Polyurethane/Silane-Functionalized ZrO2 Nanocomposite Powder Coatings: Thermal Degradation Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Thermal Decomposition Characterization
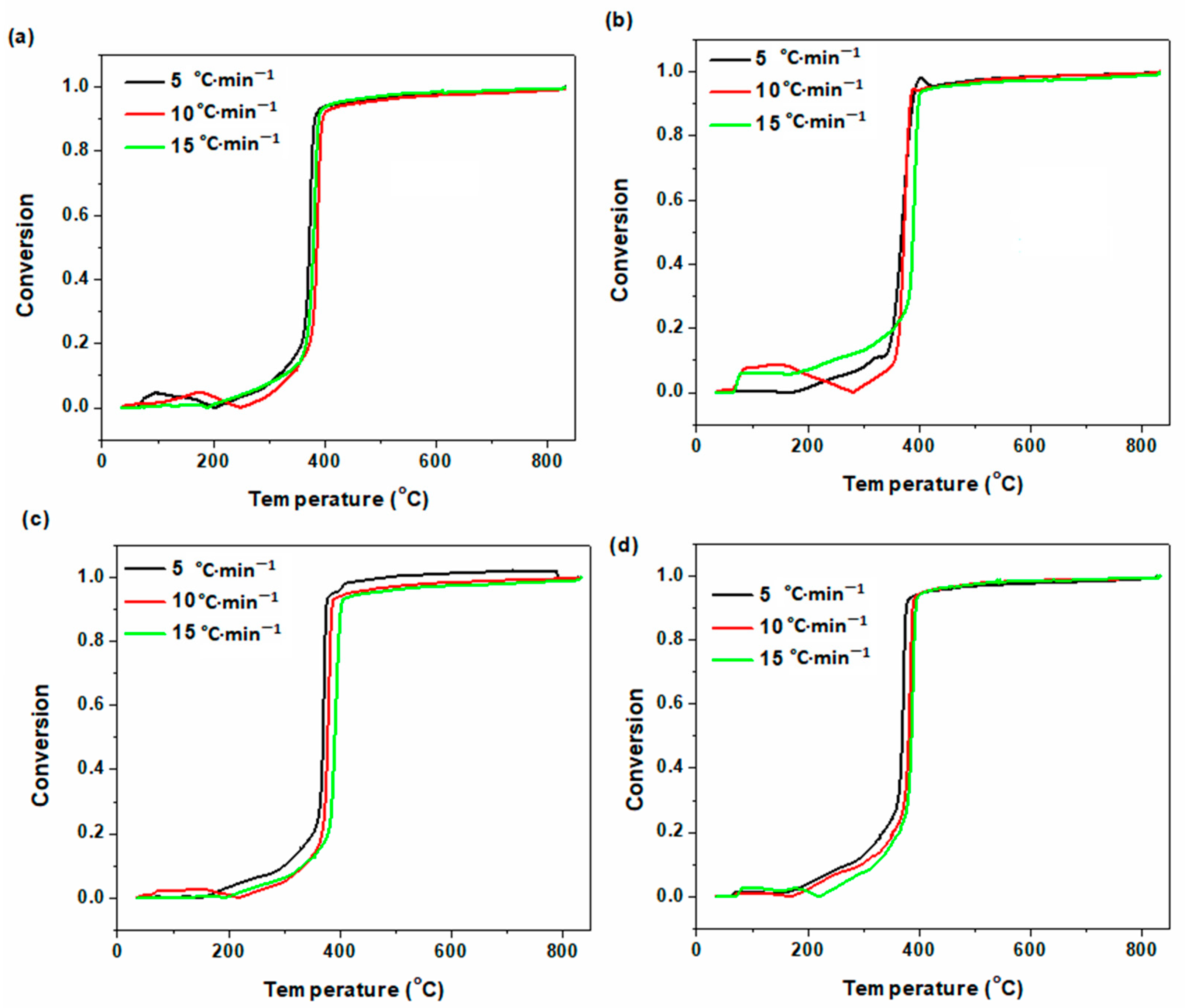
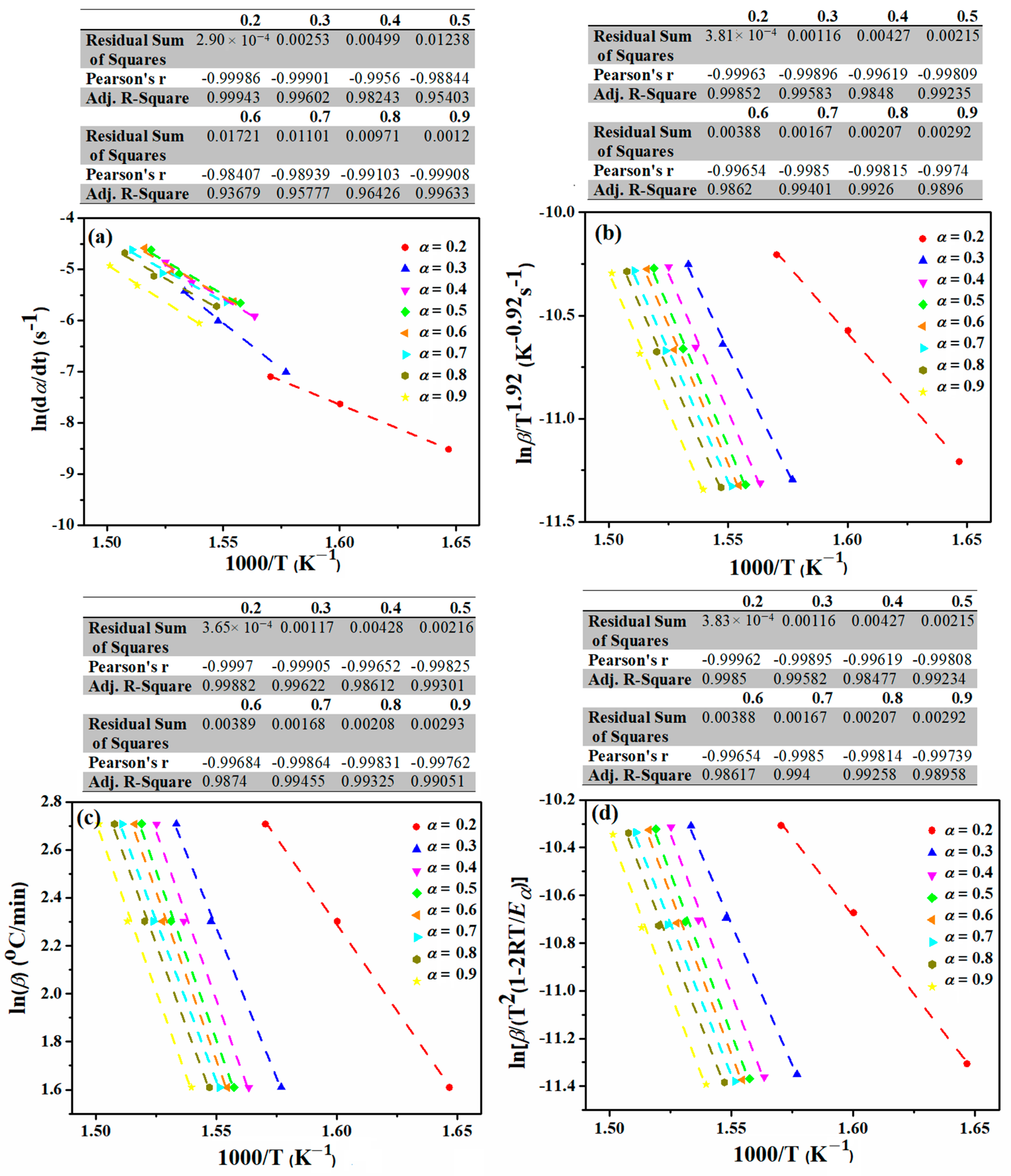
2.4. Theoretical Background
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Spyrou, E.; Loesch, H.; Wenning, A. Low-Temperature-Curable, Solid Polyurethane Powder Coating Compositions Containing Uretdione Groups. U.S. Patent 6914115, 5 July 2005. [Google Scholar]
- Schmitt, F.; Wenning, A.; Weiss, J.-V. Dimeric isocyanates in polyurethane powder coatings. Prog. Org. Coat. 1998, 34, 227–235. [Google Scholar] [CrossRef]
- Sultan, M.; Atta, S.; Bhatti, H.N.; Islam, A.; Jamil, T.; Bibi, I.; Gull, N. Synthesis, characterization, and application studies of polyurethane acrylate thermoset coatings: Effect of hard segment. Polym. Plast. Technol. Eng. 2017, 56, 1608–1618. [Google Scholar] [CrossRef]
- Lee, S.S.; Han, H.Z.; Hilborn, J.G.; Månson, J.-A.E. Surface structure build-up in thermosetting powder coatings during curing. Prog. Org. Coat. 1999, 36, 79–88. [Google Scholar] [CrossRef]
- Visakh, P.; Semkin, A.; Rezaev, I.; Fateev, A. Review on soft polyurethane flame retardant. Constr. Build. Mater. 2019, 227, 116673. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Jia, M.; Qi, B.; Zhang, H.; Lv, W.; Mao, Z.; Chang, P.; Peng, J.; Liu, Y. Study on a thermosetting polyurethane modified asphalt suitable for bridge deck pavements: Formula and properties. Constr. Build. Mater. 2020, 241, 118122. [Google Scholar] [CrossRef]
- Rossi, S.; Fedel, M.; Petrolli, S.; Deflorian, F. Accelerated weathering and chemical resistance of polyurethane powder coatings. J. Coat. Technol. Res. 2016, 13, 427–437. [Google Scholar] [CrossRef]
