Thermal-Resistant Polyurethane/Nanoclay Powder Coatings: Degradation Kinetics Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Polyurethane Nanocomposite
2.2. Characterization
2.2.1. Scanning Electron Microscopy
2.2.2. Thermal Decomposition Characterization
2.3. Methods
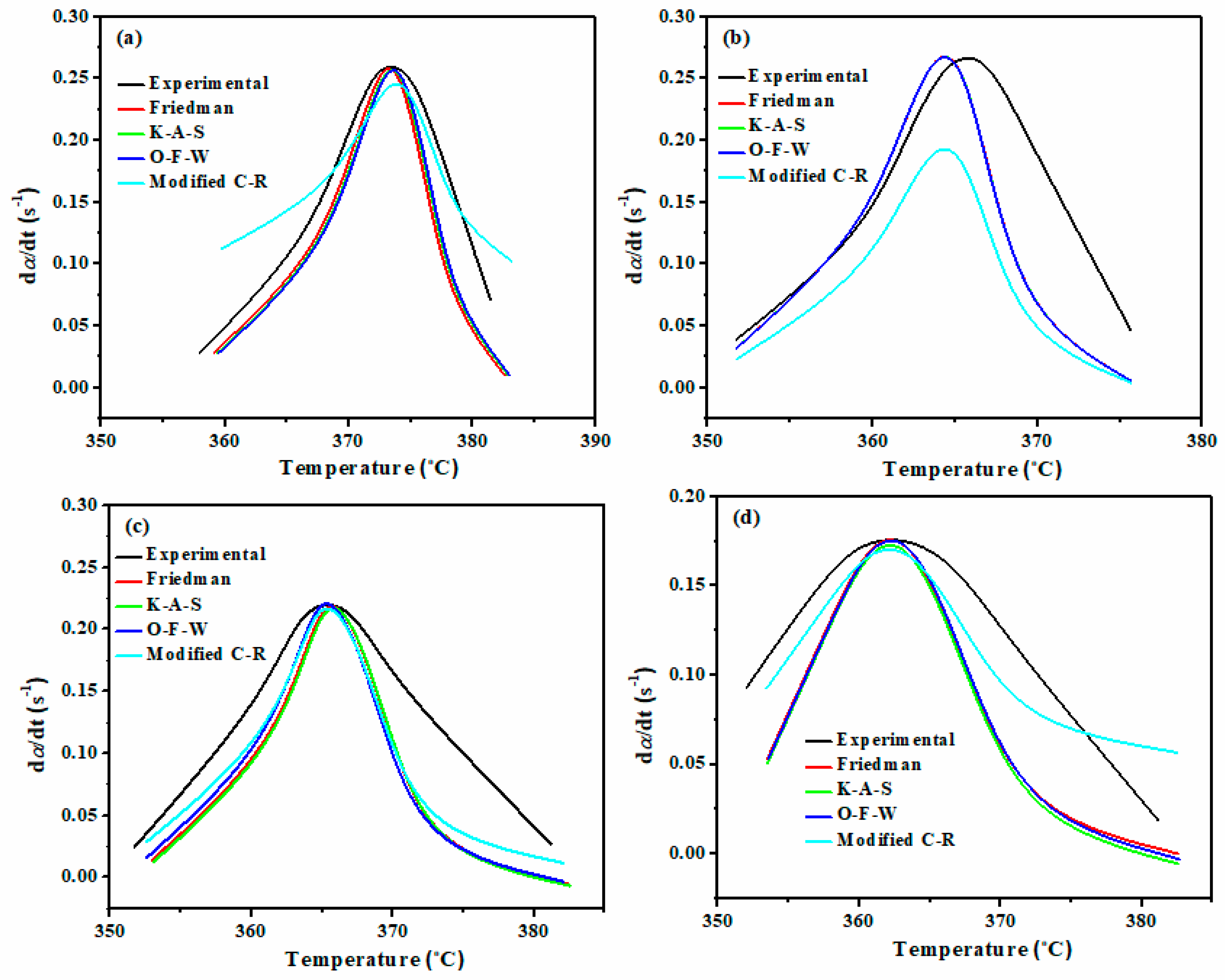
3. Results
4. Conclusions
- The presence of nano-clay in the system enhanced the PU thermal stability;
- The performance of the nanocomposite is extremely dependent on the dispersion state of the nano-clay in the PU matrix;
- The SEM images demonstrated that some aggregations exist in the nanocomposite with the highest loading while the morphologies of nanocomposites containing 1% and 3% nano-clay were uniform;
- The thermal stability of the nanocomposites had an ascending behavior by increasing nano-clay content from 1 to 3 wt.%. However, it descended when the concentration of the nano-clay reached to 5 wt.% due to aggregation of nanoplates in this content;
- The initial decomposition temperature of the systems considerably increased (more than 40 °C for T5%) due to the barrier effect of the nano-clay;
- The char content of the samples containing uniformly dispersed nanoplates was enhanced up to 10% at 600 °C;
- The value of Eα, which was calculated based on the TGA data and model-free iso-conversional methods including Friedman, FWO, KAS, and m-CR increased for all the nanocomposite sample in comparison to the pure one, which confirmed greater thermal resistance of the system against elevated temperatures;
- The Sestak-Berggren model well-fitted the experimental curve of the reaction rate, specifically in lower temperatures.
Author Contributions
Funding
Conflicts of Interest
References
- Celina, M.C. Review of polymer oxidation and its relationship with materials performance and lifetime prediction. Polym. Degrad. Stab. 2013, 98, 2419–2429. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Krongauz, V.V. Crosslink density dependence of polymer degradation kinetics: Photocrosslinked acrylates. Thermochim. Acta 2010, 503–504, 70–84. [Google Scholar] [CrossRef]
- Igor, V.K.; David, R.Z.; Nicholas, J.T. Polyurethane nanocomposites. Des. Monomers Polym. 2009, 12, 279–290. [Google Scholar] [CrossRef]
- Khobragade, P.S.; Hansora, D.P.; Naik, J.B.; Chatterjee, A. Flame retarding performance of elastomeric nanocomposites: A review. Polym. Degrad. Stab. 2016, 130, 194–244. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Rallini, M.; Luzi, F.; Torre, L.; Puglia, D.; et al. Cure Index for labeling curing potential of epoxy/LDH nanocomposites: A case study on nitrate anion intercalated Ni-Al-LDH. Prog. Org. Coat. 2019, 136, 105228. [Google Scholar] [CrossRef]
