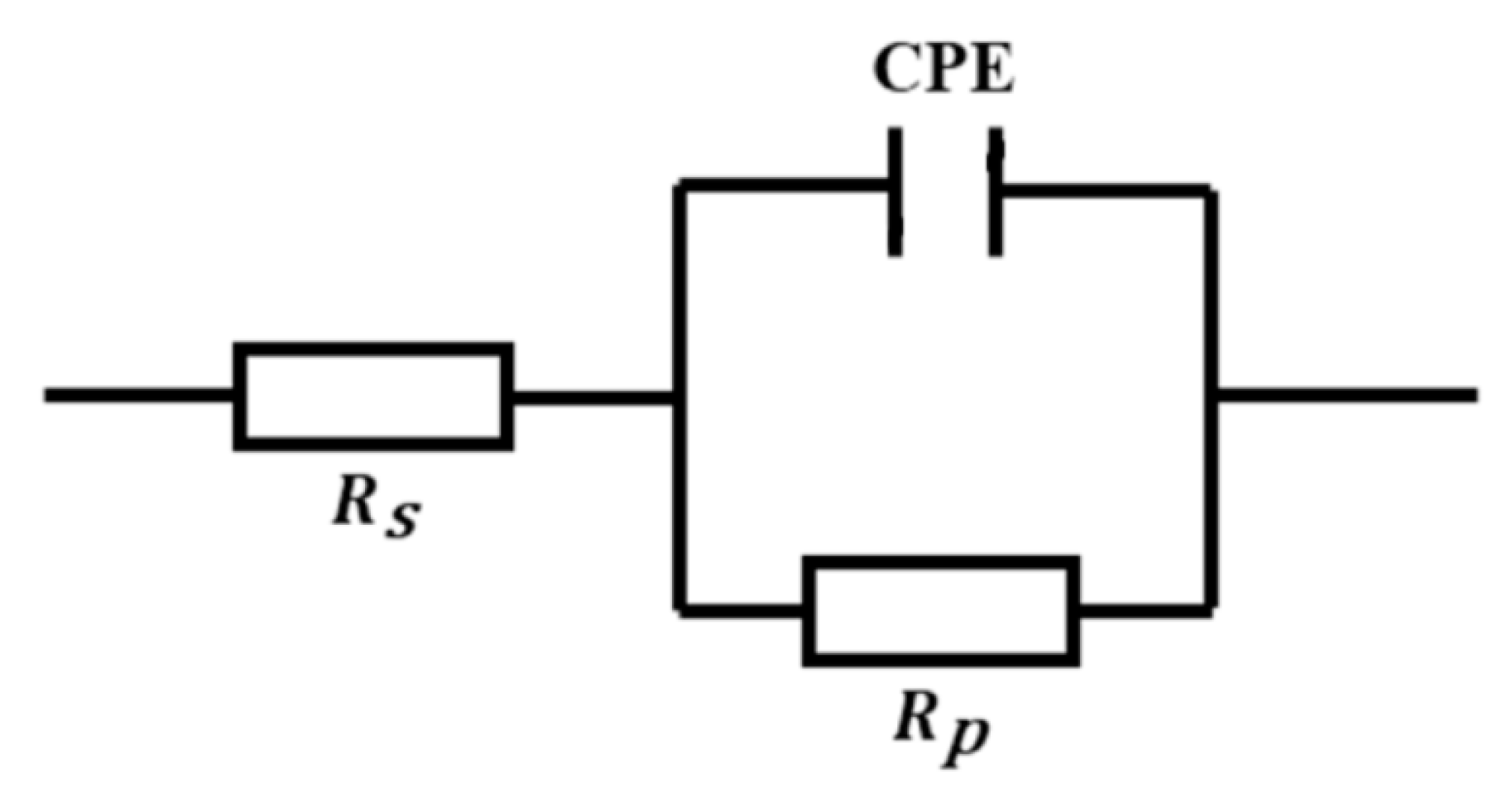
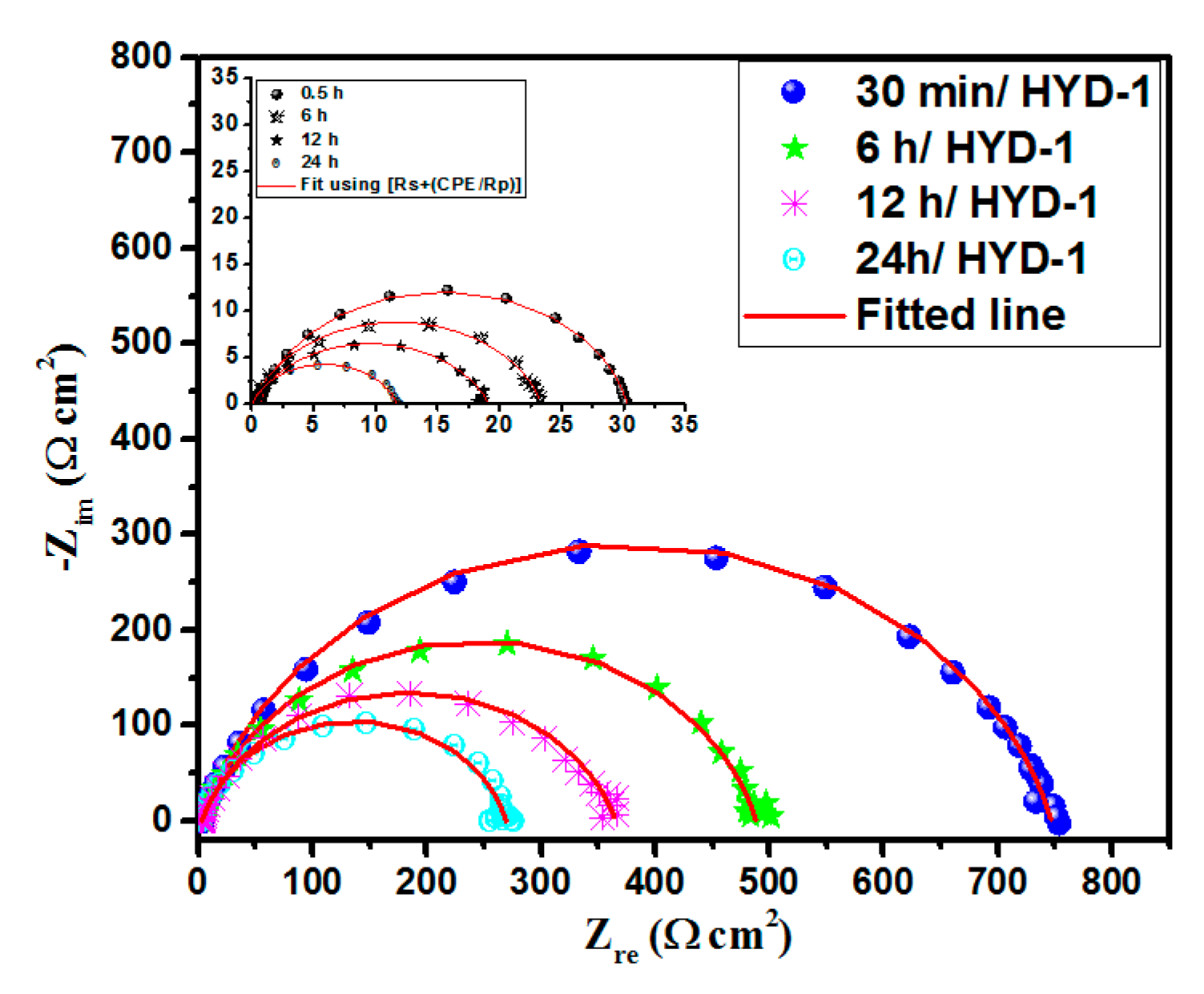
| Solution | Concentration | Temperature | Corrosion Rate | ηWL |
|---|---|---|---|---|
| K | mg/cm2 × h | % | ||
| Blank | 1.0 M HCl | 303 | 1.135 ± 0.0121 | - |
| 313 | 1.416 ± 0.0215 | - | ||
| 323 | 1.998 ± 0.0214 | - | ||
| 333 | 2.539 ± 0.0316 | - | ||
| HYD-1 | 1 × 10−4 | 303 | 0.181 ± 0.0060 | 84 |
| 313 | 0.240 ± 0.0075 | 83 | ||
| 323 | 0.359 ± 0.0065 | 82 | ||
| 333 | 0.482 ± 0.0044 | 81 | ||
| 5 × 10−4 | 303 | 0.136 ± 0.0021 | 88 | |
| 313 | 0.184 ± 0.0010 | 87 | ||
| 323 | 0.279 ± 0.0073 | 86 | ||
| 333 | 0.380 ± 0.0088 | 85 | ||
| 1 × 10−3 | 303 | 0.090 ± 0.0080 | 92 | |
| 313 | 0.127 ± 0.0045 | 91 | ||
| 323 | 0.199 ± 0.0081 | 90 | ||
| 333 | 0.279 ± 0.0031 | 89 | ||
| 5 × 10−3 | 303 | 0.056 ± 0.0075 | 95 | |
| 313 | 0.099 ± 0.0034 | 93 | ||
| 323 | 0.159 ± 0.0084 | 92 | ||
| 333 | 0.228 ± 0.0052 | 91 |
| Parameters | Ea (kJ mol−1) | ∆H* (kJ mol−1) | ∆S* (J mol−1 K−1) | (Ea − ∆H*) (kJ mol−1) | |
|---|---|---|---|---|---|
| Blank | 23.12 | 20.48 | –176.48 | 2.64 | |
| HYD-1 | 1 × 10−4 | 28 | 25.36 | −175.66 | 2.64 |
| 5 × 10−4 | 29.32 | 26.68 | −173.65 | 2.64 | |