- Chen, B.; He, M.; Huang, Z.; Wu, Z. Long-tern field test and numerical simulation of foamed polyurethane insulation on concrete dam in severely cold region. Constr. Build. Mater. 2019, 212, 618–634. [Google Scholar] [CrossRef]
- Somarathna, H.; Raman, S.N.; Mohotti, D.; Mutalib, A.A.; Badri, K. The use of polyurethane for structural and infrastructural engineering applications: A state-of-the-art review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Aliakbari, M.; Jazani, O.M.; Sohrabian, M.; Jouyandeh, M. Multi-nationality epoxy adhesives on trial for future nanocomposite developments. Prog. Org. Coat. 2019, 133, 376–386. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Rahmati, N.; Movahedifar, E.; Hadavand, B.S.; Karami, Z.; Ghaffari, M.; Taheri, P.; Bakhshandeh, E.; Vahabi, H.; Ganjali, M.R. Properties of nano-Fe3O4 incorporated epoxy coatings from Cure Index perspective. Prog. Org. Coat. 2019, 133, 220–228. [Google Scholar] [CrossRef]
- Akbari, V.; Najafi, F.; Vahabi, H.; Jouyandeh, M.; Badawi, M.; Morisset, S.; Ganjali, M.R.; Saeb, M.R. Surface chemistry of halloysite nanotubes controls the curability of low filled epoxy nanocomposites. Prog. Org. Coat. 2019, 135, 555–564. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Puglia, D.; Saeb, M.R. Epoxy/layered double hydroxide (LDH) nanocomposites: Synthesis, characterization, and Excellent cure feature of nitrate anion intercalated Zn-Al LDH. Prog. Org. Coat. 2019, 136, 105218. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Shi, Q.; Jiang, G.; Zhao, Z.; Dong, X. Study on nano-CaCO3 modified epoxy powder coatings. Prog. Org. Coat. 2006, 55, 296–300. [Google Scholar] [CrossRef]