- Vaithylingam, R.; Ansari, M.N.M.; Shanks, R.A. Recent Advances in Polyurethane Based Nanocomposites: A Review. Polym. Plast. Technol. Eng. 2017, 56, 1528–1541. [Google Scholar] [CrossRef]
- Haghayegh, M.; Mir Mohamad Sadeghi, G. Synthesis of shape memory polyurethane/clay nanocomposites and analysis of shape memory, thermal, and mechanical properties. Polym. Compos. 2012, 33, 843–849. [Google Scholar] [CrossRef]
- Peng, S.; Iroh, J.O. Synthesis and characterization of crosslinked polyurethane/clay nanocomposites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Rehab, A.; Salahuddin, N. Nanocomposite materials based on polyurethane intercalated into montmorillonite clay. Mater. Sci. Eng. A 2005, 399, 368–376. [Google Scholar] [CrossRef]
- Chen-Yang, Y.W.; Yang, H.C.; Li, G.J.; Li, Y.K. Thermal and anticorrosive properties of polyurethane/clay nanocomposites. J. Polym. Res. 2004, 11, 275–283. [Google Scholar] [CrossRef]
- Wang, X.-C.; Geng, T.; Han, J.; Liu, C.-T.; Shen, C.-Y.; Turng, L.-S.; Yang, H.E. Effects of nanoclays on the thermal stability and flame retardancy of microcellular thermoplastic polyurethane nanocomposites. Polym. Compos. 2018, 39, E1429–E1440. [Google Scholar] [CrossRef]
- Maciejewski, M. Computational aspects of kinetic analysis.: Part B: The ICTAC Kinetics Project—The decomposition kinetics of calcium carbonate revisited, or some tips on survival in the kinetic minefield. Thermochim. Acta 2000, 355, 145–154. [Google Scholar] [CrossRef]
- Jomaa, G.; Goblet, P.; Coquelet, C.; Morlot, V. Kinetic modeling of polyurethane pyrolysis using non-isothermal thermogravimetric analysis. Thermochim. Acta 2015, 612, 10–18. [Google Scholar] [CrossRef]
- Pashaei, S.; Siddaramaiah; Syed, A.A. Thermal degradation kinetics of polyurethane/organically modified montmorillonite clay nanocomposites by TGA. J. Macromol. Sci. Part A 2010, 47, 777–783. [Google Scholar] [CrossRef]
- Zhao, Y.; Mo, H.; Jiang, X.; Han, B.; Feng, F.; Wang, D.; Fu, L.; He, L.; Zhang, J.; Shen, J. Thermal stability and thermal oxidation kinetics of PU/CA-MMT composites. J. Appl. Polym. Sci. 2019, 136, 47002. [Google Scholar] [CrossRef]
- Pau, D.S.W.; Fleischmann, C.M.; Spearpoint, M.J.; Li, K.Y. Determination of kinetic properties of polyurethane foam decomposition for pyrolysis modelling. J. Fire Sci. 2013, 31, 356–384. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U. Mechanisms and kinetics of thermal decomposition of plastics from isothermal and dynamic measurements. J. Anal. Appl. Pyrolysis 1999, 50, 77–101. [Google Scholar] [CrossRef]