| 1 × 10−3 | 32.22 | 29.58 | −167.51 | 2.64 | |
| 5 × 10−3 | 39.39 | 36.75 | −147.32 | 2.64 | |
| Inhibitor | Concentration (M) | −Ecorr (mV vs. SCE) | −βc (mV dec−1) | βa (mV dec−1) | icorr (μA cm−2) | (%) |
|---|---|---|---|---|---|---|
| Blank | 1.0 | 496 ± 0.4 | 150 ± 3.5 | 92 ± 5.7 | 564 ± 2.3 | - |
| HYD-1 | 1 × 10−4 | 486 ± 0.7 | 155 ± 6.8 | 78 ± 4.1 | 82 ± 6.1 | 85 |
| 5 × 10−4 | 503 ± 0.4 | 157 ± 2.6 | 64 ± 3.4 | 68 ± 5.3 | 87 | |
| 1 × 10−3 | 490 ± 0.2 | 139 ± 2.8 | 68 ± 4.9 | 55 ± 3.4 | 90 | |
| 5 × 10−3 | 494 ± 0.5 | 142 ± 4.8 | 98 ± 6.3 | 33 ± 4.8 | 94 | |
| HYD-2 | 1 × 10−4 | 511 ± 1.7 | 146 ± 3.8 | 71 ± 5.6 | 208 ± 5.8 | 63 |
| 5 × 10−4 | 505 ± 0.5 | 136 ± 5.6 | 77 ± 8.2 | 168 ± 6.7 | 70 | |
| 1 × 10−3 | 503 ± 0.6 | 139 ± 5.9 | 74 ± 4.4 | 124 ± 3.6 | 78 | |
| 5 × 10−3 | 500 ± 0.8 | 144 ± 6.7 | 72 ± 3.9 | 89 ± 4.3 | 84 |
| Inhibitor |
Concentration (M) | Goodness of Fit (χ2) | (%) | ||||
|---|---|---|---|---|---|---|---|
| Blank | 1.0 | 29 ± 1.5 | 0.89 ± 0.005 | 1.761 ± 0.0025 | 92 | 0.33 | - |
| HYD-1 | 212 ± 1.6 | 0.80 ± 0.004 | 0.742 ± 0.0031 | 26 | 4.54 | 86 | |
| 313 ± 1.3 | 0.79 ± 0.004 | 0.557 ± 0.0019 | 18 | 4.13 | 90 | ||
| 658 ± 0.9 | 0.83 ± 0.003 | 0.296 ± 0.0012 | 12 | 3.86 | 93 | ||