- Wang, P.; Ma, Q.; Li, B.; Li, Y. Microstructure and Thermal-protective Property of CPED Coating with ZrO2 Nanoparticles Addition on Al-12Si Alloy. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 1187–1192. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Z.; Li, Y.; Sun, L.; Zhang, T. Synthesis, characterization and thermal analysis of polyaniline/ZrO2 composites. Thermochim. Acta 2006, 441, 191–194. [Google Scholar] [CrossRef]
- Mishra, T.; Kumar, A.; Verma, V.; Pandey, K.; Kumar, V. PEEK composites reinforced with zirconia nanofiller. Compos. Sci. Technol. 2012, 72, 1627–1631. [Google Scholar] [CrossRef]
- Nabiyev, A.; Olejniczak, A.; Pawlukojc, A.; Balasoiu, M.; Bunoiu, M.; Maharramov, A.; Nuriyev, M.; Ismayilova, R.; Azhibekov, A.; Kabyshev, A. Nano-ZrO2 filled high-density polyethylene composites: Structure, thermal properties, and the influence γ-irradiation. Polym. Degrad. Stab. 2020, 171, 109042. [Google Scholar] [CrossRef]
- Wang, P.; Han, J.; Yan, J.; Wang, J. Effects of ZrO2 Nanoparticles on the Microstructure and Thermal-protective Properties of PEO Coating on Al-12.5% Si Alloy. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 156–164. [Google Scholar] [CrossRef]
- Reyes-Acosta, M.; Torres-Huerta, A.M.; Dominguez-Crespo, M.A.; Flores-Vela, A.I.; Dorantes-Rosales, H.J.; Ramírez-Meneses, E. Influence of ZrO2 nanoparticles and thermal treatment on the properties of PMMA/ZrO2 hybrid coatings. J. Alloys Compd. 2015, 643, S150–S158. [Google Scholar] [CrossRef]
- Sow, C.; Riedl, B.; Blanchet, P. UV-waterborne polyurethane-acrylate nanocomposite coatings containing alumina and silica nanoparticles for wood: Mechanical, optical, and thermal properties assessment. J. Coat. Technol. Res. 2011, 8, 211–221. [Google Scholar] [CrossRef]