- Paran, S.M.R.; Vahabi, H.; Jouyandeh, M.; Ducos, F.; Formela, K.; Saeb, M.R. Thermal decomposition kinetics of dynamically vulcanized polyamide 6–acrylonitrile butadiene rubber–halloysite nanotube nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47483. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Saeb, M.R.; Thomas, S. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized ZnxFe3−xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105227. [Google Scholar] [CrossRef]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing kinetics and thermal stability of epoxy composites containing newly obtained nano-scale aluminum hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Jouyandeh, M.; Zarrintaj, P.; Ganjali, M.R.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Ghaffari, M.; Saeb, M.R. Curing epoxy with electrochemically synthesized GdxFe3−xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105245. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Seidi, F.; Xiao, H.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/polyvinylpyrrolidone functionalized superparamagnetic nano-Fe3O4 composites: Effect of Zn and Mn doping. J. Compos. Sci. 2020, 4, 55. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Khadem, S.S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/MnxFe3−xO4 nanocomposites. Prog. Org. Coat. 2020, 140, 105505. [Google Scholar] [CrossRef]
- Rastin, H.; Saeb, M.R.; Nonahal, M.; Shabanian, M.; Vahabi, H.; Formela, K.; Gabrion, X.; Seidi, F.; Zarrintaj, P.; Sari, M.G.; et al. Transparent nanocomposite coatings based on epoxy and layered double hydroxide: Nonisothermal cure kinetics and viscoelastic behavior assessments. Prog. Org. Coat. 2017, 113, 126–135. [Google Scholar] [CrossRef]
- Nonahal, M.; Rastin, H.; Saeb, M.R.; Sari, M.G.; Moghadam, M.H.; Zarrintaj, P.; Ramezanzadeh, B. Epoxy/PAMAM dendrimer-modified graphene oxide nanocomposite coatings: Nonisothermal cure kinetics study. Prog. Org. Coat. 2018, 114, 233–243. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Akbari, V.; Paran, S.M.R.; Livi, S.; Ducos, F.; Vahabi, H.; Ganjali, M.R.; Saeb, M.R. Super-crosslinked ionic liquid-intercalated montmorillonite/epoxy nanocomposites: Cure kinetics, viscoelastic behavior and thermal degradation mechanism. Polym. Eng. Sci. 2020, 60, 1940–1957. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized NixFe3−xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105198. [Google Scholar] [CrossRef]
- Venkatesh, M.; Ravi, P.; Tewari, S.P. Isoconversional kinetic analysis of decomposition of nitroimidazoles: Friedman method vs Flynn-Wall-Ozawa method. J. Phys. Chem. A 2013, 117, 10162–10169. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Ganjali, M.R.; Dubois, P. Curing epoxy with polyethylene glycol (PEG) surface-functionalized NixFe3−xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105250. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Hamad, S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Kim, S.-J.; Zarrintaj, P.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/ZnxFe3−xO4 nanocomposites. Prog. Org. Coat. 2019, 136, 105290. [Google Scholar] [CrossRef]