| 750 ± 1.2 | 0.82 ± 0.005 | 0.179 ± 0.0024 | 7 | 1.23 | 96 | ||
| HYD-2 | 86 ± 1.4 | 0.79 ± 0.007 | 1.173 ± 0.0085 | 34 | 2.18 | 65 | |
| 108 ± 1.9 | 0.81 ± 0.004 | 0.877 ± 0.0078 | 29 | 6.02 | 72 | ||
| 141 ± 1.6 | 0.83 ± 0.006 | 0.616 ± 0.0049 | 23 | 6.18 | 79 | ||
| 189 ± 1.2 | 0.79 ± 0.001 | 0.583 ± 0.0077 | 17 | 3.43 | 84 |
| Inhibitor |
Time (h) |
Goodness of Fit (χ2) | (%) | ||||
|---|---|---|---|---|---|---|---|
| Blank | 29 ± 1.5 | 0.89 ± 0.005 | 1.7610 ± 0.0025 | 92 | 0.33 | - | |
| 23 ± 2.5 | 0.84 ± 0.007 | 2.5114 ± 0.0037 | 94 | 2.45 | - | ||
| 18 ± 1.7 | 0.83 ± 0.004 | 2.9866 ± 0.0084 | 102 | 3.24 | - | ||
| 12 ± 2.9 | 0.88 ± 0.003 | 3.0891 ± 0.0031 | 144 | 1.14 | - | ||
| HYD-1 | 750 ± 1.2 | 0.82 ± 0.005 | 0.1793 ± 0.0024 | 7 | 1.23 | 96 | |
| 484 ± 1.8 | 0.84 ± 0.002 | 0.3339 ± 0.0048 | 15 | 2.14 | 95 | ||
| 352 ± 2.7 | 0.81 ± 0.003 | 0.4994 ± 0.0017 | 19 | 3.59 | 94 | ||
| 265 ± 1.6 | 0.82 ± 0.054 | 0.6095 ± 0.0089 | 24 | 3.12 | 95 |
| Inhibitor | Temperature (K) | Kads (L/mol) | R2 | (kJ/mol) | ΔHa (kJ mol−1) | ΔSa (J mol−1 K−1) |
|---|---|---|---|---|---|---|
| HYD-2 | 303 | 12,311 | 0.999 | −33.82 | - | - |
| HYD-1 | 303 | 30,910 | 0.999 | −40.12 | −79.88 | −13.09 |