- Ma, X.; Tu, R.; Ding, C.; Zeng, Y.; Wang, Y.; Fang, T. Thermal and fire risk analysis of low pressure on building energy conservation material flexible polyurethane with various inclined facade constructions. Constr. Build. Mater. 2018, 167, 449–456. [Google Scholar] [CrossRef]
- Vahabi, H.; Jouyandeh, M.; Cochez, M.; Khalili, R.; Vagner, C.; Ferriol, M.; Movahedifar, E.; Ramezanzadeh, B.; Rostami, M.; Ranjbar, Z. Short-lasting fire in partially and completely cured epoxy coatings containing expandable graphite and halloysite nanotube additives. Prog. Org. Coat. 2018, 123, 160–167. [Google Scholar] [CrossRef]
- Saeb, M.; Vahabi, H.; Jouyandeh, M.; Movahedifar, E.; Khalili, R. Epoxy-based flame retardant nanocomposite coatings: Comparison between functions of expandable graphite and halloysite nanotubes. Prog. Colorcolorants Coat. 2017, 10, 245–252. [Google Scholar]
- Yuan, H.; Shi, Y.; Xu, Z.; Lu, C.; Ni, Y.; Lan, X. Influence of nano-ZrO2 on the mechanical and thermal properties of high temperature cementitious thermal energy storage materials. Constr. Build. Mater. 2013, 48, 6–10. [Google Scholar] [CrossRef]
- Paran, S.M.R.; Vahabi, H.; Jouyandeh, M.; Ducos, F.; Formela, K.; Saeb, M.R. Thermal decomposition kinetics of dynamically vulcanized polyamide 6–acrylonitrile butadiene rubber–halloysite nanotube nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47483. [Google Scholar] [CrossRef]
- Madhi, A.; Shirkavand Hadavand, B.; Amoozadeh, A. UV-curable urethane acrylate zirconium oxide nanocomposites: Synthesis, study on viscoelastic properties and thermal behavior. J. Compos. Mater. 2018, 52, 2973–2982. [Google Scholar] [CrossRef]