- Akbari, V.; Jouyandeh, M.; Paran, S.M.R.; Ganjali, M.R.; Abdollahi, H.; Vahabi, H.; Ahmadi, Z.; Formela, K.; Esmaeili, A.; Mohaddespour, A. Effect of surface treatment of halloysite nanotubes (HNTs) on the kinetics of epoxy resin cure with amines. Polymers 2020, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Jouyandeh, M.; Yarahmadi, E.; Didehban, K.; Ghiyasi, S.; Paran, S.M.R.; Puglia, D.; Ali, J.A.; Jannesari, A.; Saeb, M.R.; Ranjbar, Z. Cure kinetics of epoxy/graphene oxide (GO) nanocomposites: Effect of starch functionalization of GO nanosheets. Prog. Org. Coat. 2019, 136, 105217. [Google Scholar] [CrossRef]
- Kornmann, X.; Lindberg, H.; Berglund, L.A. Synthesis of epoxy–clay nanocomposites: Influence of the nature of the clay on structure. Polymer 2001, 42, 1303–1310. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tikhani, F.; Shabanian, M.; Movahedi, F.; Moghari, S.; Akbari, V.; Gabrion, X.; Laheurte, P.; Vahabi, H.; Saeb, M.R. Synthesis, characterization, and high potential of 3D metal–organic framework (MOF) nanoparticles for curing with epoxy. J. Alloys Compd. 2020, 15454. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Taghizadeh, M.; Taghizadeh, A.; Vahabi, H.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Metal-organic framework (MOF)/epoxy coatings: A review. Materials 2020, 13, 2881. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tikhani, F.; Hampp, N.; Yazdi, D.A.; Zarrintaj, P.; Ganjali, M.R.; Saeb, M.R. Highly curable self-healing vitrimer-like cellulose-modified halloysite nanotube/epoxy nanocomposite coatings. Chem. Eng. J. 2020, 396, 125196. [Google Scholar] [CrossRef]
- Álvarez-Alcón, M.; López de Lacalle, L.N.; Fernández-Zacarías, F. Multiple sensor monitoring of CFRP drilling to define cutting parameters sensitivity on surface roughness, cylindricity and diameter. Materials 2020, 13, 2796. [Google Scholar] [CrossRef] [PubMed]
- Jouyandeh, M.; Ganjali, M.R.; Aghazadeh, M.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Bulk-Surface Modification of Nanoparticles for Developing Highly-Crosslinked Polymer Nanocomposites. Polymers 2020, 12, 1820. [Google Scholar] [CrossRef] [PubMed]
- Hadavand, B.S.; Jouyandeh, M.; Paran, S.M.R.; Khalili, R.; Vahabi, H.; Bafghi, H.F.; Laoutid, F.; Vijayan, P.P.; Saeb, M.R. Silane-functionalized Al2O3-modified polyurethane powder coatings: Nonisothermal degradation kinetics and mechanistic insights. J. Appl. Polym. Sci. 2020, 49412. [Google Scholar] [CrossRef]
- Tikhani, F.; Shirkavand Hadavand, B.; Fakharizadeh Bafghi, H.; Jouyandeh, M.; Vahabi, H.; Formela, K.; Hosseini, H.; Paran, S.M.R.; Esmaeili, A.; Mohaddespour, A. Polyurethane/silane-functionalized ZrO2 nanocomposite powder coatings: Thermal degradation kinetics. Coatings 2020, 10, 413. [Google Scholar] [CrossRef]