| 313 | 37,315 | 0.999 | −38.97 | |||
| 323 | 36,491 | 0.999 | −37.82 | |||
| 333 | 35,675 | 0.999 | −36.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaouiki, A.; Chafiq, M.; Lgaz, H.; Al-Hadeethi, M.R.; Ali, I.H.; Masroor, S.; Chung, I.-M. Green Corrosion Inhibition of Mild Steel by Hydrazone Derivatives in 1.0 M HCl. Coatings 2020, 10, 640. https://doi.org/10.3390/coatings10070640
Chaouiki A, Chafiq M, Lgaz H, Al-Hadeethi MR, Ali IH, Masroor S, Chung I-M. Green Corrosion Inhibition of Mild Steel by Hydrazone Derivatives in 1.0 M HCl. Coatings. 2020; 10(7):640. https://doi.org/10.3390/coatings10070640
Chicago/Turabian StyleChaouiki, Abdelkarim, Maryam Chafiq, Hassane Lgaz, Mustafa R. Al-Hadeethi, Ismat H. Ali, Sheerin Masroor, and Ill-Min Chung. 2020. "Green Corrosion Inhibition of Mild Steel by Hydrazone Derivatives in 1.0 M HCl" Coatings 10, no. 7: 640. https://doi.org/10.3390/coatings10070640
APA StyleChaouiki, A., Chafiq, M., Lgaz, H., Al-Hadeethi, M. R., Ali, I. H., Masroor, S., & Chung, I.-M. (2020). Green Corrosion Inhibition of Mild Steel by Hydrazone Derivatives in 1.0 M HCl. Coatings, 10(7), 640. https://doi.org/10.3390/coatings10070640