- Hadavand, B.S.; Ataeefard, M.; Bafghi, H.F. Preparation of modified nano ZnO/polyester/TGIC powder coating nanocomposite and evaluation of its antibacterial activity. Compos. Part B Eng. 2015, 82, 190–195. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U. Mechanisms and kinetics of thermal decomposition of plastics from isothermal and dynamic measurements. J. Anal. Appl. Pyrolysis 1999, 50, 77–101. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Shabanian, M.; Ghiyasi, S.; Vahabi, H.; Badawi, M.; Formela, K.; Puglia, D.; Saeb, M.R. Curing behavior of epoxy/Fe3O4 nanocomposites: A comparison between the effects of bare Fe3O4, Fe3O4/SiO2/chitosan and Fe3O4/SiO2/chitosan/imide/phenylalanine-modified nanofillers. Prog. Org. Coat. 2018, 123, 10–19. [Google Scholar] [CrossRef]
- Rastin, H.; Saeb, M.R.; Nonahal, M.; Shabanian, M.; Vahabi, H.; Formela, K.; Gabrion, X.; Seidi, F.; Zarrintaj, P.; Sari, M.G.; et al. Transparent nanocomposite coatings based on epoxy and layered double hydroxide: Nonisothermal cure kinetics and viscoelastic behavior assessments. Prog. Org. Coat. 2017, 113, 126–135. [Google Scholar] [CrossRef]
- Saeb, M.R.; Rastin, H.; Shabanian, M.; Ghaffari, M.; Bahlakeh, G. Cure kinetics of epoxy/β-cyclodextrin-functionalized Fe3O4 nanocomposites: Experimental analysis, mathematical modeling, and molecular dynamics simulation. Prog. Org. Coat. 2017, 110, 172–181. [Google Scholar] [CrossRef]
- Saeb, M.R.; Nonahal, M.; Rastin, H.; Shabanian, M.; Ghaffari, M.; Bahlakeh, G.; Ghiyasi, S.; Khonakdar, H.A.; Goodarzi, V.; Vijayan, P.P.; et al. Calorimetric analysis and molecular dynamics simulation of cure kinetics of epoxy/chitosan-modified Fe3O4 nanocomposites. Prog. Org. Coat. 2017, 112, 176–186. [Google Scholar] [CrossRef]