- Barick, A.; Tripathy, D. Preparation and characterization of thermoplastic polyurethane/organoclay nanocomposites by melt intercalation technique: Effect of nanoclay on morphology, mechanical, thermal, and rheological properties. J. Appl. Polym. Sci. 2010, 117, 639–654. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.; Naik, Y. Effect of a nanoclay on the mechanical, thermal and flame retardant properties of rigid polyurethane foam. J. Macromol. Sci. Part A Pure Appl. Chem. 2009, 46, 704–712. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Moo Espinosa, J.I.; Cauich-Rodríguez, J.V.; Ávila-Ortega, A.; Vázquez-Torres, H.; Marcos-Fernández, A.; San Roman, J. TGA/FTIR studies of segmented aliphatic polyurethanes and their nanocomposites prepared with commercial montmorillonites. Polym. Degrad. Stab. 2009, 94, 1666–1677. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Yang, X.; Wang, X. Thermal and mechanical properties of polyurethane/montmorillonite nanocomposites based on a novel reactive modifier. Polym. Degrad. Stab. 2004, 86, 549–555. [Google Scholar] [CrossRef]
- Cheng, A.; Wu, S.; Jiang, D.; Wu, F.; Shen, J. Study of elastomeric polyurethane nanocomposites prepared from grafted organic–montmorillonite. Colloid Polym. Sci. 2006, 284, 1057–1061. [Google Scholar] [CrossRef]
- Ramesh, S.; Punithamurthy, K. The effect of organoclay on thermal and mechanical behvaiours of thermoplastic polyurethane nanocomposites. Dig. J. Nanomater. Biostruct. 2017, 12, 331–338. [Google Scholar]
- Criado, J.M. Kinetic analysis of DTG data from master curves. Thermochim. Acta 1978, 24, 186–189. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vimalathithan, P.K.; Barile, C.; Casavola, C.; Arunachalam, S.; Battisti, M.G.; Friesenbichler, W.; Vijayakumar, C.T. Thermal degradation kinetics of polypropylene/clay nanocomposites prepared by injection molding compounder. Polym. Compos. 2019, 40, 3634–3643. [Google Scholar] [CrossRef]






| Designation | T5% (°C) | T10% (°C) | TP (°C) | Residue (%) |
|---|---|---|---|---|
| Blank PU | 235.3 | 309.1 | 372.8 | 21.8 |
| PU/Nanoclay-1 | 277.4 | 328.1 | 365.2 | 30.2 |
| PU/Nanoclay-3 | 269.3 | 322.3 | 365.8 | 28.7 |
| PU/Nanoclay-5 | 274.3 | 320.1 | 362.4 | 19.8 |
| Reaction Model | f(α) | g(α) | Model Code |
|---|---|---|---|
| Power law | 2α1/2 | α1/2 | P2 |
| Power law | 3α2/4 | α1/3 | P3 |
| Power law | 4α3/4 | α1/4 | P4 |
| Avrami-Erofeev: Two-dimensional nucleation | 2(1 − α)[−ln(1 − α)]1/2 | [−ln(1 − α)]1/2 | A2 |
| Avrami-Erofeev: Three-dimensional nucleation | 3(1 − α)[−ln(1 − α)]2/4 | [−ln(1 − α)]1/3 | A3 |