- Nonahal, M.; Rastin, H.; Saeb, M.R.; Sari, M.G.; Moghadam, M.H.; Zarrintaj, P.; Ramezanzadeh, B. Epoxy/PAMAM dendrimer-modified graphene oxide nanocomposite coatings: Nonisothermal cure kinetics study. Prog. Org. Coat. 2018, 114, 233–243. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Venkatesh, M.; Ravi, P.; Tewari, S.P. Isoconversional kinetic analysis of decomposition of nitroimidazoles: Friedman method vs Flynn-Wall-Ozawa method. J. Phys. Chem. A 2013, 117, 10162–10169. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ebrahimi-Kahrizsangi, R.; Abbasi, M. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans. Nonferrous Met. Soc. China 2008, 18, 217–221. [Google Scholar] [CrossRef]
- Turmanova, S.C.; Genieva, S.; Dimitrova, A.; Vlaev, L. Non-isothermal degradation kinetics of filled with rise husk ash polypropene composites. Express Polym. Lett. 2008, 2, 133–146. [Google Scholar] [CrossRef]
- Huang, C.C.; Wu, T.S.; Leu, A.L. Determination of kinetic parameters for decomposition reaction from a single DTA curve. Thermochim. Acta 1991, 188, 119–128. [Google Scholar] [CrossRef]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.; Gaan, S. Recent studies on the decomposition and strategies of smoke and toxicity suppression for polyurethane based materials. RSC Adv. 2016, 6, 74742–74756. [Google Scholar] [CrossRef]
- Gallo, E.; Schartel, B.; Acierno, D.; Russo, P. Flame retardant biocomposites: Synergism between phosphinate and nanometric metal oxides. Eur. Polym. J. 2011, 47, 1390–1401. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Vimalathithan, P.K.; Barile, C.; Casavola, C.; Arunachalam, S.; Battisti, M.G.; Friesenbichler, W.; Vijayakumar, C.T. Thermal degradation kinetics of polypropylene/clay nanocomposites prepared by injection molding compounder. Polym. Compos. 2019, 40, 3634–3643. [Google Scholar] [CrossRef]
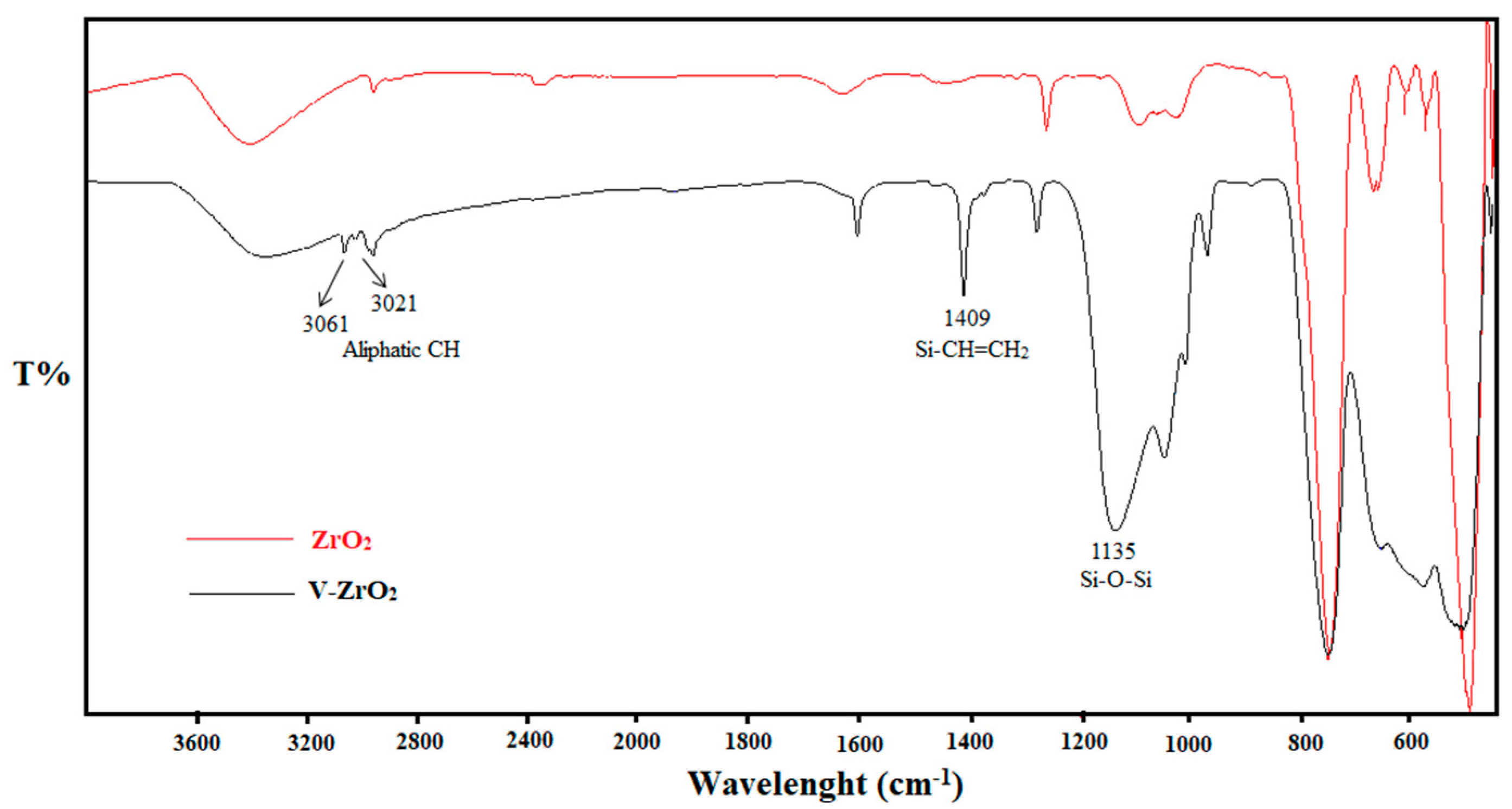

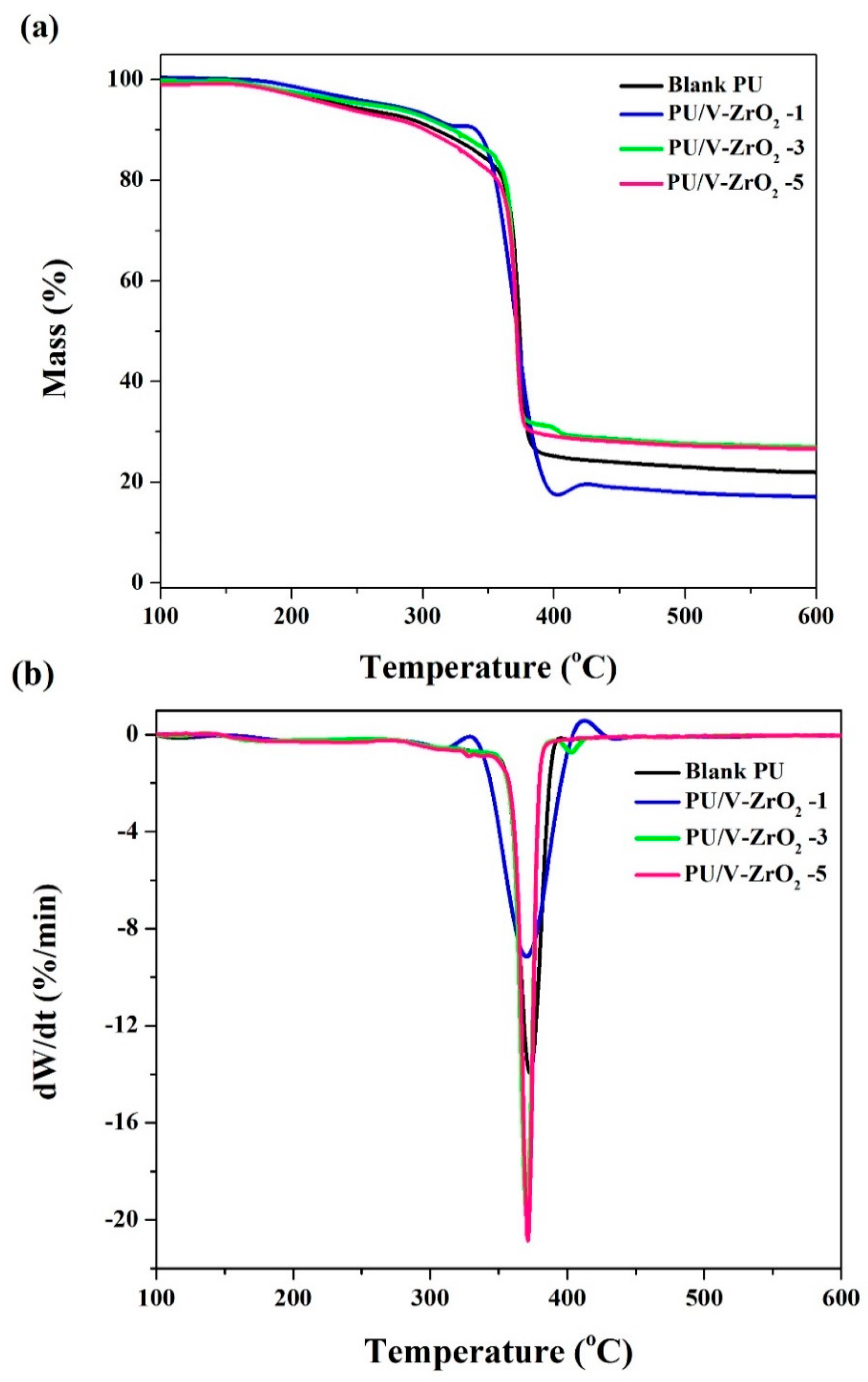
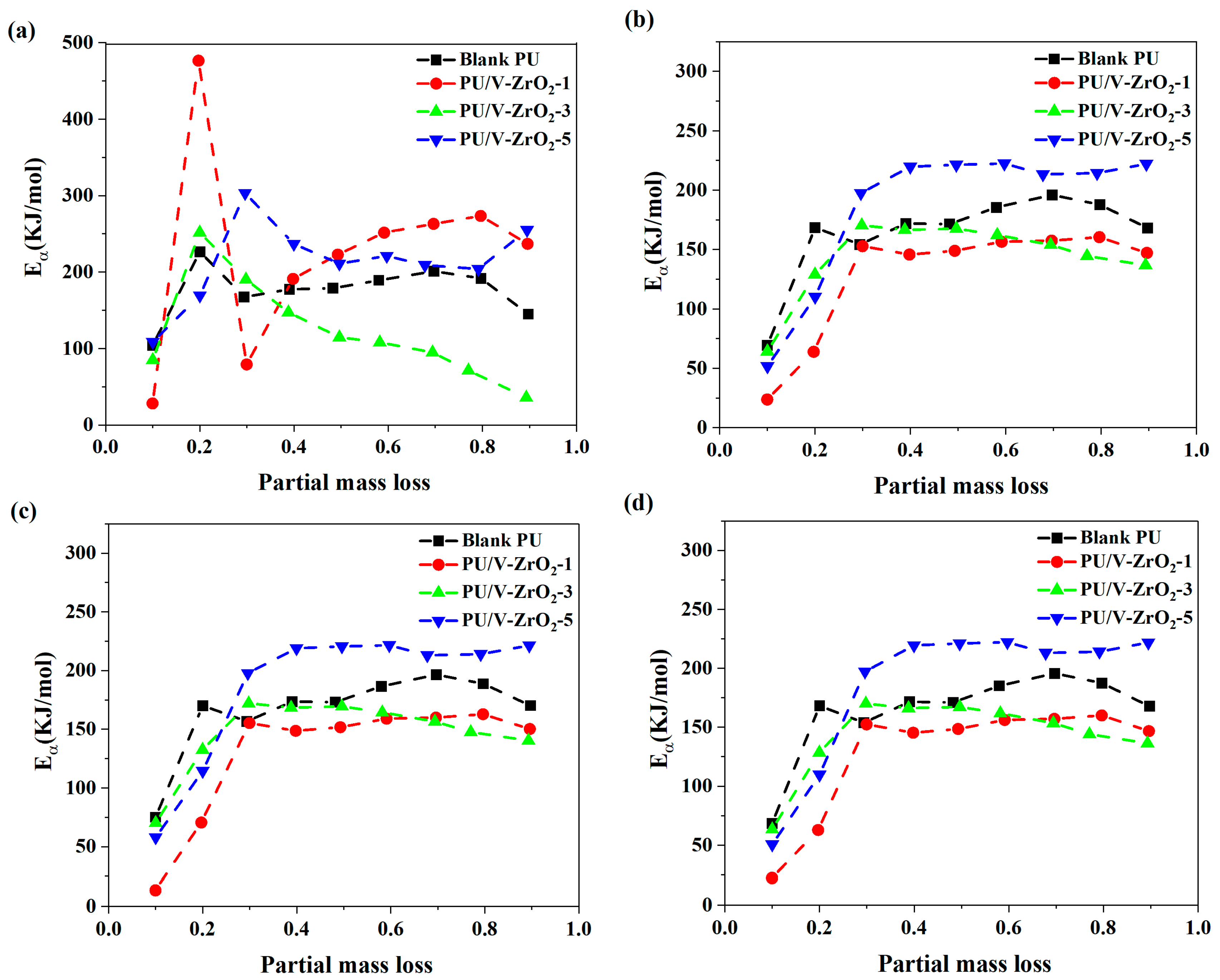
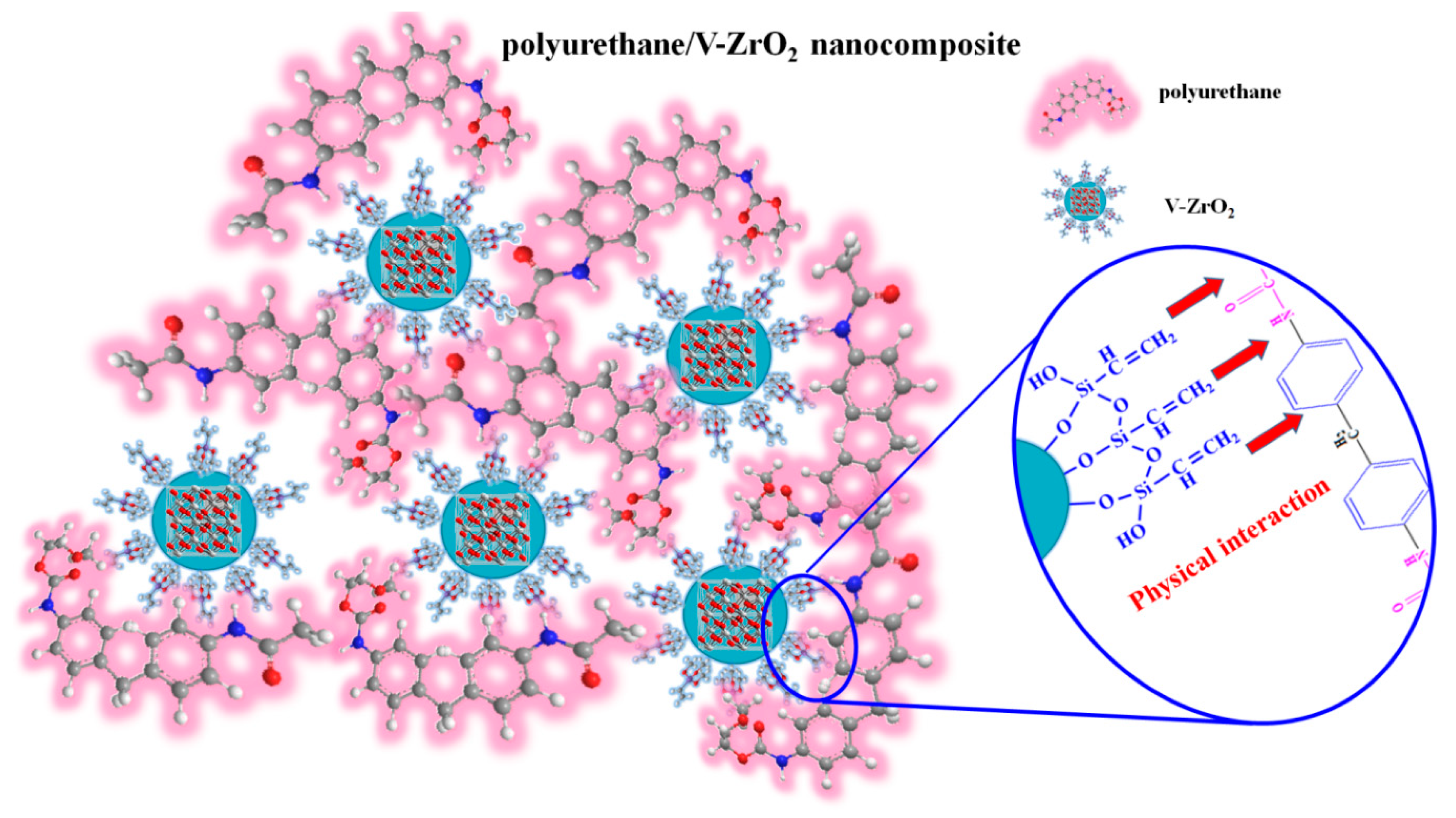
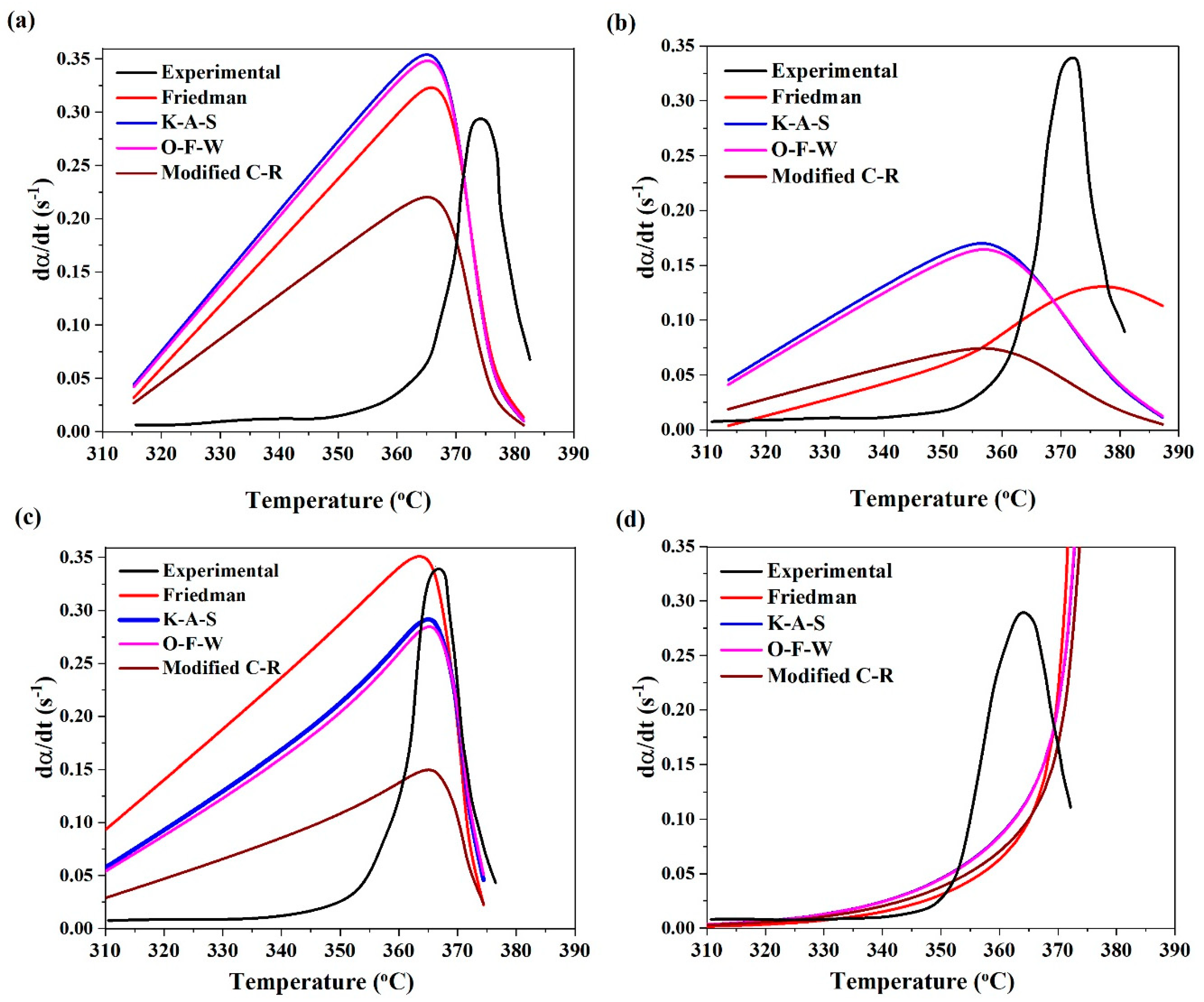

| Designation | T5% (°C) | T10% (°C) | TP (°C) | Residue (%) |
|---|---|---|---|---|
| Blank | 237.47 | 309.60 | 372.86 | 21.09 |
| ZrO2-1 | 272.60 | 340.83 | 370.16 | 17.11 |
| ZrO2-3 | 261.78 | 323.24 | 370.47 | 27.04 |
| ZrO2-5 | 230.80 | 301.86 | 371.45 | 26.64 |
| Designation | Blank | ZrO2-1 | ZrO2-3 | ZrO2-5 | |
|---|---|---|---|---|---|
| Friedman | Eα (kJ·mol−1) | 184.67 | 249.33 | 126.81 | 226.23 |
| Ln A (min−1) | 38.12 | 46.57 | 27.55 | 43.66 | |
| n | 7.22 | 2.93 | 7.03 | 3.89 | |
| KAS | Eα (kJ·mol−1) | 175.38 | 141.58 | 153.84 | 202.58 |
| Ln A (min−1) | 36.42 | 26.47 | 32.43 | 39.58 | |
| n | 7.26 | 3.00 | 6.78 | 4.38 | |
| FWO | Eα (kJ·mol−1) | 177.01 | 144.88 | 156.54 | 202.79 |
| Ln A (min−1) | 36.71 | 27.08 | 32.92 | 39.61 | |
| n | 7.25 | 3.00 | 6.75 | 4.37 | |
| m-CR | Eα (kJ·mol−1) | 175.02 | 141.08 | 153.42 | 202.26 |
| Ln A (min−1) | 36.35 | 26.37 | 32.35 | 39.52 | |
| n | 7.26 | 3.00 | 6.78 | 4.39 | |
| Designation | Blank | ZrO2-1 | ZrO2-3 | ZrO2-5 | |
|---|---|---|---|---|---|
| Friedman | Eα (kJ·mol−1) | 184.67 | 249.33 | 126.81 | 226.23 |
| Ln A (min−1) | 38.12 | 46.57 | 27.55 | 43.66 | |
| m | 3.55 | 0.77 | 3.96 | 1.96 | |
| n | 3.68 | 2.16 | 3.07 | 1.93 | |
| KAS | Eα (kJ·mol−1) | 175.38 | 141.58 | 153.84 | 202.58 |
| Ln A (min−1) | 36.42 | 26.47 | 32.43 | 39.58 | |
| m | 3.58 | 1.12 | 3.79 | 2.28 | |
| n | 3.68 | 1.88 | 2.99 | 2.10 | |
| FWO | Eα (kJ·mol−1) | 177.01 | 144.88 | 156.54 | 202.79 |
| Ln A (min−1) | 36.71 | 27.08 | 32.92 | 39.61 | |