| Avrami-Erofeev: Four-dimensional nucleation | 4(1 − α)[−ln(1 − α)]3/4 | [−ln(1 − α)]1/4 | A4 |
| Contracting cylinder: Two-dimensional phase boundary reaction | 2(1 − α)1/2 | 1 − (1 − α)1/2 | R2 |
| Contracting sphere: Three-dimensional phase boundary reaction | 3(1 − α)2/3 | 1 − (1 − α)1/3 | R3 |
| Two-dimensional diffusion | [−ln(1 − α)]−1 | (1 − α)ln(1 − α) + α | D2 |
| Three-dimensional diffusion | 3/2(1 − α)2/3[1 − (1 − α)1/3]−1 | [1 − (1 − α)1/3]2 | D3 |
| Mampel (first order) | 1 − α | −ln(1 − α) | F1 |
| One-dimensional diffusion | 1/2α−1 | α2 | D1 |
| Ginstling-Brounshtein | 3/2((1 − α)−1/3 − 1) | 1 − (2α/3) − (1 − α)2/3 | D4 |
| Second order | (1 − α)2 | (1 − α)−1 − 1 | F2 |
| Third order | (1 − α)3 | [(1 − α)−1 − 1]/2 | F3 |
| Designation | Blank PU | PU/Nanoclay-1 | PU/Nanoclay-3 | PU/Nanoclay-5 | |
|---|---|---|---|---|---|
| Friedman | Eα (kJ/mol.) | 173.37 | 180.16 | 203.44 | 219.19 |
| Ln A (min−1) | 36.05 | 38.23 | 43.83 | 44.89 | |
| n | 3.59 | 3.77 | 4.56 | 2.86 | |
| m | 3.68 | 4.19 | 5.42 | 4.50 | |
| KAS | Eα (kJ/mol.) | 189.91 | 175.84 | 202.77 | 173.39 |
| Ln A (min−1) | 39.08 | 37.43 | 43.70 | 36.11 | |
| n | 3.53 | 3.78 | 4.56 | 2.87 | |
| m | 3.68 | 4.19 | 5.42 | 4.31 | |
| FWO | Eα (kJ/mol.) | 190.90 | 177.43 | 203.03 | 175.09 |
| Ln A (min−1) | 39.27 | 37.72 | 43.75 | 36.43 | |
| n | 3.53 | 3.77 | 4.56 | 2.87 | |
| m | 3.67 | 4.19 | 5.42 | 4.31 | |
| m-CR | Eα (kJ/mol.) | 189.57 | 175.49 | 202.47 | 173.02 |
| Ln A (min−1) | 39.02 | 37.36 | 43.65 | 36.03 | |
| n | 3.53 | 3.77 | 4.56 | 2.87 | |
| m | 3.68 | 4.19 | 5.42 | 4.31 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouyandeh, M.; Hadavand, B.S.; Tikhani, F.; Khalili, R.; Bagheri, B.; Zarrintaj, P.; Formela, K.; Vahabi, H.; Saeb, M.R. Thermal-Resistant Polyurethane/Nanoclay Powder Coatings: Degradation Kinetics Study. Coatings 2020, 10, 871. https://doi.org/10.3390/coatings10090871
Jouyandeh M, Hadavand BS, Tikhani F, Khalili R, Bagheri B, Zarrintaj P, Formela K, Vahabi H, Saeb MR. Thermal-Resistant Polyurethane/Nanoclay Powder Coatings: Degradation Kinetics Study. Coatings. 2020; 10(9):871. https://doi.org/10.3390/coatings10090871
Chicago/Turabian StyleJouyandeh, Maryam, Behzad Shirkavand Hadavand, Farimah Tikhani, Reza Khalili, Babak Bagheri, Payam Zarrintaj, Krzyszof Formela, Henri Vahabi, and Mohammad Reza Saeb. 2020. "Thermal-Resistant Polyurethane/Nanoclay Powder Coatings: Degradation Kinetics Study" Coatings 10, no. 9: 871. https://doi.org/10.3390/coatings10090871
APA StyleJouyandeh, M., Hadavand, B. S., Tikhani, F., Khalili, R., Bagheri, B., Zarrintaj, P., Formela, K., Vahabi, H., & Saeb, M. R. (2020). Thermal-Resistant Polyurethane/Nanoclay Powder Coatings: Degradation Kinetics Study. Coatings, 10(9), 871. https://doi.org/10.3390/coatings10090871