| m | 3.58 | 1.11 | 3.77 | 2.27 | |
| n | 3.68 | 1.89 | 2.99 | 2.10 | |
| m-CR | Eα (kJ·mol−1) | 175.02 | 141.08 | 153.42 | 202.26 |
| Ln A (min−1) | 36.35 | 26.37 | 32.35 | 39.52 | |
| m | 3.58 | 1.11 | 3.78 | 2.28 | |
| n | 3.68 | 1.89 | 3.00 | 2.11 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhani, F.; Shirkavand Hadavand, B.; Fakharizadeh Bafghi, H.; Jouyandeh, M.; Vahabi, H.; Formela, K.; Hosseini, H.; Paran, S.M.R.; Esmaeili, A.; Mohaddespour, A.; et al. Polyurethane/Silane-Functionalized ZrO2 Nanocomposite Powder Coatings: Thermal Degradation Kinetics. Coatings 2020, 10, 413. https://doi.org/10.3390/coatings10040413
Tikhani F, Shirkavand Hadavand B, Fakharizadeh Bafghi H, Jouyandeh M, Vahabi H, Formela K, Hosseini H, Paran SMR, Esmaeili A, Mohaddespour A, et al. Polyurethane/Silane-Functionalized ZrO2 Nanocomposite Powder Coatings: Thermal Degradation Kinetics. Coatings. 2020; 10(4):413. https://doi.org/10.3390/coatings10040413
Chicago/Turabian StyleTikhani, Farimah, Behzad Shirkavand Hadavand, Hamed Fakharizadeh Bafghi, Maryam Jouyandeh, Henri Vahabi, Krzyszof Formela, Hossein Hosseini, Seyed Mohammad Reza Paran, Amin Esmaeili, Ahmad Mohaddespour, and et al. 2020. "Polyurethane/Silane-Functionalized ZrO2 Nanocomposite Powder Coatings: Thermal Degradation Kinetics" Coatings 10, no. 4: 413. https://doi.org/10.3390/coatings10040413
APA StyleTikhani, F., Shirkavand Hadavand, B., Fakharizadeh Bafghi, H., Jouyandeh, M., Vahabi, H., Formela, K., Hosseini, H., Paran, S. M. R., Esmaeili, A., Mohaddespour, A., & Saeb, M. R. (2020). Polyurethane/Silane-Functionalized ZrO2 Nanocomposite Powder Coatings: Thermal Degradation Kinetics. Coatings, 10(4), 413. https://doi.org/10.3390/coatings10040413










